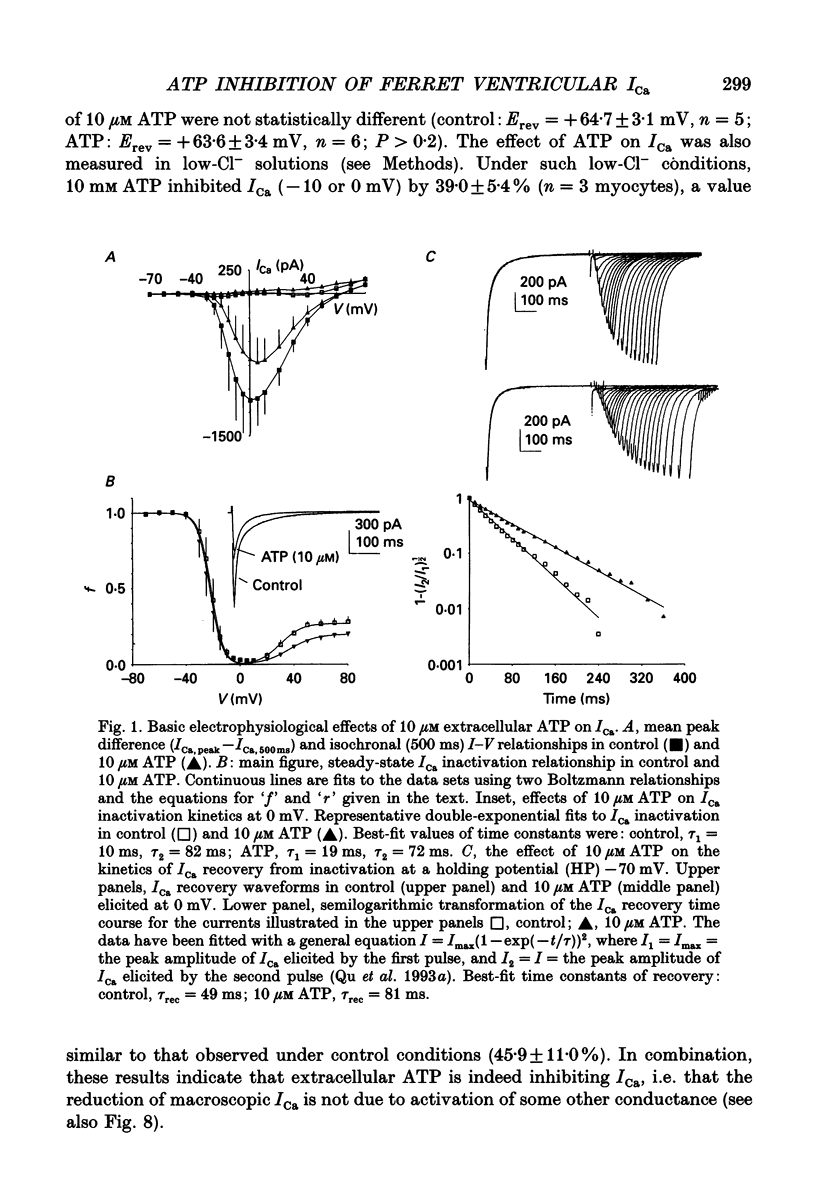
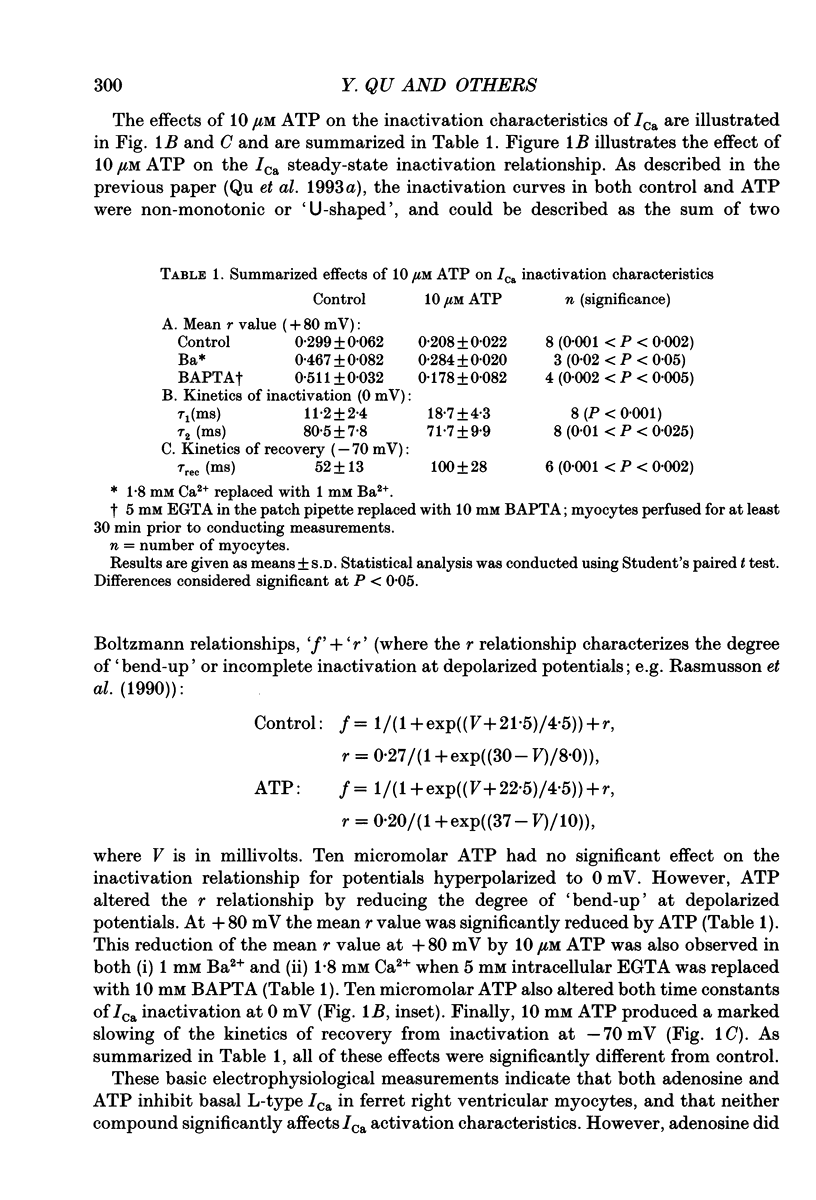
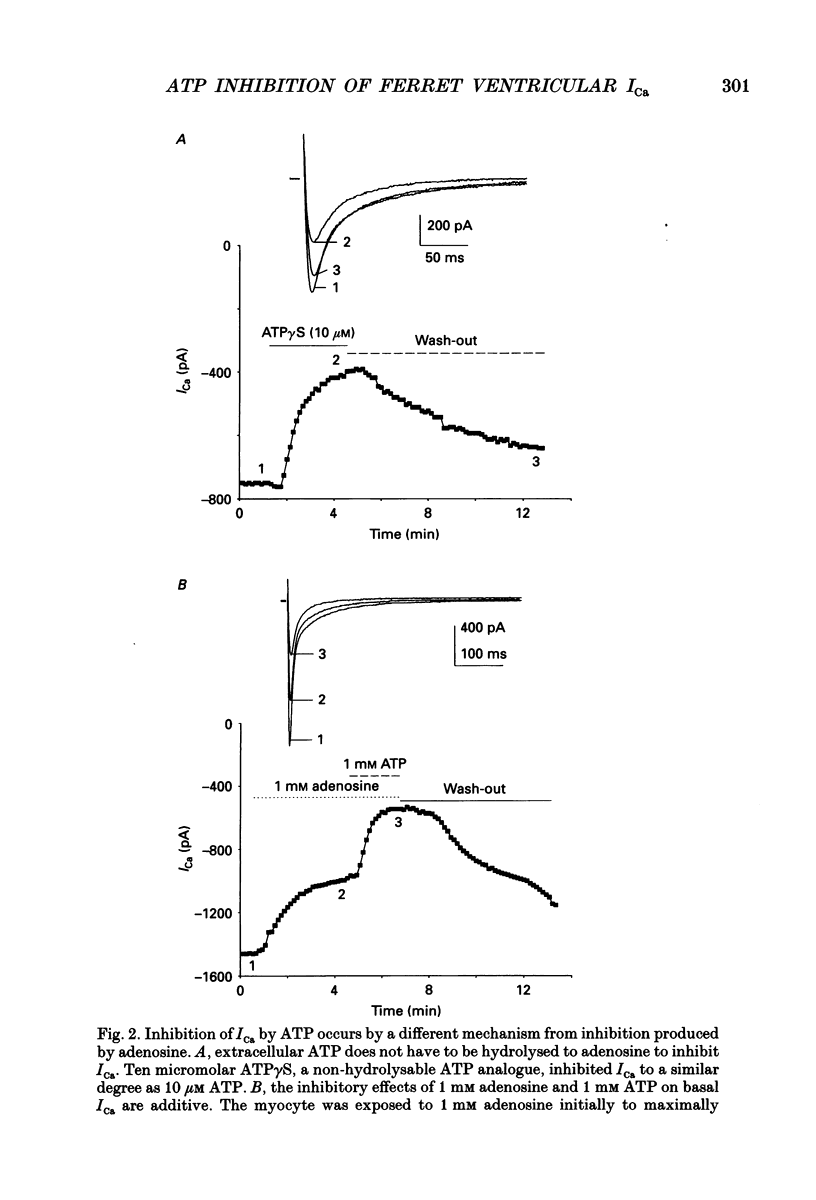
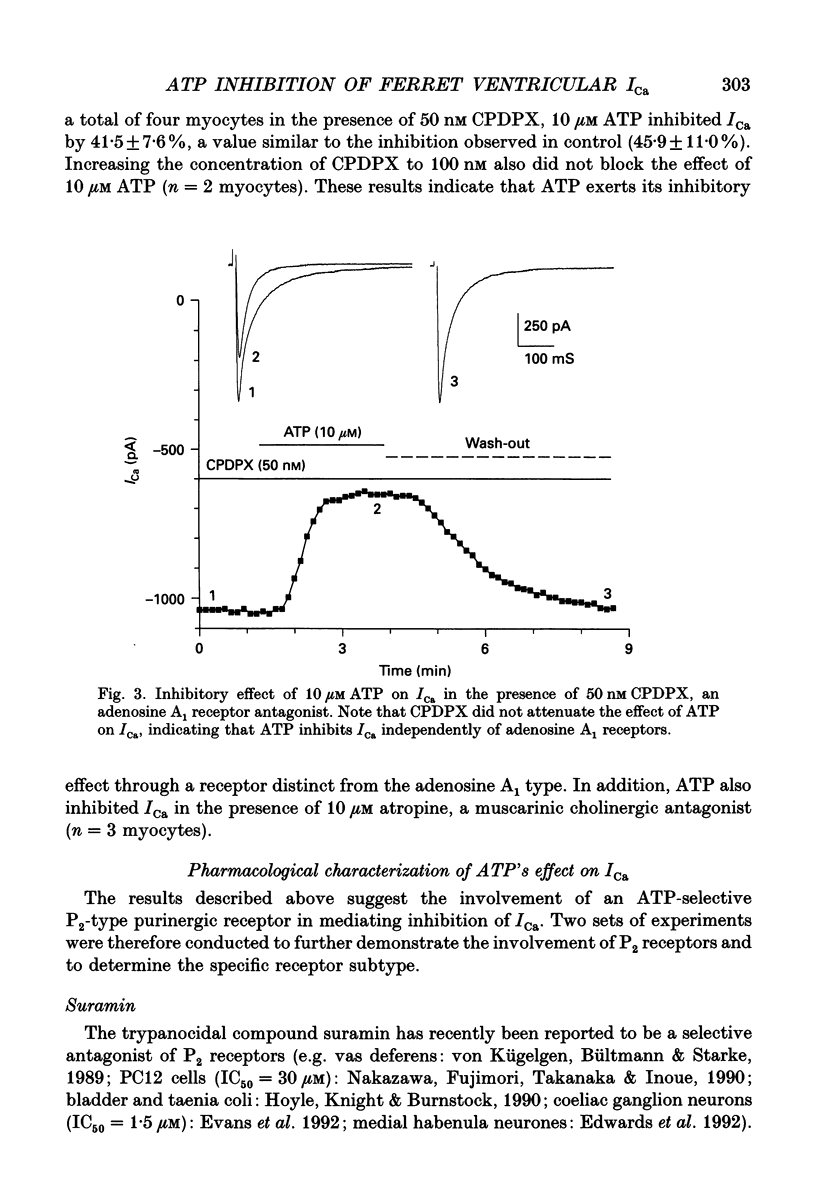
Abstract
1. The effects of extracellular adenosine triphosphate (ATP) on the basal L-type Ca2+ current (ICa) were investigated in ferret isolated right ventricular myocytes using the gigaohm seal voltage clamp in the whole-cell and cell-attached configurations. 2. Micromolar levels of extracellular ATP reversibly inhibited ICa in a concentration-dependent manner, without any significant changes in the voltage dependence of either the peak ICa I-V relationship or steady-state activation curve. 3. In contrast, micromolar levels of extracellular ATP did significantly alter the inactivation characteristics of ICa. Ten micromolar ATP: (i) increased the degree of steady-state inactivation of ICa; (ii) altered the time constants of ICa inactivation at 0 mV; and (iii) decreased the time constant of ICa recovery from inactivation at -70 mV. 4. The inhibitory effect of ATP on ICa was not blocked by atropine, a muscarinic cholinergic