Abstract
The HEALthy Brain and Child Development (HBCD) Study, a multi-site prospective longitudinal cohort study, will examine human brain, cognitive, behavioral, social, and emotional development beginning prenatally and planned through early childhood. Electroencephalography (EEG) is one of two brain imaging modalities central to the HBCD Study. EEG records electrical signals from the scalp that reflect electrical brain activity. In addition, the EEG signal can be synchronized to the presentation of discrete stimuli (auditory or visual) to measure specific cognitive processes with excellent temporal precision (e.g., event-related potentials; ERPs). EEG is particularly helpful for the HBCD Study as it can be used with awake, alert infants, and can be acquired continuously across development. The current paper reviews the HBCD Study’s EEG/ERP protocol: (a) the selection and development of the tasks (Video Resting State, Visual Evoked Potential, Auditory Oddball, Face Processing); (b) the implementation of common cross-site acquisition parameters and hardware, site setup, training, and initial piloting; (c) the development of the preprocessing pipelines and creation of derivatives; and (d) the incorporation of equity and inclusion considerations. The paper also provides an overview of the functioning of the EEG Workgroup and the input from members across all steps of protocol development and piloting.
Keywords: HBCD, EEG, Infants, Longitudinal cohort, Protocols, Resting EEG, Visual evoked potentials, Auditory oddball, Face processing
Highlights
-
•
The HEALthy Brain and Child Development (HBCD) Study is a multi-site longitudinal study.
-
•
This paper outlines the role of EEG in the study and the development of the EEG protocol.
-
•
Tasks selected: Video Resting State, Visual Evoked Potential, Auditory Oddball, Face Processing.
1. Introduction to HBCD
The HEALthy Brain and Child Development (HBCD) Study is a multi-site prospective longitudinal cohort study that will examine child development across multiple domains, beginning with the recruitment of pregnant participants and extending through early childhood (Nelson et al., 2024; Volkow et al., 2024). One core focus of the HBCD Study is to examine the impact of prenatal substance exposure, as well as pre- and post-natal environmental contexts, on long-term health and behavior. Central to the initial formulation and design of the study by the National Institutes of Health (NIH) was the proposal to examine brain development, both structure and function, beginning in early infancy (Volkow et al., 2021). Detailed sampling of brain activity using electroencephalography (EEG) was explicitly required in the initial NIH call for proposals. EEG is acquired by placing small sensors or electrodes on the scalp, and the electrical signals recorded off the scalp can be sampled quickly (e.g., 1000 Hz), amplified, and stored for subsequent analysis (Luck, 2014). When synchronized to auditory or visual stimuli presented to a participant, EEG signals can be processed to capture the associated brain responses with a temporal accuracy of milliseconds. For example, EEG signals time-locked to stimuli can provide event-related potentials (ERPs) that illuminate psychological processes even in the absence of overt behavior (Luck, 2014).
The long history of EEG in developmental studies, with the ability to measure brain activity starting early in infancy and the ability to acquire brain responses to auditory and visual stimuli in awake and alert infants and young children, makes EEG an ideal brain imaging assessment method in the HBCD Study. EEG can also be used in conjunction with magnetic resonance imaging (MRI; Dean et al., 2024), as in the HBCD Study, to capture and integrate developmentally sensitive measures of brain structure and function across both space and time.
In the current paper, we describe the theoretical and practical roles of EEG in the HBCD Study. We highlight the work of the EEG Workgroup (WG-EEG) in the process and note the considerations taken in choosing the tasks used and the practicalities of launching a study of this scale and complexity. In doing so, we also note the collaborative structure of the WG-EEG, the diversity and inclusion considerations needed, and the decisions made in the process. Finally, we note the specifics of the tasks chosen, the results of piloting, and the details of the tasks and variables the field can expect with the initial HBCD Study data releases.
2. EEG within the context of the HBCD Study
EEG was included within the broader scope of the HBCD Study to capture variation in task-based neural functioning in awake infants and children. These data can be used in a stand-alone manner to assess normative brain development, as well as examined for the impact of early exposures and adversity. In addition, EEG can be coupled with the rich corpus of data collected by the HBCD Study to examine multiple complex and nested relations across development that could leverage EEG variables as a predictor, moderator, mediator, or outcome.
Several factors make EEG particularly valuable in a cohort starting early in development, such as HBCD (Norton et al., 2021). First, EEG is non-invasive, making it infant- and child-friendly, and therefore well-suited for longitudinal studies, enabling researchers to track changes in brain activity over time, elucidating the underlying neural processes associated with cognitive, socioemotional, and psychiatric outcomes. Relative to MRI, EEG is also more tolerant to motion artifacts and has fewer physical constraints, allowing for a more active and engaged participant during data collection. In addition, familiar caregivers can remain with the child in most EEG protocols, bolstering participant tolerance of data acquisition.
Second, in addition to lending support for studies across longer developmental windows, EEG provides excellent temporal resolution at the millisecond level, capturing rapid changes in concurrent brain activity in the timescale in which cognition occurs. Third, EEG source localization techniques, when combined with MRI (see Dean et al., 2024), improve spatial resolution to provide information about the general location and timing of neural activity in specific brain regions (Conte and Richards, 2022, Liu et al., 2019, Xie et al., 2022). Finally, and particularly important for the scale of the HBCD Study, EEG is relatively affordable and mobile. This allowed all HBCD Study sites to purchase identical equipment, software, and peripherals (e.g., speakers, lighting, and monitors) to help ensure the comparability of the data collected.
EEG will provide essential data for inquiries across multiple research sub-fields. Given the focus on early exposures and experiences, EEG data will allow researchers to examine individual differences in brain activity patterns before behavioral differences may become evident. Researchers can thus identify stable individual traits or track changes in neural signatures over time, examining how the brain adapts and develops in response to experiences, learning opportunities, and environmental inputs.
3. Operationalizing HBCD Study goals in the EEG protocol
Incorporating a robust EEG protocol in a study as complex as HBCD required a two-pronged approach, focused on both the specific EEG-based tasks selected and the scientific infrastructure that would support data collection across 27 different testing sites. The HBCD EEG Workgroup (WG-EEG) is led by Dr. Nathan A. Fox, from the University of Maryland (UMD), who is an Associate Director of the HBCD Data Coordinating Center (HDCC; Nelson et al., 2024). To carry out the work of embedding EEG across the longitudinal study, representatives from all HBCD Study sites came together to form the WG-EEG. Here we describe how the WG-EEG carried out its mission and decision making.
3.1. WG-EEG structure and function
At the outset of HBCD Study planning, the WG-EEG met weekly as a large group to discuss the scientific and practical concerns of creating an EEG protocol that could be robustly and reliably implemented at an unprecedented scale. In addition to the large group meetings, sub-groups were formed to meet twice monthly to focus on specific areas of work. One group focused on task design and site training, while the second group focused on the data processing pipelines. The WG-EEG members, in turn, worked with the staff of the EEG Core team at the UMD. The EEG Core supported task design and coding, equipment purchasing, site training, and data quality control checks. To integrate the EEG protocol with the larger HBCD Study, the WG-EEG Chair and Co-Chair (Dr. Koraly Pérez-Edgar from The Pennsylvania State University) met weekly and then twice monthly with the leadership of all the Workgroups (Nelson et al., 2024) to make sure that components of the study were compatible. Conversations within and across Workgroups were also supported and overseen by the HBCD Consortium Administrative Core (HCAC).
3.2. Study protocol and task selection
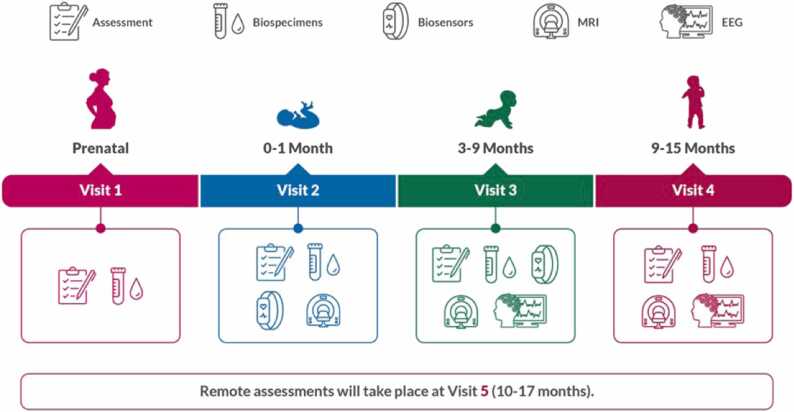
One of the first considerations was selecting the timepoints at which EEG tasks would be deployed across the longitudinal study. We aimed to efficiently capture meaningful variation and developmental change in neural activity in domains important for early cognitive, auditory, visual, linguistic, and social-emotional development. To enable integration of EEG with the other central brain imaging measure, MRI, members of the WG-EEG worked with members of the MRI Workgroup (WG-MRI) to ensure that the EEG and MRI data were collected in a way that allows for linked analysis by the scientific community. Therefore, the decision was made that study visits involving EEG data collection would occur within two weeks of an MRI scan. Ideally, study visits are scheduled such that EEG and MRI data are collected a few days apart, if not the same day. The central goal was to design tasks that were both developmentally appropriate and feasible across the early ages of study, given time constraints, staffing, and family burden. As such, EEG would be collected at every in-person visit from Visit 3, which occurs at three to nine months of age, onwards (see Fig. 1).
