Abstract
Viral protein U (Vpu) is a 17-kDa phosphoprotein that enhances the release of viral particles from human immunodeficiency virus type 1-infected cells. This study shows that the effect of Vpu on efficient particle release depends on the rate of cell proliferation. Cells arrested by contact inhibition, chemical arresting agents, or terminal differentiation (i.e., macrophages) all exhibited a striking dependence on Vpu for efficient particle release, as shown by examination of particle production from transfections with full-length clones, infections, and the vaccinia virus expression system. In contrast, actively proliferating cells did not exhibit enhanced particle release with Vpu expression. This study demonstrates the necessity of Vpu for efficient viral particle release from quiescent cells.
Viral protein U (Vpu) is a unique gene product of human immunodeficiency virus (HIV) type 1 (HIV-1) with two well-described functions: CD4 degradation and enhancement of viral particle release (32, 35). Several studies have shown that Vpu is dispensable for HIV-1 infection in vitro (30). However, in vivo studies have suggested that Vpu may be a critical factor in viral pathogenesis. Although HIV strains replicating in vitro often exhibit mutations that abrogate Vpu expression, in vivo the Vpu open reading frame remains intact (12). In addition, macaque models of simian-human immunodeficiency virus infections have shown that during the nonpathogenic stages of disease, the Vpu reading frame is often disrupted, but as the virus evolves and becomes more pathogenic, the Vpu reading frame is often found to be intact (24–26). Finally, HIV-1 infection of certain primary cells, such as terminally differentiated, nondividing macrophages, generally requires Vpu (2, 33).
Unlike many other retroviruses, HIV-1 is capable of infecting nondividing cells. Infection of macrophages represents a crucial step in HIV-1 pathogenesis (5, 20). For instance, initial infection with HIV-1 generally occurs via the macrophage-tropic R5 viruses (18, 29, 37). Macrophages represent a longer-lived population of cells that may be a site of viral latency, posing problems for viral eradication with current antiretroviral therapy (19). Recently, resting CD4 T cells have been identified as an additional site of viral latency (40). While infection of resting T cells is very inefficient, these cells represent the majority of cells encountered by newly transmitted virus (40). Moreover, HIV-1 has also been shown to induce G2 growth arrest of infected cells via Vpr (7), leading to enhanced viral transcription (17). Thus, infection of quiescent cells is a major determinant of HIV-1 pathogenicity in vivo.
The role of cell growth and replication has been studied in relation to viral entry and transport of DNA into the nuclei of nondividing cells (9, 17, 23). This study examines viral assembly and release in proliferating and nonproliferating cells. It shows that efficient viral release in growth-arrested cells requires the presence of Vpu.
Efficient viral release is dependent on Vpu in cells that are arrested by contact inhibition.
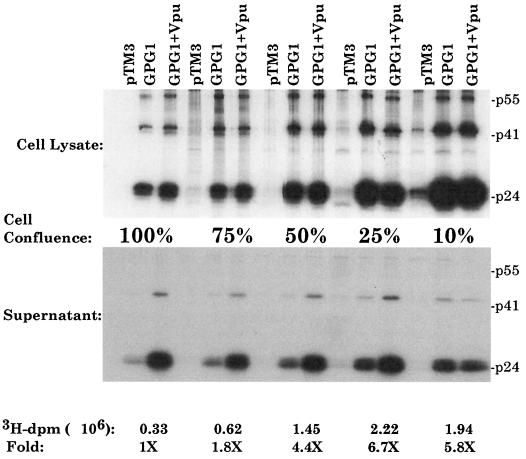
We initially observed that viral assembly and Vpu responses were dependent on cell growth conditions, e.g., the CO2 concentration of the tissue culture incubator (data not shown). This effect was not due to alterations of the pH of the cultures but appeared to correlate with the rate of cell proliferation (data not shown). To examine the effects of growth arrest, we initially used contact inhibition through cell confluence as a means of halting cells from cycling. 293T cells were grown at 10, 25, 50, 75, and 100% cell confluencies. Figure 1 shows that cells were arrested at high confluencies, as measured by [3H]thymidine (ICN) incorporation into DNA. Levels of incorporation at 100% confluence were similar to those in cells arrested with nocodazole (data not shown). At lower confluencies, these cells were actively dividing.
FIG. 1.