receptor antagonist, or CPDPX (8-cyclopentyl-3,4-dipropylxanthine), an A1 adenosine receptor antagonist. In contrast, the inhibitory effect of 10 microM ATP could be nearly completely antagonized by 100 microM suramin, a purinergic P2 receptor antagonist. 5. The potency order of ATP analogues in inhibiting ICa was 2-methyl-thio-ATP > ATP > alpha,beta-methylene-ATP, indicating involvement of a P2Y-type ATP receptor. 6. Pretreatment of cells with pertussis toxin (PTX) did not prevent the ATP-induced decrease in ICa. However, (i) ATP produced an irreversible decrease of ICa in the presence of intracellular GTP gamma S, and (ii) the inhibitory effect was significantly attenuated in the presence of intracellular GDP beta S, indicating the involvement of a PTX-insensitive G protein in the P2Y receptor-coupling process. 7. Neither (i) replacing extracellular Ca2+ with 1 mM Ba2+, nor (ii) intracellular perfusion of 10 mM BAPTA for at least 30 min attenuated the inhibitory effect of ATP on the current through Ca2+ channels, suggesting that the inhibitory effect was not obligatorily dependent upon influx of Ca2+ or changes in [Ca2+]i. 8. Ensemble-average current behaviour constructed from cell-attached patch recordings of single L-type Ca2+ channels (110 mM BaCl2) demonstrated that when 10 microM ATP was added to the superfusate on the outside of the patch electrode the inhibition of ICa was still observed, providing evidence for the involvement of intracellular diffusible second messenger(s).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




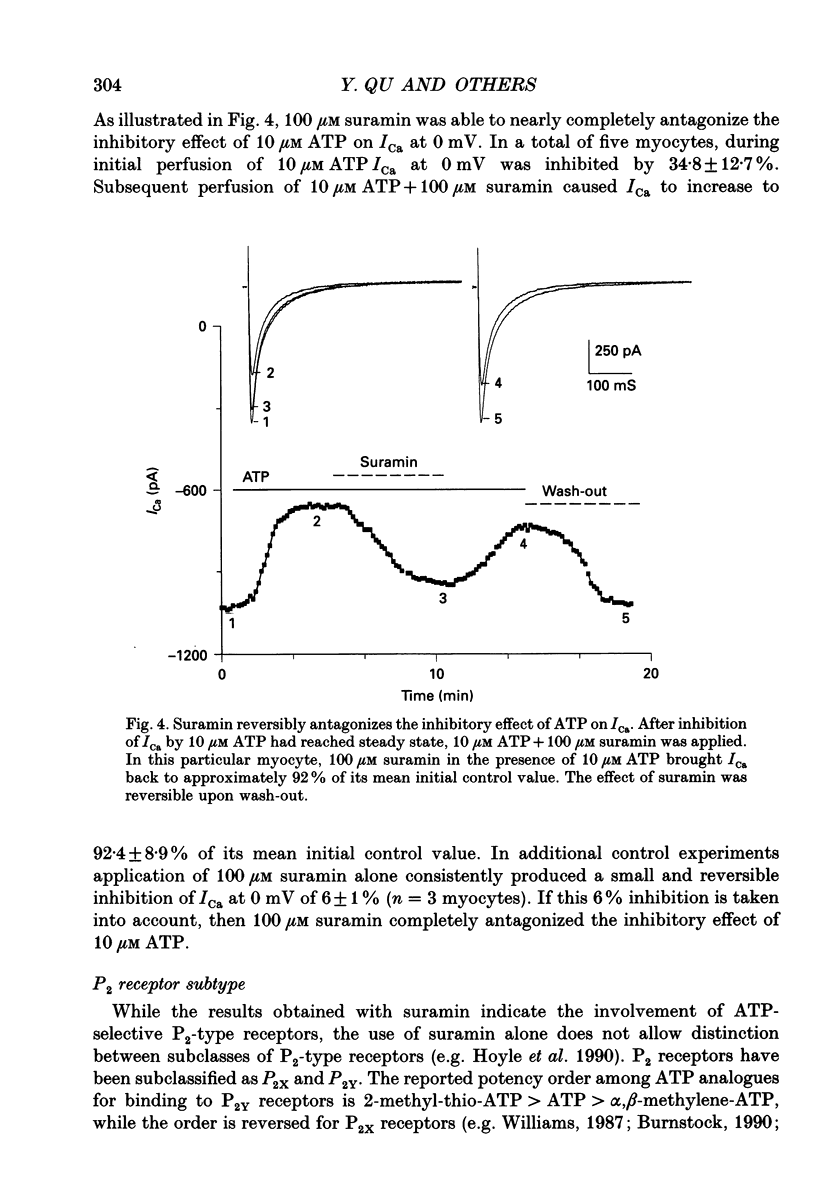
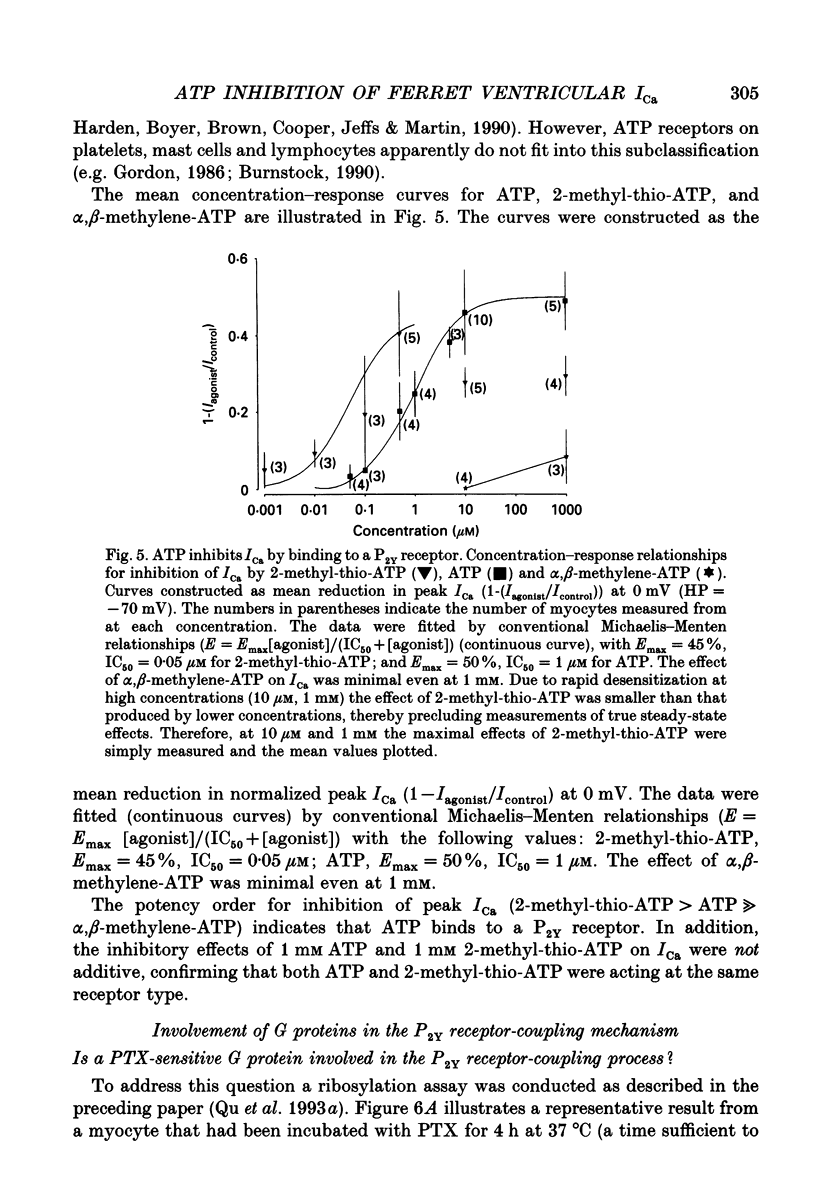
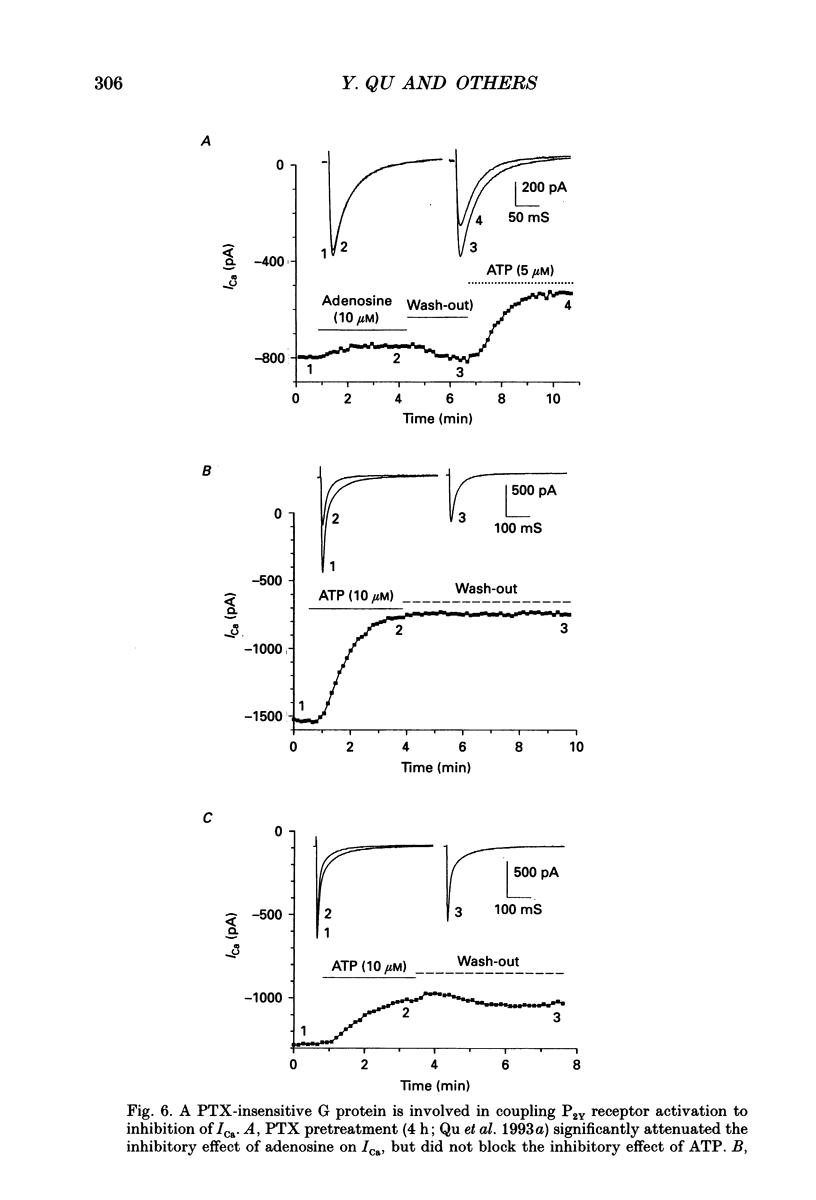

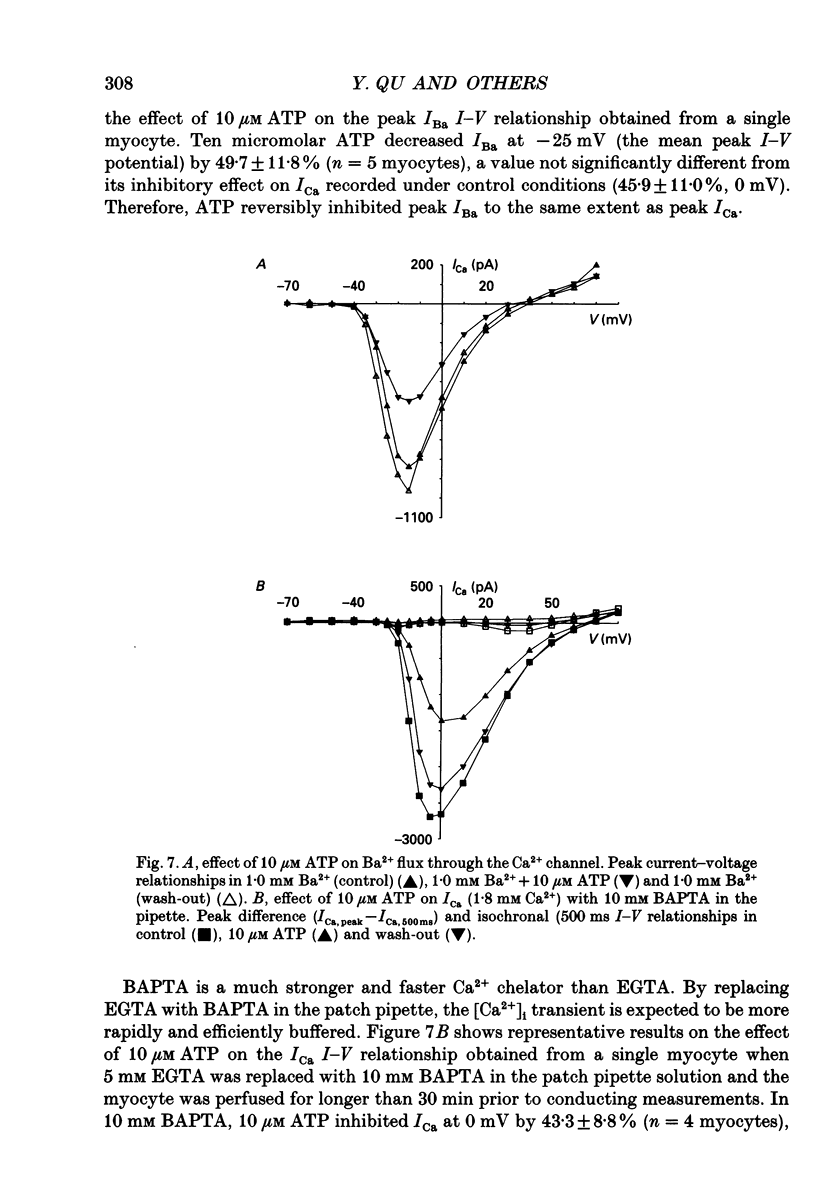







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
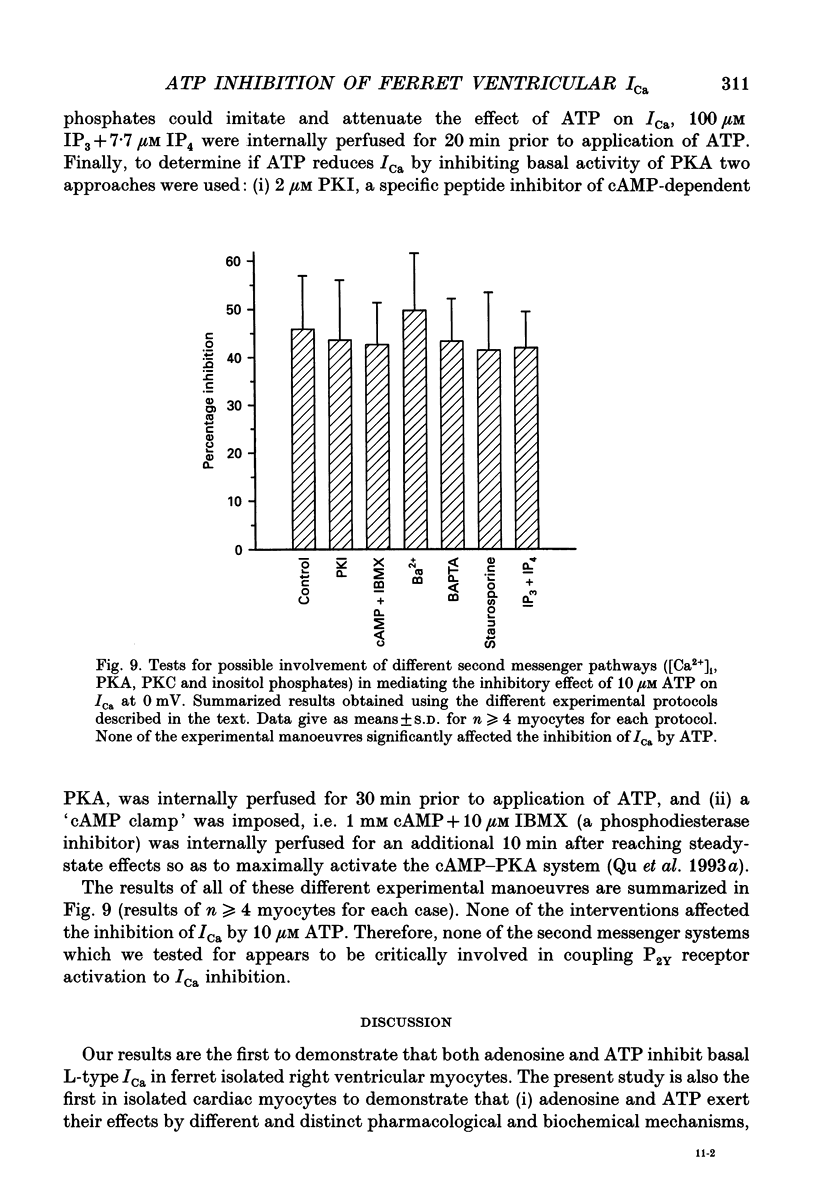
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Björnsson O. G., Monck J. R., Williamson J. R. Identification of P2Y purinoceptors associated with voltage-activated cation channels in cardiac ventricular myocytes of the rat. Eur J Biochem. 1989 Dec 8;186(1-2):395–404. doi: 10.1111/j.1432-1033.1989.tb15222.x. [DOI] [PubMed] [Google Scholar]
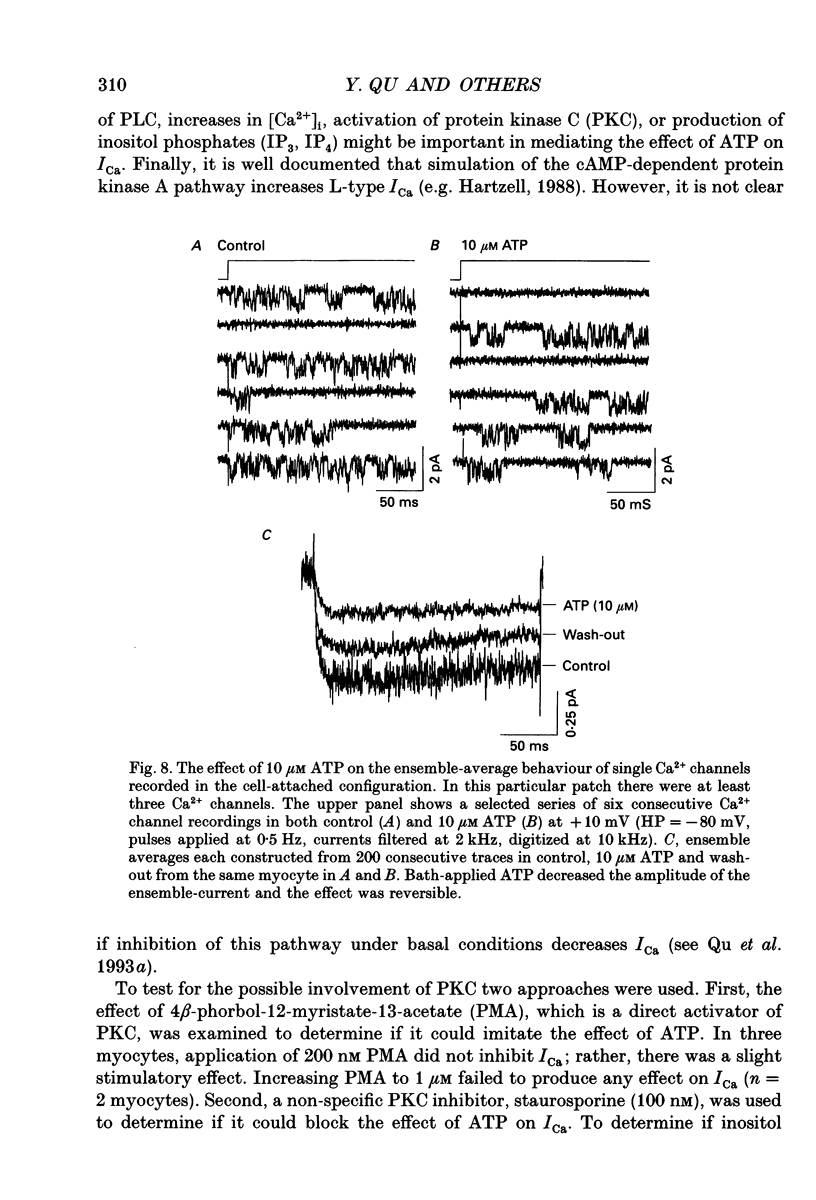