Fig. 1.
Schematic of the first set of HBCD Study visits. EEG is collected at Visits 3 and 4.
We then examined how to best create an EEG protocol that could be embedded within the larger HBCD Study. In doing so, we looked to balance the burden on the infant participants and their caregivers, the study site research staff, and the UMD Core that would be processing and checking the incoming data. Given the length of many of the study visits, we wished to maximize the amount of data collected from our participants while minimizing the amount of time needed. As such, our goal was to create a developmentally appropriate protocol that could be implemented in 45–60 minutes, with no more than 30 minutes of active participation time for the infant or child.
The WG-EEG targeted domains that reflected core developmental processes, had a rich history in the larger developmental literature, were known to be sensitive to early exposure to substances or adversity, and could be effectively deployed across a wide range of settings by researchers with varying levels of expertise. Fortunately, EEG provides a vast array of potential variables that can be derived from the same underlying signal, varying with the specific combinations of either stimulus presentation timing, stimulus type, scalp location of the electrodes, or type of analysis. Derivatives collected via simultaneous electrocardiogram (ECG) collection will complement this approach with information regarding the development of the autonomic branch of the nervous system (Sania et al., 2023).
In consultation with both the WG-EEG members and the broader HBCD Study consortium, we selected four core tasks. They include (1) a Video Resting State, (2) a Visual Evoked Potential task, (3) an Auditory Oddball task, and (4) a Face Processing task. (Specific task rationale and parameters are provided in Section 4.)
3.3. EEG equipment and software considerations
We also considered how to best implement the tasks across all sites, while also ensuring a robust, reliable, and consistent data collection process across the over 7000 participants. Although this is a concern for any multi-site study, the breadth, depth, and complexity of the HBCD Study, and the WG-EEG’s inability to have hands-on control of all data collection, demanded systems and procedures that were not overly cumbersome for site staff or for the staff involved with dissemination, training, and quality control.
To maximize our ability to easily prepare the infant for data collection, monitor data quality in real time, and present stimuli in a standardized manner, we decided to use MagStim EGI (Eugene, OR, USA) for the data acquisition hardware and software. The WG-EEG worked closely with MagStim representatives to create a standardized package of equipment and software (see Table 1) that was sent to all sites, installed in a relatively uniform manner, and deployed for data collection. The initial configuration included a Net Station Amps 400 system with 128-channel nets and an ECG electrode to record the electrical activity of the heart. This allows for simultaneous ECG collection, which will complement the EEG-based variables with measures of autonomic nervous system activity. For stimulus presentation, E-Prime software was selected (version 3.0; Psychology Software Tools, 2016) with an additional stimulus presentation computer and monitor, and a camera for recording the child during EEG data collection (to be used for quality control). To ensure accuracy and precision of stimulus presentation timing across all sites and visits, we employed a Cedrus StimTracker integrated with the MagStim software and hardware to measure the onset of visual and auditory stimuli.
Table 1.
Specific metrics and descriptors for the equipment, settings, and tasks used in the initial study launch. Note, the ISI listed here for the Auditory Oddball task is used in Visit 3. In later visits, the ISI is 600 ms.
 |
The UMD EEG Core with the consultation of the WG-EEG provided each site with all stimuli and code needed to run each task. In addition, we worked closely with the developers of the Longitudinal Online Research and Imaging System (LORIS) to develop a protocol to upload, validate, organize, conduct quality control, and ultimately generate derivatives from the collected data. This included the development of software, called the “BIDS Wizard,” to align with the brain imaging data structure (BIDS; Gorgolewski et al., 2016) and scale our preprocessing pipeline to provide feedback to sites in a timely manner (described in detail in Section 5). One of the issues in installing hardware and software across multiple sites was the information technology (IT) protections and restrictions that many institutions had regarding downloading and installing software on computers. As such, we worked to choose specifications that were likely to be acceptable to as many sites as possible (Table 1).
3.4. Site set-up and training
In disseminating the tasks, software, and hardware, the EEG Core staff, working with the WG-EEG, instituted a hands-on support process to ensure that data generated by the HBCD Study were of high quality and comparable across all study sites. Sites varied in their experience with EEG in general, infant EEG specifically, and the use of MagStim for data collection. Approximately 80 % of sites had at least some study members with pediatric EEG expertise. Representatives from MagStim traveled to every location to help support the installation and initial testing of the EEG software and hardware, as some sites had not previously used MagStim equipment. The EEG Core from UMD then set up an individual video meeting with each site. During those meetings, sites demonstrated their physical lab setup and the necessary ancillary equipment for EEG acquisition. They also conducted a mock EEG acquisition, usually with a research assistant from their team acting as the participant.
Results of these video visits allowed the UMD staff to identify sites that needed additional training to optimize their data collection configurations. In-person visits were then conducted to those sites. The UMD staff supervised a final “sign-off” visit via video call with all sites wherein the site demonstrated the entire EEG acquisition protocol and received feedback on protocol accuracy and basic EEG competencies, such as correct EEG net placement, proficiency in the protocol, and troubleshooting. Sites had to achieve at least 85 % accuracy according to a standardized assessment rubric before they could begin collecting pilot data with infants. It should be noted that there was, and is, some variability in the physical set-up among sites. Some sites have two dedicated rooms for EEG acquisition, one for the participant, presentation monitor, speakers, and StimTracker, and a second control room where the EEG acquisition computer with MagStim software and E-Prime computer and monitor are housed. Some sites have only one room but use curtains to separate participants from researchers. There are some sites that have all the equipment on a cart that is wheeled in and out depending on use. Sites also vary in the acoustic and electric shielding of the participant spaces, but a decibel meter and electric noise tri-field recorder/sniffer are used to ensure minimal effects on data.
3.5. Piloting the EEG protocol
After initial training and confirmation of setup for hardware and software, sites were tasked with running at least three pilot participants in the age range for the first EEG visit (Visit 3; 3–9 months). The EEG Core staff, along with the WG-EEG, reviewed incoming data for quality, examining the data collected against the standard literature. Special care was taken to note deviations in either protocol or expected data quality evident with a task or a site. The equipment manuals and standard operating procedures documents were revised based on what was learned from the video and in-person visits and piloting.
De-identified records from each pilot visit were uploaded via our custom-developed software (BIDS Wizard) to the online database where the data were organized and stored. Any identifiable materials were sent to a secure server at the University of Minnesota, part of the HBCD Data Coordinating Center. UMD staff downloaded the files and ran two preprocessing pipelines (described below) to determine the amount of usable data for each task. As part of protocol, sites also had to provide five pictures of the EEG net placement per participant displaying angles from the front, back, above, left, and right. Finally, the site had to correctly upload the files to the online database (LORIS; see Section 5). To “pass” piloting, each site was required to acquire 30 usable EEG trials per stimulus condition for at least three of the four tasks from each of the three infants. This threshold was determined by conducting preliminary reliability analyses (as described in Morales et al., 2022) with existing pediatric datasets. Additionally, sites had to obtain usable EEG data for each of the four tasks at least once among the three pilot infants.
By the end of piloting, over 200 data files had been uploaded and processed, with small variations in participant numbers by task (Table 2). Infants were evenly split between male and female. The age range was 2.9–12.2 months, as some sites were allowed to test older infants to maintain the training timeline for full study launch.
Table 2.
Number of infants who contributed data to the piloting process. This information was used to determine task parameters for the full HBCD Study.
| Video Resting State | Visual Evoked Potential | Auditory Oddball | Face Processing | |
|---|---|---|---|---|
| Infants With Task Data Collected | 234 | 227 | 224 | 225 |
| Files Excluded Due to Technical Issues | 27 | 26 | 18 | 34 |
|
Files Processed (No Technical Issues) |
207 | 201 | 206 | 191 |
| Passed Preprocessing QC | 193 | 200 | 197 | 113 |
Data derivatives and metrics were computed for the pilot phase. Overall, the tasks proved robust, and the vast majority of infants were able to attempt all four tasks (range: 91.95–96.55 %). Task completion times also fit with general expectations, with average lengths of 2.95 (SD = 0.90) minutes for the Video Resting State, 1.13 (SD = 0.29) minutes for the Visual Evoked Potential, 11.85 (SD = 3.19) minutes for the Auditory Oddball, and 8.74 (SD = 4.95) minutes for the Face Processing task.