Relationship of cell confluence to the effect of Vpu on particle release. 293T cells were plated in 35-mm wells at 100, 75, 50, 25, and 10% confluencies. Each well was labeled with 50 μCi of [3H]thymidine/ml overnight. The next day, equal numbers of cells were lysed on glass filters (Fisher) and washed three times with radioimmunoprecipitation buffer, and [3H]thymidine incorporation was determined. The data at the bottom show levels of 3H disintegrations per minute at each confluence, as well as the fold above 100% confluence. A duplicate set of cells grown at the same confluencies were used to assess Gag protein synthesis and viral particle release as described previously (3). For infection-transfection, cells were first infected with a recombinant vaccinia virus expressing T7 polymerase, vTF7-3, at a multiplicity of infection of 10. At 30 min postinfection, cells were transfected with 10 μg of total DNA by use of Lipofectamine. At 5 h posttransfection, the medium was removed and 50 μCi of Tran35S label/ml was added. Cell lysates and supernatants were harvested and prepared for immunoprecipitation analysis with anti-p24 (CA) antisera. The proteins were examined by SDS–12.5% PAGE. pTM3 is a plasmid lacking gag and pol sequences. The top panel shows levels in cell lysates. The bottom panel shows the amount of Gag released into supernatants at each confluence.
As previously described, the vaccinia virus expression system was then used to express Gag-Pol (GPG1) alone or in the presence of Vpu (pTM-Vpu) (11). Briefly, 293T cells were infected with a recombinant vaccinia virus, vTF7-3, expressing the T7 polymerase. After 0.5 h, the cells were transfected with 4 μg of total DNA by use of Lipofectamine (GIBCO-BRL). GPG1 is sufficient for virus-like particle production from a variety of cells (36). However, Vpu is necessary for the efficient release of particles from certain cell types, such as HeLa or 293T cells (14, 33). Figure 1 demonstrates the effect of Vpu in cells infected-transfected at various cell confluencies. The top panel of Fig. 1 shows levels of protein expression in cell lysates. Although different levels were observed at the various confluencies, essentially equivalent levels were seen in cells expressing GPG1 alone or GPG1 plus Vpu at each confluence examined. The amount of Vpu protein increased proportionally with that of the Gag protein (data not shown). The bottom panel of Fig. 1 shows levels of Gag protein released into supernatants as virus-like particles (11). Densitometric analysis showed that in cultures at 100, 75, and 50% confluencies, Vpu enhanced particle release 5.6-, 2.1-, and 2.4-fold, respectively. In contrast, at 25 and 10% confluencies, Vpu enhanced particle release only 1.4- and 0.8-fold, respectively.
Vpu is necessary for efficient particle release from cells arrested by cell cycle-arresting agents such as nocodazole and mimosine.
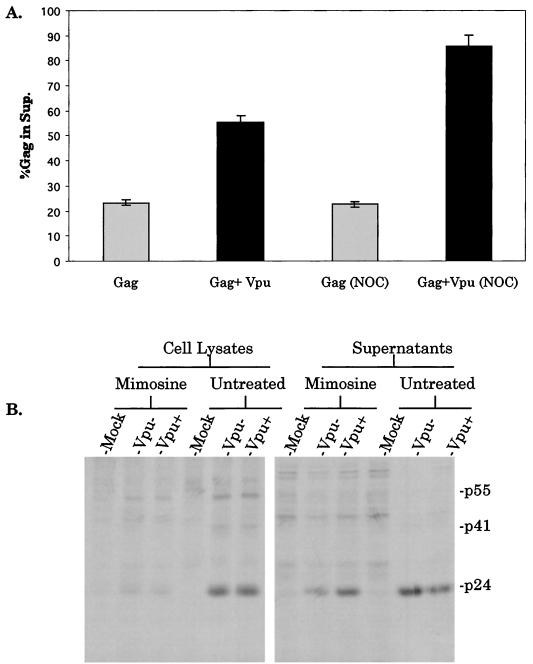
To determine if contact inhibition enhanced the effect of Vpu by arresting cell growth, we used the cell cycle inhibitor nocodazole to block cells from exiting the G2 phase of the cell cycle. Cells were grown to 25% confluence, at which Vpu does not efficiently promote particle release. At lower levels of confluence, cells exhibited toxicity in the presence of nocodazole and could not be used in these studies (data not shown). The cells grown to 25% confluence were left untreated or were treated with nocodazole at a concentration at which cell proliferation was halted, as determined by [3H]thymidine incorporation (data not shown). The cells were then transfected with GPG1 or GPG1 plus Vpu as described above. The proportion of Gag released was calculated from densitometric measurements of the p24, p41, and p55 bands on film. Figure 2 shows the results of this experiment. When Vpu was present, there was some enhancement of particle production. However, the enhancement was much more striking when nocodazole was also present.
FIG. 2.
Effects of nocodazole and mimosine on Vpu activity. (A) Cells were grown to 25% confluence in the presence or absence of the cell cycle inhibitor nocodazole (NOC) (10 μg/ml). Cells were infected, transfected, and metabolically labeled as described in the text. Anti-p24 (CA) antiserum immunoprecipitates were analyzed by SDS-PAGE and densitometry, and the proportion of total Gag proteins found in the supernatants (Sup.) was determined. Error bars represent standard deviations for the experiment performed in triplicate. (B) Cells were grown to 25% confluence in isoleucine-deficient medium for 48 h. This medium was then replaced with complete medium containing 400 μM mimosine. After 48 h, treated cells and untreated cells, also plated at 25% confluence, were transfected, labeled, and analyzed by immunoprecipitation and SDS-PAGE as described in the text.