- Boeynaems J. M., Pirotton S., Van Coevorden A., Raspe E., Demolle D., Erneux C. P2-purinergic receptors in vascular endothelial cells: from concept to reality. J Recept Res. 1988;8(1-4):121–132. doi: 10.3109/10799898809048982. [DOI] [PubMed] [Google Scholar]
- Born G. V., Kratzer M. A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol. 1984 Sep;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Giles W. R., Shibata E. F. Ion transfer characteristics of the calcium current in bull-frog atrial myocytes. J Physiol. 1988 Sep;403:239–266. doi: 10.1113/jphysiol.1988.sp017248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Christie A., Sharma V. K., Sheu S. S. Mechanism of extracellular ATP-induced increase of cytosolic Ca2+ concentration in isolated rat ventricular myocytes. J Physiol. 1992 Jan;445:369–388. doi: 10.1113/jphysiol.1992.sp018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. G., Forrester T. Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol. 1981 Mar;312:143–158. doi: 10.1113/jphysiol.1981.sp013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger R. S., Raffaeli S., Moreno-Sanchez R., Sakai M., Capogrossi M. C., Spurgeon H. A., Hansford R. G., Lakatta E. G. Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Calcium. 1988 Aug;9(4):193–199. doi: 10.1016/0143-4160(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Cowen D. S., Meuller L. M. Activation of inositol phospholipid breakdown in HL60 cells by P2-purinergic receptors for extracellular ATP. Evidence for mediation by both pertussis toxin-sensitive and pertussis toxin-insensitive mechanisms. J Biol Chem. 1988 Dec 5;263(34):18108–18117. [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden T. K., Boyer J. L., Brown H. A., Cooper C. L., Jeffs R. A., Martin M. W. Biochemical properties of a P2Y-purinergic receptor. Ann N Y Acad Sci. 1990;603:256–266. doi: 10.1111/j.1749-6632.1990.tb37677.x. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Evidence that ATP is a neurotransmitter in the frog heart. Eur J Pharmacol. 1986 May 27;124(3):285–289. doi: 10.1016/0014-2999(86)90229-3. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legssyer A., Poggioli J., Renard D., Vassort G. ATP and other adenine compounds increase mechanical activity and inositol trisphosphate production in rat heart. J Physiol. 1988 Jul;401:185–199. doi: 10.1113/jphysiol.1988.sp017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. Reversible and selective antagonism by suramin of ATP-activated inward current in PC12 phaeochromocytoma cells. Br J Pharmacol. 1990 Sep;101(1):224–226. doi: 10.1111/j.1476-5381.1990.tb12117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- Needleman P., Minkes M. S., Douglas J. R., Jr Stimulation of prostaglandin biosynthesis by adenine nucleotides. Profile of prostaglandin release by perfused organs. Circ Res. 1974 Apr;34(4):455–460. doi: 10.1161/01.res.34.4.455. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Paddle B. M., Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11(3):110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Robaye B., Lagneau C., Boeynaems J. M. Adenine nucleotides modulate phosphatidylcholine metabolism in aortic endothelial cells. J Cell Physiol. 1990 Mar;142(3):449–457. doi: 10.1002/jcp.1041420303. [DOI] [PubMed] [Google Scholar]
- Qu Y., Campbell D. L., Whorton A. R., Strauss H. C. Modulation of basal L-type Ca2+ current by adenosine in ferret isolated right ventricular myocytes. J Physiol. 1993 Nov;471:269–293. doi: 10.1113/jphysiol.1993.sp019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Himmel H. M., Campbell D. L., Strauss H. C. Effects of extracellular ATP on ICa, [Ca2+]i, and contraction in isolated ferret ventricular myocytes. Am J Physiol. 1993 Mar;264(3 Pt 1):C702–C708. doi: 10.1152/ajpcell.1993.264.3.C702. [DOI] [PubMed] [Google Scholar]
- Ragazzi E., Wu S. N., Shryock J., Belardinelli L. Electrophysiological and receptor binding studies to assess activation of the cardiac adenosine receptor by adenine nucleotides. Circ Res. 1991 Apr;68(4):1035–1044. doi: 10.1161/01.res.68.4.1035. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Rasmusson R. L., Clark J. W., Giles W. R., Robinson K., Clark R. B., Shibata E. F., Campbell D. L. A mathematical model of electrophysiological activity in a bullfrog atrial cell. Am J Physiol. 1990 Aug;259(2 Pt 2):H370–H389. doi: 10.1152/ajpheart.1990.259.2.H370. [DOI] [PubMed] [Google Scholar]
- Scamps F., Rybin V., Puceat M., Tkachuk V., Vassort G. A Gs protein couples P2-purinergic stimulation to cardiac Ca channels without cyclic AMP production. J Gen Physiol. 1992 Oct;100(4):675–701. doi: 10.1085/jgp.100.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P., Hopp H. H., Isenberg G. Ca2+ influx through ATP-gated channels increments [Ca2+]i and inactivates ICa in myocytes from guinea-pig urinary bladder. J Physiol. 1991;440:479–496. doi: 10.1113/jphysiol.1991.sp018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- el-Moatassim C., Dornand J., Mani J. C. Extracellular ATP and cell signalling. Biochim Biophys Acta. 1992 Feb 19;1134(1):31–45. doi: 10.1016/0167-4889(92)90025-7. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Bültmann R., Starke K. Effects of suramin and alpha, beta-methylene ATP indicate noradrenaline-ATP co-transmission in the response of the mouse vas deferens to single and low frequency pulses. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):760–763. doi: 10.1007/BF00169686. [DOI] [PubMed] [Google Scholar]