4. EEG Tasks in the HBCD Study
The tasks and stimuli are publicly available and can be found in the following link: https://github.com/ChildDevLab/Tasks
4.1. Video Resting State
Video Resting State provides information on spontaneous perturbations in electrical activity (Deco et al., 2011, Deco et al., 2013), with ontogenetic changes reflecting developing large-scale neural networks associated with self-regulatory, cognitive, and affective processes and developmental outcomes (Gabard-Durnam et al., 2019; Jones et al., 2020; Whedon et al., 2020). Similar baseline tasks have been extensively used in the infant EEG literature to investigate language, cognitive, and socioemotional outcomes and have shown to produce a reliable signal (Brito et al., 2022). The metrics one can derive from the resting EEG signal include power across the frequency spectrum (Gabard-Durnam et al., 2019), relative power between different scalp location (e.g., frontal alpha asymmetry (Davidson and Fox, 1982), neural connectivity across different scalp regions (Debnath et al., 2021), nonlinear dynamics (Rodriguez-Bermudez and Garcia-Laencina, 2015), and both periodic and aperiodic signals (Donoghue et al., 2020). Importantly, early adversity and prenatal exposure to alcohol and other substances are associated with “spontaneous” EEG activity (Pini et al., 2024, Shuffrey et al., 2020). Moreover, many of these outputs have robust psychometric properties (e.g. internal consistency and test-retest reliability) across development to facilitate examining individual differences (Anaya et al., 2021, Lopez et al., 2023). Thus, the task is highly appropriate for HBCD goals.
The task presents a 3-minute silent video on a computer monitor in front of the child. The video for Visit 3 is a compilation of clips taken from infant-friendly videos that display colorful toys and abstract images. The video for later visits contains alternating 30 second clips of construction scenes and marble runs. The transitions between clips cross-dissolve to avoid harsh shifts between scenes. Images shift in the video in order to maintain a calm, but alert, infant throughout data collection. Using a video also helps ensure greater standardization of experience for infants in the study.
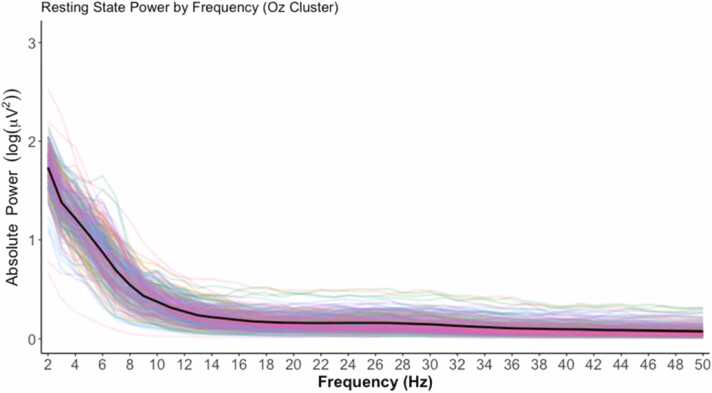
In piloting, we observed a clear logarithmic decrease which followed a “1/frequency” pattern in single Hz bins when plotting power across increasing frequencies. We also transformed the data into relative power by dividing each of the frequency bins by the sum total across these frequencies. At this time, we are providing a figure of the power spectrum with single Hz bins from 2 to 50 Hz rather than power in different frequency bands (e.g., alpha, beta). The width and boundaries of these bands change with age, even across the age range of the current pilot data (Cuevas & Bell, 2022).
Fig. 2.
Representation of EEG signal collected during the Video Resting State Task as power spectra for relative power in the 2–50 Hz in the occipital (OZ) cluster. The black line represents the average across all participants and the lines in color represent each individual.
4.2. Auditory oddball
Auditory Oddball tasks have been used successfully in infants, children, and adults to probe detection of differences between standard (frequent) and deviant (rarely presented) sounds, which can be in the form of speech stimuli or tones. The difference between ERPs to standard and deviant sounds produces a negative wave in adults called the mismatch negativity (MMN), and a positive wave in infants, referred to as the mismatch response (MMR). The amplitude/latency of these difference waves have been linked to language (Choudhury and Benasich, 2011), temperament/personality (Gurrera et al., 2001, Marshall et al., 2009), internalizing problems (Reeb-Sutherland et al., 2009), externalizing/attention problems (Gumenyuk et al., 2005), and disorders including autism (Lepistö et al., 2005, Schwartz et al., 2018) and reading ability/dyslexia (Leppänen et al., 2010, Norton et al., 2021). The topography of the MMN difference wave differs based on stimulus type, but typically includes an early component from scalp regions over auditory cortex followed by a later component over frontal areas.
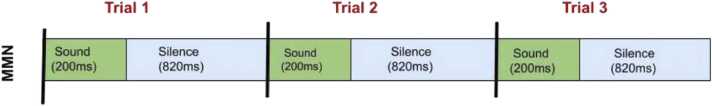
The HBCD task consists of natural speech consonant-vowel syllable stimuli that are presented in a ratio of 85 % standard and 15 % deviant (see Fig. 3). We sought advice regarding the use of tones vs. speech syllables, ultimately deciding to use the syllables as they have been widely studied in infants and most closely linked to language outcomes. The sounds themselves (previously used in Norton et al., 2021) were provided by WG-EEG member Dr. Elizabeth Norton (Northwestern University), using a task structure provided by Dr. April Benasich (Rutgers University). The task consists of 667 trials wherein the participant hears either a /ba/ or /da/ syllable. One sound is a “standard”, which is played in 567 trials and the other sound is a “deviant”, which is randomly played in 100 trials. Whether the /ba/ or /da/ syllable is the standard sound is randomized across participants and for each visit. Each sound has a duration of 200 ms and the interstimulus interval is 820 ms for Visit 3 and 600 ms for Visit 4. The ISI was changed between age visits to account for developmental differences in processing speed that impact the generation of an MMN (Morr et al., 2002). There is a silent video played on an iPad in front of the participant throughout the task. For Visit 3, the video displays colorful toys and abstract images. Later, the video consists of safari, aquarium, and dancing fruit videos which switch every 30 seconds. The task is 11 minutes long for Visit 3 and 8.5 minutes for later visits.
Fig. 3.
Sequencing of stimuli sounds, /ba/ or /da/, in the MMN task. The ISI noted is used in Visit 3. The ISI is 600 ms for later visits.
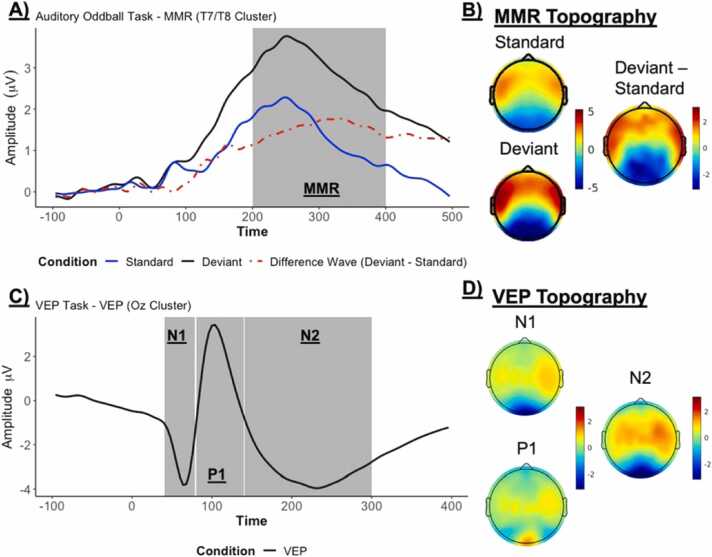
In piloting, we observed a clear mismatch response (MMR). This was indicated as a more positive response for deviant compared to standard syllable stimuli over frontal and temporal electrodes between 200 and 400 ms (see Fig. 4A & 4B). This positive MMR response over frontolateral sites is consistent with existing studies with a similar paradigm (Hämäläinen et al., 2019, Ortiz-Mantilla et al., 2023).
Fig. 4.
Selected ERP Waveforms for the Auditory Oddball (MMN/MMR) and Visual Evoked Potential (VEP) tasks. A) Auditory Oddball task ERP plots by condition for the Deviant, Standard, and their difference wave (Deviant – Standard) and B) their topography during the 200–400 ms time window. Note that only standard stimuli immediately preceding a deviant syllable were used. C) ERP plots for the VEP with the N1, P1, and N2 components labeled, and D) their topography during their respective time windows.