The Vpu effect on release was also analyzed using the G1-arresting agent mimosine. Isogenic proviral clones that were Vpu+ and Vpu− were transfected into arrested or untreated cells. Transfection of proviral clones allowed for additional analysis of viral assembly without vaccinia virus infection. The parental clone, p125, has been previously described (20, 39). The Vpu− virus contains a T→C mutation in the initiator codon, introduced by site-directed mutagenesis, to close the Vpu open reading frame. 293T cells were seeded at 25% confluence in isoleucine-deficient medium in six-well plates. At 48 h after starvation, the medium was replaced with 400 μM mimosine-containing complete medium. At 48 h after addition of the arresting agent, these cells and untreated cells, also plated at 25% confluence, were transfected with 4 μg of proviral DNA by use of Lipofectamine. At 24 h posttransfection, the cells were labeled with 50 μCi of Tran35S label (ICN)/ml overnight. The next day, cell lysates and supernatants were analyzed as described above by radioimmunoprecipitation, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and autoradiography. Figure 2B shows that at a low confluence, Vpu had no effect on viral release in untreated cells. However, in cells arrested with mimosine, Vpu+ virus was more efficiently released than Vpu− virus (3.2-fold).
Thus, in situations where Vpu has no or little effect, i.e., low cell confluence, the addition of a cell cycle inhibitor such as nocodazole or mimosine can mimic the effect of cell arrest induced by contact inhibition. In addition, blocking cells in G1 with mimosine or G2 with nocodazole facilitates the effect of Vpu on particle assembly.
Vpu enhances particle release in differentiated macrophages in a dose-dependent manner.
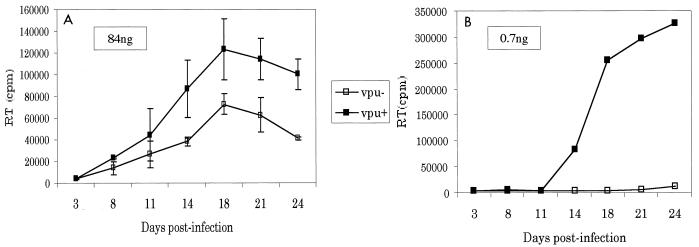
We next examined the effect of cell proliferation on viral release in a more physiologically relevant setting. Terminally differentiated, noncycling macrophages were infected with equal amounts of p24 from Vpu− and Vpu+ viruses generated in 293T cells transfected with molecular proviral clones. Infections were initiated with 84 ng of p24 or with fivefold dilutions down to 0.7 ng of p24. At high viral inocula, Vpu+ virus was released two- to threefold more efficiently than Vpu− virus (Fig. 3A). However, at lower viral inocula, the Vpu effect was far more significant, with over a 1,000-fold increase in viral release from cells infected with 670 pg of Vpu+ virus compared to cells infected with 670 pg of Vpu− virus (Fig. 3B). These results may explain previous controversies. Some groups have reported a three- to fourfold increase in viral release from macrophages in the presence versus the absence of Vpu, while other investigators have reported a more dramatic effect similar to that seen with the lower viral inocula used above (2, 33, 39). Thus, the differences reported earlier may reflect experimental variations in cell proliferation and multiplicity of infection. Moreover, these findings emphasize the important role of Vpu in quiescent cells.
FIG. 3.
Effects on macrophages. Macrophages were isolated by adherence from fresh peripheral blood mononuclear cells. The macrophages were infected with equal amounts of p24 from viruses obtained from transfection of 293T cells with isogenic proviral clones that were Vpu+ and Vpu− (ATG→ACG) in the context of an HIV strain 125 backbone, which is a chimera of the NL4-3 HXB-2 and ADA strains of HIV, as described previously (39). Supernatants were harvested on the indicated days and analyzed for reverse transcriptase (RT) activity as described previously (39). Error bars show standard deviations.
The work presented in this study demonstrates that HIV-1 assembly is inefficient in growth-arrested cells. The lack of efficient assembly was seen when cells were grown at low CO2 concentrations, at high confluencies, in the presence of cell cycle inhibitors such as nocodazole or mimosine, or in terminally differentiated, nondividing cells such as macrophages. However, coexpression of the Vpu protein restored efficient particle assembly under each of these conditions.