4.3. Visual Evoked Potential (VEP)
Visual Evoked Potential (VEP) tasks, like the pattern-reversal VEP task employed in HBCD, often consist of a series of visually contrasting images (e.g., black and white checkerboard) that oscillate at a specific frequency. The stimulus presentation elicits its namesake ERP that matures over the first three years of life. The VEP has been associated with concurrent and later developmental outcomes as a function of prenatal substance exposures (Margolis et al., 2024), early visual enrichment or deprivation (Jensen et al., 2019), vision system maturation (Lippé et al., 2009), neurodevelopmental disorders (e.g., ASD and ADHD; Cremone-Caira et al., 2023; Nazhvani et al., 2013), and reading and learning disabilities (Shandiz et al., 2017).
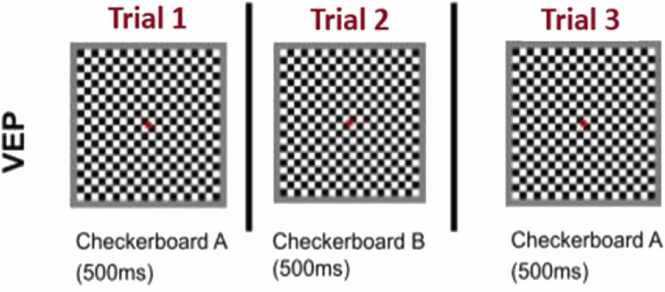
The task consists of a series of black and white checkerboard images. The checkerboard pattern reverses at 2 Hz (i.e. each checkerboard is presented for 500 ms), with the white tiles turning black and vice versa (see Fig. 3). For this task, the participants view 120 checkerboards in total. Infants sit approximately 60 cm from the screen. The task is 60 seconds in duration. This task does not change across the early visits.
Fig. 5.
Visual representation of the stimuli used in the VEP task.
In piloting, we observed a clear VEP response, comprised of three deflections in the occipital area (Oz), corresponding to the visual cortex. Specifically, this task generates the N1 (the first negative peak), the P1 (the first positive peak), and the N2 (the second negative peak), which were clearly observed in our pilot data (see Fig. 4C & 4D).
4.4. Face Processing
Face Processing is an important skill used for successfully navigating the social world. The development of face processing begins early in life and is predicted to be impacted by visual and social experiences (Markant and Scott, 2018, Scherf and Scott, 2012). The neural structures supporting face processing begin to specialize across the first year of life and continue to develop into childhood and adolescence (Di Lorenzo et al., 2020, Scherf and Scott, 2012, Scott and Arcaro, 2023). Faces engage and impact several behavioral and neural systems including attention, perception, language development, memory, emotion, attachment and social processing (Markant and Scott, 2018). Variations in face processing are also associated with neurodevelopmental disorders (e.g., autism spectrum disorder, Tye et al., 2022; Webb et al., 2017). ERPs generated by face processing tasks can capture a wide range of experience-dependent perceptual, cognitive and social effects (Barry-Anwar et al., 2020). As such, we included a task that captures neural responses to faces as a function of standard (or canonical) orientation (upright versus inverted) as well as the predicted cortical prioritization of faces relative to novel objects.
Stimuli for this task include grayscale faces from female-presenting adults (Pickron et al., 2024) and novel objects called Sheinbugs (T. Jones et al., 2020). Stimuli were processed using Adobe Photoshop and the SHINE toolbox in Matlab (Willenbockel et al., 2010) to equate luminance, projected image size, eye location and orientation, under the direction Dr. Lisa Scott and her team at the University of Florida (see Fig. 6). Each face was superimposed on a grayscale background of either greenery, sand, ocean water, pool water, or the sky (based on Guy et al., 2016). The backgrounds were made at UMD by the EEG Core with the advice of Dr. John Richards (University of South Carolina) and Dr. Scott. To encourage infants to maintain fixation on the center of the screen throughout the task, the background images were incrementally blurred from the center to the edge of the screen, resulting in five levels of visual clarity (Guy et al., 2016, Mallin and Richards, 2012, Xie and Richards, 2016). The final stimulus set consists of 30 “Sheinbug” novel objects, and 36 face stimuli provided by individuals from white, Black, Asian, Latine, and Indigenous communities.
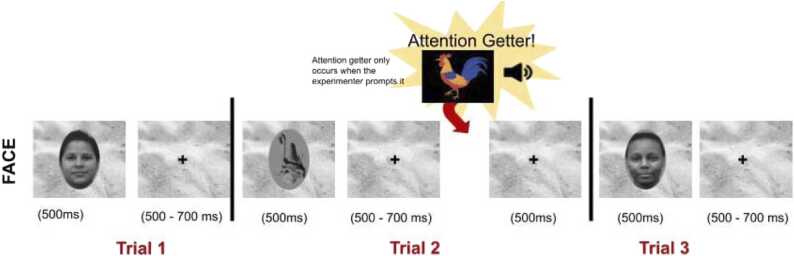
Fig. 6.
Schematic of the Faces task used in Visits 3, 4, and 6, depicting the Face/Object block. The attention getter is one of several toys, paired with a sound, displayed by a research assistant to return infant gaze to the screen. Trials advance by RA button press when infant is attending to the screen.
The task consists of two blocks. In one block, the participants view 50 upright faces and 50 inverted faces. In the other block, there are 50 upright faces and 50 abstract objects. The stimuli are drawn randomly from the set and are presented for 500 ms with a jittered 500–700 ms interstimulus interval. Trials progress with a button press from the experimenter based on the infant’s attention to the computer screen. Attention getters are triggered by the experimenter, as necessary, to keep the participant's attention oriented to the screen. The images are presented randomly with replacement, and no face can be displayed twice in a row. This task does not change across the early visits.
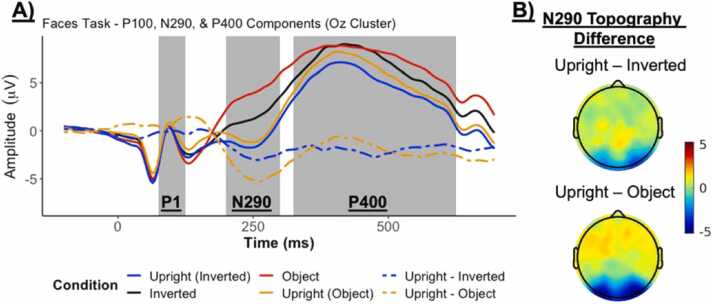
In piloting, we observed the expected P1 response in occipital clusters across all conditions. We also observed an N290 response to upright faces in occipital clusters, regardless of the block, such that there was a negative deflection compared to objects and inverted faces (Fig. 7). Finally, we also observed the expected P400 response with a similar shape across conditions.
Fig. 7.
Selected ERP Waveforms for the Face Processing task: A) ERP plots by condition for the Upright faces during the Inverted faces block, Objects, Inverted faces, and Upright faces during the Object block with the P1, N290, and P400 components labeled. Also displayed are the difference waves between Upright – Inverted and Upright – Object as dashed lines and B) their topography during the N290 time window (200–300 ms).
4.5. EEG tasks in the HBCD Study protocol at Launch
Tasks are presented in a pseudorandom order: Video Resting State is always presented first, with the remaining three tasks presented in a random order set centrally by the HDCC and communicated to the site staff before the participant visit. The decision to always begin the EEG protocol with the Video Resting State was made by the WG-EEG to avoid carryover effect from a task. The order of the remaining three tasks is re-randomized for every subsequent study visit. The randomization ensured that no one task was “disadvantaged” by always being last and potentially systematically being skipped or providing poor data. In reviewing the published literature and the pilot data, we found no evidence of order effects. During the EEG protocol, the infant participant sits in a high-chair or on the caregiver’s lap. Importantly, this initial protocol was optimized for the young infants and children in the first set of visits. For visits at older ages, the WG-EEG will work to modify the protocol to match the participants’ developmental stage.
5. Transfer protocols for EEG data
To develop protocols for data transfer, preprocessing, and ultimately generating outputs, we established an EEG preprocessing subcommittee led by Dr. Santiago Morales (University of Southern California). The subcommittee opted to standardize all acquired datasets into the BIDS format for preprocessing and analysis. A custom build of the LORIS platform was selected to store and stage data (Das et al., 2012).
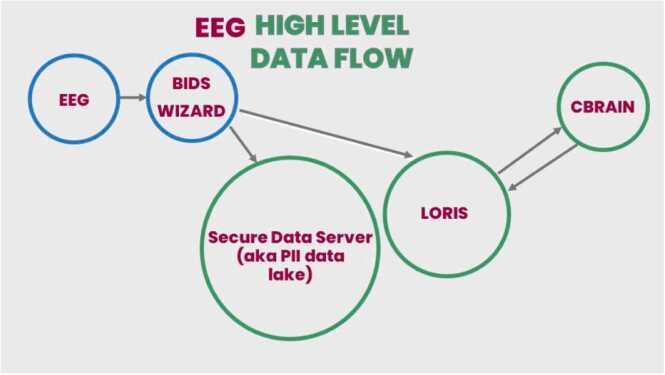
The BIDS Wizard was then developed to address the challenge of uploading and validating raw EEG data from different sites. It takes the raw MagStim (.MFF) and E-Prime (.EDAT and.txt) files acquired from a site and transforms them to a more commonly used BIDS-compliant format (.SET files). During the upload process, the BIDS Wizard also runs local checks to make sure that the correct number of files were uploaded, naming conventions were followed, and expected number of events and tasks were acquired in the expected manner (see Table 3 for list of checks). If any of these checks fail, research assistants are asked to document any deviations to the protocol in a standardized manner. Moreover, the BIDS Wizard also uploads the photos taken to confirm EEG net placement and original data (.MFF files), which contain videos of the EEG session. These files are sent to a separate secure server at UMN that is HIPAA-compliant, given that the photos and videos of the EEG acquisition contain personal identifying information (PII). Multiple iterations of the BIDS Wizard program were developed and modified as issues arose while sites attempted to upload data. A schematic of the data flow developed by the EEG subcommittee and the LORIS team is illustrated in Fig. 8.
Table 3.
List of local checks run via the BIDS Wizard to ensure that data meet basic quality checks before upload to the shared server, LORIS.
| Checks | What is this check? | What happens if it fails? |
|---|---|---|
| Naming Checks | Ensures file names match naming convention | Ask site to rename before uploading |
| File Checks | Ensures we get all the files we expect (i.e., participants, tasks, pictures, etc.) | Ask site to explain missing or additional files |
| Check for number of events/flags | Ensures number of flags/trials are what we expect for each task and condition | Ask site why there is a discrepancy in number of events and automatically inform Core about the mismatch |
| BIDS Conversion | Ensures file is in BIDS format | Ask the site to format data before uploading. If errors with conversion, contact Core |
| Check for Flag Delays | Ensure that the E-Prime flags are not delayed | Ask site if there was a deviation from the protocol in this visit (e.g., starting E-Prime before NS). Inform Core |
Fig. 8.
Conceptual schematic of the data flow used by HBCD to move EEG data from the collection site to the central storage and processing servers.
To determine and optimize the parameters and metrics for the launch of the full study and data releases, we processed the pilot data with a variety of settings (e.g., filter settings, and artifact detection threshold). We also compared, “in-house” pipelines from WG-EEG members, the Harvard Automated Processing Pipeline for EEG (HAPPE v.2.2; Monachino et al., 2022) and the Maryland Analysis of Developmental EEG (MADE) pipeline (Debnath et al., 2020, Leach et al., 2020). Because each pipeline and preprocessing approach has strengths and limitations, we initially wanted to preprocess the data with both pipelines. These comparisons revealed that the HAPPE pipeline generally kept more data, but significantly reduced the amplitude of some condition effects in the ERP tasks. Due to practical constraints, we had to select one preprocessing pipeline for initial data releases. We decided to utilize MADE for the initial releases of HBCD Study data. A paper by members of the EEG WG is currently being written to describe the pipeline comparisons and metrics that were used to decide between pipelines.
6. Processing Pipelines
Once data are uploaded to LORIS, files are run through the MADE pipeline (Debnath et al., 2020, Leach et al., 2020). The pipeline is implemented by the high-performance computing (HPC) facility at the University of Minnesota through CBRAIN – a web-based software that integrates LORIS with the HPC. The details of this preprocessing approach have been described in detail in Debnath et al. (2020). In short, channels that include the electrocardiogram data are excluded, and the continuous EEG data are high-pass filtered at 0.3 Hz and low-pass filtered at 50 Hz. All tasks are then merged into one large recording to increase the size of the data and improve independent component analysis (ICA) decomposition. Bad channels are identified and removed using the EEGLAB plug-in FASTER (Nolan et al., 2010). To further remove ocular and muscle artifacts, ICA is performed on an identical copy of the dataset.
To improve ICA decomposition, this copied dataset is high pass filtered at 1 Hz and segmented into 1 s epochs. Then, noisy segments of the data and EMG-like activity are rejected using a voltage threshold of +/-1000 μV and spectral threshold (range −100 dB to +30 dB) within the 20–40 Hz frequency band. If a channel contains artifact in more than 20 % of the epochs, that channel is removed from both the ICA copied dataset and the original dataset. ICA is then run on the copied dataset, and ICA weights are subsequently applied back to the original dataset (Debner et al., 2010). Artifactual independent components are identified and removed from the original dataset using the Adjusted-ADJUST algorithm, which was optimized for pediatric data (Leach et al., 2020, Mognon et al., 2011).
The data are then segmented into task-specific epochs for two additional steps of artifact rejection. For event-related data preprocessed for ERPs, a low-pass filter of 30 Hz is applied prior to segmenting, and all epochs are time-locked to the presentation of the stimuli (i.e., checkerboard, face/object, or sound). The average of the pre-event baseline period is subtracted from each epoch. The resting state data are segmented into 2-second epochs with 50 % overlap. To capture the presence of residual ocular activity which may be left after ICA, any epochs in which ocular channel (E8, E14, E21, and E25) voltages exceed ±200 μV are removed and interpolated. Second, non-ocular channels that exceeded ±200 μV are interpolated at the epoch level. However, if over 10 % of the channels (not considering globally rejected channels) exceeded ±200 μV, the epoch is rejected instead. Any remaining missing channels are then interpolated using the spherical spline method (Perrin et al., 1989). The data are then referenced to the average reference.
6.1. Derivatives provided in the initial data releases
For the initial annual data releases, the HBCD Study will provide processed variables in addition to raw data files. In short, for the Video Resting State, we will provide absolute and relative power in 1-Hz bins. For all ERP variables, we will provide mean amplitude, peak amplitude, peak latency for each component for every participant, as well as the number of artifact-free trials. For the VEP task, we will provide these derivatives for the occipital N1, P1, and N2 responses. For the Auditory Oddball task, we will provide the frontal and temporal ERPs for the standard trials (all available), the standard trials just prior to presentation of a deviant, and the deviant trials. In addition, a difference wave (MMR/MMN) will be computed. Data for the P1 and N2 sensory components (frontocentral midline) will also be provided. For the Face Processing task, we will provide metrics for the occipital P1, N290, P400, and the frontocentral Nc (not shown). Finally, semi-processed ‘raw’ data that have been labelled and formatted into BIDS will also be available for those interested in deriving other variables.
7. Diversity, equity, and inclusion considerations
Much of the extant developmental EEG data are derived from individuals who are disproportionately white, urban, and middle- or higher-socio-economic status (Penner et al., 2023, Rowley and Camacho, 2015, Webb et al., 2017). In addition, researchers have historically avoided acquiring EEG from individuals with voluminous, porous, and/or curly hair due to an improper assumption that thin straight hair was ideal for acquiring EEG signals (Choy et al., 2022, Louis et al., 2022, Parker and Ricard, 2022), contributing to the systematic exclusion of Black, Latine, and Indigenous communities from neuroscience research (Brown et al., 2023). Even when more diverse participants are included, a lack of training with diverse hair types often leads to poorer data quality and greater exclusion rates. As such, at the outset, the WG-EEG was keenly aware of the need to improve our approach and we continue to devote time, training, and implementation towards ensuring that all families can participate in the study in an equitable manner. In doing so, we worked closely with the Diversity, Equity, and Inclusion Workgroup (WG-DEI; Murray et al., 2024) and sought advice from a wide range of consortium members.
As with the other aspects of the study, we approached the issue of equity and inclusion from multiple angles. First, following recommendations from another large-scale study with diverse participants (Baby’s First Years), we worked with MagStim to modify the EEG nets to extend the pedestal length (distance from scalp contact to net), to ensure that the electrodes can reach through voluminous hair to establish good scalp contact for all participants (Adams et al., 2024, Mlandu et al., 2024). Each site was instructed to supplement their standard array of nets by purchasing a set of nets with long pedestals. Second, we worked with Dr. Laurel Gabard-Durnam (Northeastern University), Dr. Caitlin Hudac (University of South Carolina), and Dr. Sonya Troller-Renfree (Teachers College, Columbia University) to train research assistants in net placement strategies and procedures using the MagStim-EGI nets (guidelines available in Hudac et al., 2022). Drs. Gabard-Durnam and Hudac led dedicated training workshops, and Drs. Gabard-Durnam, Hudac, and Troller-Renfree advised WG-EEG and the EEG staff in creating written standard operating procedures. The SOP and scripts were reviewed and approved by members of the WG-DEI.
Third, as part of this process, we adapted existing scripts provided by Dr. Troller-Renfree (Adams et al., 2024) to help research assistants discuss any caregiver concerns prior to the scheduled EEG visit. We are also working with the Communications, Engagement, and Dissemination Workgroup (WG-CED; Cole et al., 2024) to create family-facing documents. We made sure to emphasize the social and cultural importance of hair, particularly for Black families. The documentation and training explicitly emphasize that caregivers are the experts on the infant’s hair and comfort needs. Staff are advised to learn about how the child’s hair is styled (e.g., braids, twists, puffs) and any other hair-related needs (e.g., hair should not get wet) before the visit. We work with caregivers to schedule visits and modify net placement to meet data collection needs without disturbing the child’s hair. If the caregiver wishes, we can schedule a visit around the time they were already planning to adjust styles (e.g., removing and rebraiding hair). At some of the HBCD sites, researchers are partnering with curly hair specialists to offer guidance to staff, participating families, and/or preferred community hairstylists on preparing curly hair for successful and equitable EEG data collection. Other sites also offer post visit styling to interested participants.
We also made sure that the actual tasks were equally accessible to all participants. One primary focus was on the Face Processing task. The literature is clear that infant responses to faces are shaped by their daily experiences and exposures to different phenotypic traits (Bar-Haim et al., 2006). As such, our proposal was to create a data set with exemplars of faces from different communities that would reflect the families of infants likely to participate in the study. The original stimulus set included faces from individuals who self-identified as white, Black, Asian, and Latine. During the consultation process, the WG-DEI noted that the set did not include face stimuli from individuals from Indigenous or Native communities. Given the distribution of sites in HBCD (Nelson et al., 2024), this could disproportionately and adversely impact a subset of infants. As a result, the staff of the EEG core at UMD worked with members of the consortium site from Oklahoma State University, particularly Jordan Love, to identify female-identifying adults from the local Indigenous community. Pictures of the volunteers were selected and then added to the extant face set. All faces were then processed under the supervision of Dr. Scott to ensure uniformity across the stimuli. Similarly, the Auditory Oddball stimuli /ba/ and /da/ were selected as those sounds exist and occur with similar frequency in English and Spanish, the two languages in which the study is conducted (Anunziata et al., 2024).
8. Conclusions
The WG-EEG began its work in the Fall of 2021 to create the EEG protocol within the larger HBCD Study. The membership grew steadily with the addition of representatives from all sites, and experts were included to address the evolution of procedural, contextual, and technical needs. The membership now represents all facets of HBCD, including Principal Investigators, Co-Investigators, Post-doctoral Fellows, Project Coordinators, and Research Staff. As a result, we have input from multiple perspectives in the study and can identify and incorporate changes as needed. With the launch of full-study data collection in July 2023, the WG-EEG is focused on assessing and ensuring continued high data quality. In addition, researchers and staff are implementing the data processing pipeline that will generate derivatives for each data release. Importantly, the specifics for the current protocol noted above are not set in stone. We will continue to monitor and modify tasks and procedures as needed for future visits. Indeed, we are keenly aware that the initial protocol was optimized for use with children through ∼36 months and will not remain suitable for use across time, as participants age into later visits. As such, the WG-EEG is currently assessing potential changes to the current tasks, removal or addition of tasks to match developmental repertoires. In doing so, we will continue to closely collaborate with HBCD Study leadership, the other Workgroups, and the members of the WG-EEG.
CRediT authorship contribution statement
Sonya V. Troller-Renfree: Writing – review & editing, Validation, Resources, Methodology. Enda Tan: Validation, Software, Methodology, Data curation. Lauren C. Shuffrey: Writing – review & editing. Lisa S. Scott: Writing – review & editing, Validation, Supervision, Software, Resources, Methodology, Investigation, Funding acquisition, Conceptualization. Tara M. Rutter: Writing – review & editing. Timothy P. Roberts: Writing – review & editing, Methodology, Investigation. Rachel Reetzke: Writing – review & editing, Resources, Methodology, Investigation. Natalie Brito: Writing – original draft, Writing – review & editing. Elizabeth S. Norton: Writing – review & editing, Resources, Methodology, Funding acquisition. The HBCD EEG Workgroup: Project administration, Conceptualization. Ernest V. Pendapati: Writing – review & editing, Software, Methodology, Data curation. Lydia Yoder: Writing – review & editing, Software, Methodology, Investigation, Formal analysis, Data curation. Laura J. Larson-Prior: Supervision, Investigation. Maeve R. Boylan: Writing – review & editing, Methodology, Investigation. Martín Antúnez: Writing – review & editing, Visualization, Validation, Software, Methodology, Formal analysis, Data curation. Bailey M. Garner: Writing – review & editing, Methodology, Investigation. Alena Quinn: Writing – review & editing, Resources, Methodology, Investigation. James F. Cavanaugh: Software, Methodology. Nicolò Pini: Writing – review & editing, Software, Methodology. Alana M Campbell: Writing – review & editing, Resources, Investigation. Olufemi Shakuur Nyabingi: Writing – review & editing, Software, Methodology, Investigation. Santiago Morales: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jessica Norris: Software, Data curation. Koraly Perez-Edgar: Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Maria Isabella Natale Castillo: Writing – review & editing, Resources, Methodology. Nathan A Fox: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Marco McSweeney: Visualization, Validation, Software, Methodology, Formal analysis, Data curation. Savannah McNair: Software, Data curation. Britley Learnard: Writing – review & editing. Alexandra P. Key: Writing – review & editing, Investigation. Caitlin Hudac: Writing – review & editing, Supervision, Resources, Methodology. Rachel Stosur: Software, Data curation. Laurel Joy Gabard-Durnam: Writing – review & editing, Validation, Software, Resources, Methodology, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
The data that has been used is confidential.
References
- Adams E.J., Scott M.E., Amarante M., Ramírez C.A., Rowley S.J., Noble K.G., Troller-Renfree S.V. Fostering inclusion in measures of pediatric brain activity: Implications for education and policy. Npj Sci. Learn. 2024 doi: 10.1038/s41539-024-00240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaya B., Ostlund B., LoBue V., Buss K., Pérez-Edgar K. Psychometric properties of infant electroencephalography: Developmental stability, reliability, and construct validity of frontal alpha asymmetry and delta-beta coupling. Dev. Psychobiol. 2021;63(6) doi: 10.1002/dev.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anunziata F., Cisneros C., Castillo M.I.N., Perez A., Rodriguez V., De La Cruz S., Estrada K., Durbal A., Jaramillo M., Marquez L.E., Nunez J., Peralta-Carcelen M., Wisnowski J.L., the HEALthy Brain & Child Development (HBCD) Spanish Language Committee ¿Donde están? Hispanic/Latine inclusion, diversity and representation in the Healthy Brain and Child Development Study (HBCD) Dev. Cogn. Neurosci. 2024 [Google Scholar]
- Bar-Haim Y., Ziv T., Lamy D., Hodes R.M. Nature and Nurture in Own-Race Face Processing. Psychol. Sci. 2006;17(2):159–163. doi: 10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Barry-Anwar R., Riggins T., Scott L.S. Electrophysiology in developmental populations: Key methods and findings. Oxf. Handb. Dev. Cogn. Neurosci. 2020 [Google Scholar]
- Brito N.H., Werchan D., Brandes-Aitken A., Yoshikawa H., Greaves A., Zhang M. Paid maternal leave is associated with infant brain function at 3 months of age. Child Dev. 2022;93(4):1030–1043. doi: 10.1111/cdev.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Rollock D., Foti D. Conducting electroencephalography with black individuals: barriers, recommendations, and impact on generalizability. Policy Insights Behav. Brain Sci. 2023;10(2):178–185. doi: 10.1177/23727322231197739. [DOI] [Google Scholar]
- Choudhury N., Benasich A.A. Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. 2011;122(2):320–338. doi: 10.1016/j.clinph.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Choy T., Baker E., Stavropoulos K. Systemic Racism in EEG Research: Considerations and Potential Solutions. Affect. Sci. 2022;3(1):14–20. doi: 10.1007/s42761-021-00050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K.M., Jordan C.J., Parkinson M., Estrada K., Hoffman E.A., Croff J.M., Freund M.P., Howlett K.D., The Communications, Engagement, and Dissemination Workgroup Communications, engagement, and dissemination strategies for the HEALthy Brain and Child Development Study (HBCD) Dev. Cogn. Neurosci. 2024 doi: 10.1016/j.dcn.2024.101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte S., Richards J.E. Cortical Source Analysis of Event-Related Potentials: A Developmental Approach. Dev. Cogn. Neurosci. 2022;54 doi: 10.1016/j.dcn.2022.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremone-Caira A., Braverman Y., MacNaughton G.A., Nikolaeva J.I., Faja S. Reduced Visual Evoked Potential Amplitude in Autistic Children with Co-Occurring Features of Attention-Deficit/Hyperactivity Disorder. J. Autism Dev. Disord. 2023 doi: 10.1007/s10803-023-06005-7. [DOI] [PubMed] [Google Scholar]
- Cuevas K., Bell M.A. In: In The Oxford Handbook of EEG Frequency. Gable P., Miller M., Bernat E., editors. Oxford University Press; 2022. EEG frequency development across infancy and childhood; pp. 293–323. [Google Scholar]
- Das S., Zijdenbos A.P., Harlap J., Vins D., Evans A.C. LORIS: A web-based data management system for multi-center studies. Front. Neuroinformatics. 2012;5:37. doi: 10.3389/fninf.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Fox N.A. Asymmetrical Brain Activity Discriminates Between Positive and Negative Affective Stimuli in Human Infants. Science. 1982;218(4578):1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Dean D.C., Tisdall M.D., Wisnowski J.L., Fezko E., Gagoski B., Alexandar A.L., Edden R.A.E., Gao W., Hendrickson T.J., Howell B.R., Huang H., Humphreys K.L., Riggins T., Sylvester C.M., Weldon K.B., Yacoub E., Ahtam B., Beck N., Banerjee S., Elison J.T. Quantifying brain development in Quantifying Brain Development in HEALthy Brain and Child Development (HBCD) Study: The Magnetic Resonance Imaging and Spectroscopy Protocol. Dev. Cogn. Neurosci. 2024 doi: 10.1016/j.dcn.2024.101452. [DOI] [PubMed] [Google Scholar]
- Debnath R., Buzzell G.A., Morales S., Bowers M.E., Leach S.C., Fox N.A. The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology. 2020;57(6) doi: 10.1111/psyp.13580. [DOI] [PubMed] [Google Scholar]
- Debnath R., Miller N.V., Morales S., Seddio K.R., Fox N.A. Investigating brain electrical activity and functional connectivity in adolescents with clinically elevated levels of ADHD symptoms in alpha frequency band. Brain Res. 2021;1750 doi: 10.1016/j.brainres.2020.147142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debner S., Thorne J., Schneider T.T., Viola F.C. In: Simultaneous EEG and fMRI. Ullsperger M., Debner S., editors. Oxford University Press; 2010. Using ICA for the analysis of multi-channel EEG data; pp. 121–135. [DOI] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 2011;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Resting brains never rest: Computational insights into potential cognitive architectures. Trends Neurosci. 2013;36(5):268–274. doi: 10.1016/j.tins.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo R., van den Boomen C., Kemner C., Junge C. Charting development of ERP components on face-categorization: Results from a large longitudinal sample of infants. Dev. Cogn. Neurosci. 2020;45 doi: 10.1016/j.dcn.2020.100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T., Haller M., Peterson E.J., Varma P., Sebastian P., Gao R., Noto T., Lara A.H., Wallis J.D., Knight R.T. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020;23(12):1655–1665. doi: 10.1038/s41593-020-00744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Wilkinson C., Kapur K., Tager-Flusberg H., Levin A.R., Nelson C.A. Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nat. Commun. 2019;10(1):1–12. doi: 10.1038/s41467-019-12202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K.J., Auer T., Calhoun V.D., Craddock R.C., Das S., Duff E.P., Flandin G., Ghosh S.S., Glatard T., Halchenko Y.O., Handwerker D.A., Hanke M., Keator D., Li X., Michael Z., Maumet C., Nichols B.N., Nichols T.E., Pellman J., Poldrack R.A. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci. Data. 2016;3(1) doi: 10.1038/sdata.2016.44. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumenyuk V., Korzyukov O., Escera C., Hämäläinen M., Huotilainen M., Häyrinen T., Oksanen H., Näätänen R., Von Wendt L., Alho K. Electrophysiological evidence of enhanced distractibility in ADHD children. Neurosci. Lett. 2005;374(3):212–217. doi: 10.1016/j.neulet.2004.10.081. [DOI] [PubMed] [Google Scholar]
- Gurrera R.J., O’Donnell B.F., Nestor P.G., Gainski J., McCarley R.W. The P3 auditory event–related brain potential indexes major personality traits. Biol. Psychiatry. 2001;49(11):922–929. doi: 10.1016/S0006-3223(00)01067-2. [DOI] [PubMed] [Google Scholar]
- Guy M.W., Zieber N., Richards J.E. The Cortical Development of Specialized Face Processing in Infancy. Child Dev. 2016;87(5):1581–1600. doi: 10.1111/cdev.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen J.A., Ortiz-Mantilla S., Benasich A. Change detection to tone pairs during the first year of life–predictive longitudinal relationships for EEG-based source and time-frequency measures. NeuroImage. 2019;198:83–92. doi: 10.1016/j.neuroimage.2019.05.034. [DOI] [PubMed] [Google Scholar]
- Hudac C.M., Wallace J.S., Ward V.R., Friedman N.R., Delfin D., Newman S.D. Dynamic cognitive inhibition in the context of frustration: Increasing racial representation of adolescent athletes using mobile community-engaged EEG methods. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.918075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S.K., Kumar S., Xie W., Tofail F., Haque R., Petri W.A., Nelson C.A. Neural correlates of early adversity among Bangladeshi infants. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-39242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.J., Goodwin A., Orekhova E., Charman T., Dawson G., Webb S.J., Johnson M.H. Infant EEG theta modulation predicts childhood intelligence. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-67687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T., Hadley H., Cataldo A.M., Arnold E., Curran T., Tanaka J.W., Scott L.S. Neural and behavioral effects of subordinate-level training of novel objects across manipulations of color and spatial frequency. Eur. J. Neurosci. 2020;52(11):4468–4479. doi: 10.1111/ejn.13889. [DOI] [PubMed] [Google Scholar]
- Leach S.C., Morales S., Bowers M.E., Buzzell G.A., Debnath R., Beall D., Fox N.A. Adjusting ADJUST: Optimizing the ADJUST algorithm for pediatric data using geodesic nets. Psychophysiology. 2020;57 doi: 10.1111/psyp.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepistö T., Kujala T., Vanhala R., Alku P., Huotilainen M., Näätänen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Res. 2005;1066(1–2):147–157. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Leppänen P.H., Hämäläinen J.A., Salminen H.K., Eklund K.M., Guttorm T.K., Lohvansuu K., Puolakanaho A., Lyytinen H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010;46(10):1362–1376. doi: 10.1016/j.cortex.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Lippé S., Kovacevic N., McIntosh A.R. Differential Maturation of Brain Signal Complexity in the Human Auditory and Visual System. Front. Hum. Neurosci. 2009;3:48. doi: 10.3389/neuro.09.048.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Bai X., Pérez-Edgar K.E. Integrating high-density ERP and fMRI measures of face-elicited brain activity in 9–12-year-old children: An ERP source localization study. NeuroImage. 2019;184:599–608. doi: 10.1016/j.neuroimage.2018.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez K.L., Monachino A.D., Vincent K.M., Peck F.C., Gabard-Durnam L.J. Stability, change, and reliable individual differences in electroencephalography measures: A lifespan perspective on progress and opportunities. NeuroImage. 2023;275 doi: 10.1016/j.neuroimage.2023.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C.C., Webster C.T., Gloe L.M., Moser J.S. Hair me out: Highlighting systematic exclusion in psychophysiological methods and recommendations to increase inclusion. Front. Hum. Neurosci. 2022;16:1058953. doi: 10.3389/fnhum.2022.1058953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J. MIT Press; 2014. An Introduction to the Event-Related Potential Technique. [Google Scholar]
- Mallin B.M., Richards J.E. Peripheral stimulus localization by infants of moving stimuli on complex backgrounds. Infancy. 2012;17(6):692–714. doi: 10.1111/j.1532-7078.2011.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis E.T., Davel L., Bourke N.J., Bosco C., Zieff M.R., Monachino A.D., Mazubane T., Williams S.R., Miles M., Jacobs C.A. Longitudinal effects of prenatal alcohol exposure on visual neurodevelopment over infancy. Dev. Psychol. 2024 doi: 10.1037/dev0001727. 〈https://psycnet.apa.org/record/2024-66755-001〉 [DOI] [PubMed] [Google Scholar]
- Markant J., Scott L.S. Attention and perceptual learning interact in the development of the other-race effect. Curr. Dir. Psychol. Sci. 2018;27(3):163–169. [Google Scholar]
- Marshall P.J., Reeb B.C., Fox N.A. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Dev. Sci. 2009;12(4):568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlandu N., McCormick S.A., Davel L., Zieff M.R., Bradford L., Herr D., Jacobs C.A., Khumalo A., Knipe C., Madi Z., Mazubane T., Methola B., Mhlakwaphalwa T., Miles M., Nabi Z.G., Negota R., Nkubungu K., Pan T., Samuels R., Gabard-Durnam L.J. Evaluating a novel high-density EEG sensor net structure for improving inclusivity in infants with curly or tightly coiled hair. Dev. Cogn. Neurosci. 2024;67 doi: 10.1016/j.dcn.2024.101396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A., Jovicich J., Bruzzone L., Buiatti M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48(2):229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Monachino A.D., Lopez K.L., Pierce L.J., Gabard-Durnam L.J. The HAPPE plus Event-Related (HAPPE+ER) software: A standardized preprocessing pipeline for event-related potential analyses. Dev. Cogn. Neurosci. 2022;57 doi: 10.1016/j.dcn.2022.101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S., Bowers M.E., Leach S.C., Buzzell G.A., Fifer W., Elliott A.J., Fox N.A. Time–frequency dynamics of error monitoring in childhood: An EEG study. Dev. Psychobiol. 2022;64(3) doi: 10.1002/dev.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morr M.L., Shafer V.L., Kreuzer J.A., Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear. 2002;23(2):118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Murray T.M., Slopen N., the HEALthy Brain and Child Development (HBCD) Diversity, Equity, and Inclusion (DEI) Workgroup Investment, integration, and innovation: fostering diversity, equity, and inclusion across the HEALthy Brain and Child Development Study (HBCD) Consortium. Dev. Cogn. Neurosci. 2024 doi: 10.1016/j.dcn.2024.101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazhvani A.D., Boostani R., Afrasiabi S., Sadatnezhad K. Classification of ADHD and BMD patients using visual evoked potential. Clin. Neurol. Neurosurg. 2013;115(11):2329–2335. doi: 10.1016/j.clineuro.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Nelson C.A., Frankeberger J., Chambers C.D. An Introduction to the HEALthy Brain and Child Development Study. Dev. Cogn. Neurosci. 2024 doi: 10.1016/j.dcn.2024.101441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H., Whelan R., Reilly R.B. FASTER: Fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods. 2010;192(1):152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Norton E.S., Beach S.D., Eddy M.D., McWeeny S., Ozernov-Palchik O., Gaab N., Gabrieli J.D. ERP mismatch negativity amplitude and asymmetry reflect phonological and rapid automatized naming skills in English-speaking kindergartners. Front. Hum. Neurosci. 2021;15 doi: 10.3389/fnhum.2021.624617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton E.S., MacNeill L.A., Harriott E.M., Allen N., Krogh-Jespersen S., Smyser C.D., Rogers C.E., Smyser T.A., Luby J., Wakschlag L. EEG/ERP as a pragmatic method to expand the reach of infant-toddler neuroimaging in HBCD: Promises and challenges. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.100988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Roesler C.P., Realpe-Bonilla T., Benasich A.A. Experience-dependent effects of passive auditory exposure in infants impact theta phase synchrony and predict later language. Cereb. Cortex. 2023 doi: 10.1093/cercor/bhad063. bhad063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker T.C., Ricard J.A. Structural racism in neuroimaging: Perspectives and solutions. Lancet Psychiatry. 2022;9(5) doi: 10.1016/S2215-0366(22)00079-7. [DOI] [PubMed] [Google Scholar]
- Penner F., Wall K.M., Guan K.W., Huang H.J., Richardson L., Dunbar A.S., Groh A.M., Rutherford H.J.V. Racial disparities in EEG research and their implications for our understanding of the maternal brain. Cogn., Affect., Behav. Neurosci. 2023;23(1):1–16. doi: 10.3758/s13415-022-01040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Echallier J.F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pickron C.B., Brown A., Hudac C.M., Scott L.S. Gazing faces: A new racially diverse eye gaze stimulus set (iMAP-DG) Behavior Research Methods. 2024 doi: 10.3758/s13428-024-02504-2. [DOI] [PubMed] [Google Scholar]
- Pini N., Sania A., Rao S., Shuffrey L.C., Nugent J.D., Lucchini M., McSweeney M., Hockett C., Morales S., Yoder L., Ziegler K., Perzanowski M.S., Fox N.A., Elliott A.J., Myers M.M., Fifer W.P. In Utero Exposure to Alcohol and Tobacco and Electroencephalogram Power During Childhood. JAMA Netw. Open. 2024;7(1) doi: 10.1001/jamanetworkopen.2023.50528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools, I. (2016). E-Prime 3.0 [Computer software]. 〈https://support.pstnet.com/〉.
- Reeb-Sutherland B.C., Vanderwert R.E., Degnan K.A., Marshall P.J., Pérez-Edgar K., Chronis-Tuscano A., Pine D.S., Fox N.A. Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. J. Child Psychol. Psychiatry. 2009;50(11):1365–1372. doi: 10.1111/j.1469-7610.2009.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Bermudez G., Garcia-Laencina P.J. Analysis of EEG signals using nonlinear dynamics and chaos: a review. Appl .Math. nf. Sci. 2015;9(5):2309. [Google Scholar]
- Rowley S.J., Camacho T.C. Increasing diversity in cognitive developmental research: issues and solutions. J. Cogn. Dev. 2015;16(5):683–692. doi: 10.1080/15248372.2014.976224. [DOI] [Google Scholar]
- Sania A., Myers M.M., Pini N., Lucchini M., Nugent J.D., Shuffrey L.C., Rao S., Barbosa J., Angal J., Elliott A.J. Prenatal smoking and drinking are associated with altered newborn autonomic functions. Pediatr. Res. 2023;93(1):242–252. doi: 10.1038/s41390-022-02060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Scott L.S. Connecting developmental trajectories: Biases in face processing from infancy to adulthood. Dev. Psychobiol. 2012;54(6):643–663. doi: 10.1002/dev.21013. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Shinn-Cunningham B., Tager-Flusberg H. Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci. Biobehav. Rev. 2018;87:106–117. doi: 10.1016/j.neubiorev.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L.S., Arcaro M.J. A domain-relevant framework for the development of face processing. Nat. Rev. Psychol. 2023;2(3):183–195. [Google Scholar]
- Shandiz J.H., Heyrani M., Sobhani-Rad D., Salehinejad Z., Shojaei S., Khoshsima M.J., Azimi A., Yekta A.A., Yazdi S.H.H. Pattern Visual Evoked Potentials in Dyslexic Children. J. Ophthalmic Vis. Res. 2017;12(4):402–406. doi: 10.4103/jovr.jovr_106_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuffrey L.C., Myers M.M., Isler J.R., Lucchini M., Sania A., Pini N., Nugent J.D., Condon C., Ochoa T., Brink L. Association between prenatal exposure to alcohol and tobacco and neonatal brain activity: Results from the safe passage study. JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.4714. e204714–e204714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye C., Bussu G., Gliga T., Elsabbagh M., Pasco G., Johnsen K., Charman T., Jones E.J., Buitelaar J., Johnson M.H. Understanding the nature of face processing in early autism: a prospective study. J. Psychopathol. Clin. Sci. 2022;131(6):542. doi: 10.1037/abn0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Gordon J.A., Bianchi D.W., Chiang M.F., Clayton J.A., Klein W.M., Koob G.F., Koroshetz W.J., Pérez-Stable E.J., Simoni J.M., Tromberg B.J., Hommer R., Spotts E.L., Xu B., Zehr J.L., Cole K.M., Dowling G., Freund M.P., Howlett K.D.…Weiss S.R.B. The HEALthy Brain and Child Development (HBCD) study: NIH collaboration to understand the impacts of prenatal and early life experiences on brain development. Developmental Cognitive Neuroscience. 2024;69:101423. doi: 10.1016/j.dcn.2024.101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Gordon J.A., Freund M.P. The Healthy Brain and Child Development Study—Shedding Light on Opioid Exposure, COVID-19, and Health Disparities. JAMA Psychiatry. 2021;78(5):471–472. doi: 10.1001/jamapsychiatry.2020.3803. [DOI] [PubMed] [Google Scholar]
- Webb S.J., Neuhaus E., Faja S. Face Perception and Learning in Autism Spectrum Disorders. Q. J. Exp. Psychol. 2017;70(5):970–986. doi: 10.1080/17470218.2016.1151059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whedon M., Perry N.B., Bell M.A. Relations between frontal EEG maturation and inhibitory control in preschool in the prediction of children’s early academic skills. Brain Cogn. 2020;146 doi: 10.1016/j.bandc.2020.105636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbockel V., Sadr J., Fiset D., Horne G.O., Gosselin F., Tanaka J.W. Controlling low-level image properties: The SHINE toolbox. Behav. Res. Methods. 2010;42(3):671–684. doi: 10.3758/BRM.42.3.671. [DOI] [PubMed] [Google Scholar]
- Xie W., Richards J.E. Effects of interstimulus intervals on behavioral, heart rate, and event-related potential indices of infant engagement and sustained attention. Psychophysiology. 2016;53(8):1128–1142. doi: 10.1111/psyp.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Toll R.T., Nelson C.A. EEG functional connectivity analysis in the source space. Dev. Cogn. Neurosci. 2022;56 doi: 10.1016/j.dcn.2022.101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.