Vpu functions described in the literature include both degradation of newly synthesized CD4 and enhancement of the release of viral particles from the surface of infected cells (32). While the significance and mechanism of CD4 degradation have largely been delineated, the significance and mechanism of enhanced particle assembly remain unclear. Several interesting observations concerning this activity have been reported in the literature: the effect of Vpu is dependent on cell type (2, 33); variations in the effect have been reported, with some investigators showing it to be fairly moderate (2- to 3-fold) while others have reported a more dramatic effect (>1,000-fold) (2, 33); Vpu can enhance the release of divergent retroviral Gag proteins (16); and HIV-2 Env may have an effect on viral assembly similar to that of HIV-1 Vpu (4).
While intriguing, these observations indicate a broad effect of Vpu on the assembly process. Vpu enhances the release of divergent Gag proteins from viruses that do not contain a Vpu-like protein, making a direct interaction of HIV-1 Gag and Vpu unlikely. The finding that another protein (HIV-2 Env) has activity similar to that of Vpu is also indicative of a nonspecific mechanism of action. Moreover, the variability of Vpu action in different cell types and in the magnitude of the effect on viral release implicates factors in the cell or cellular environment which may modulate the assembly process and the effect of Vpu on viral assembly. The work presented here furthers the idea of a more generalized mechanism of action of Vpu on the assembly process, one that depends on the rate of growth of the cells.
The role of Vpu in modulating the cellular environment is intriguing. Recently, a group of small, hydrophobic viral proteins have been grouped together and termed viroporins. They include poliovirus 2BC and 3AB, influenza virus M2, togavirus 6K, rotavirus NSP4, hepatitis A virus 3A, 2B, and 2BC, hepatitis C virus E1, and coxsackievirus 2B proteins (3, 6, 22). These proteins regulate various points of the viral life cycle by altering membrane permeability. In particular, the coxsackievirus 2B, hepatitis virus 2BC, and influenza virus M2 proteins alter ion and membrane permeabilities and enhance virion assembly and the release of each of these viruses (22, 31, 38). Vpu, which has been shown to form channels and alter permeability in bacteria, oocytes, and human cells, may act in a manner similar to that of these other small, hydrophobic proteins (8, 13, 15, 34). It may function by altering the membrane permeability or environment in cells that are less permissive for HIV-1 assembly.
Intriguingly, a recent report demonstrated that HIV, like several other viruses, buds selectively through lipid raft structures at the plasma membrane (28). Additionally, raft formation may be related to cell activation, with dramatic reorganization of membrane raft structures occurring during cell activation (10, 21, 27). Moreover, raft structures may also be regulated by ionic changes occurring in cells (1). Thus, it will be interesting to study the effect of Vpu on raft formation. Studying the effect of Vpu on assembly may provide greater insight into the assembly process. It may also provide more information on membranes, their structure, and how viruses can alter membranes to their replicative advantage.
Acknowledgments
We thank Nancy Vander Heyden for work on macrophage infections and reverse transcriptase assays and Charlie Rice for helpful discussions.
This work was supported by PHS grants AI34736, CA83659, and AI24745 and training grant 5 T32 HL 07088-23 (to A.D.).
REFERENCES
- 1.Babiychuk E B, Draeger A. Annexins in cell membrane dynamics. Ca(2+)-regulated association of lipid microdomains. J Cell Biol. 2000;150:1113–1124. doi: 10.1083/jcb.150.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 3.Barco A, Carrasco L. Identification of regions of poliovirus 2bc protein that are involved in cytotoxicity. J Virol. 1998;72:3560–3570. doi: 10.1128/jvi.72.5.3560-3570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bour S, Strebel K. The human immunodeficiency virus (HIV) type 2 envelope protein is a functional complement to HIV type 1 Vpu that enhances particle release of heterologous retroviruses. J Virol. 1996;70:8285–8300. doi: 10.1128/jvi.70.12.8285-8300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr J M, Hocking H, Li P, Burrell C J. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–329. doi: 10.1006/viro.1999.0047. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y S, Liao C L, Tsao C H, Chen M C, Liu G I, Chen L K, Lin Y L. Membrane permeabilization by small hydrophobic nonstructural proteins of Japanese encephalitis virus. J Virol. 1999;73:6257–6264. doi: 10.1128/jvi.73.8.6257-6264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M Z, Elder R T, Yu M, O'Gorman M G, Selig L, Benarous R, Yamamoto A, Zhao Y Q. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J Virol. 1999;73:3236–3245. doi: 10.1128/jvi.73.4.3236-3245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coady M J, Daniel N G, Tiganos E, Allain B, Friborg J, Lapointe J Y, Cohen E A. Effects of Vpu expression on Xenopus oocyte membrane conductance. Virology. 1998;244:39–49. doi: 10.1006/viro.1998.9087. [DOI] [PubMed] [Google Scholar]
- 9.Collman R G, Yi Y J. Cofactors for human immunodeficiency virus entry into primary macrophages. J Infect Dis. 1999;179:S422–S426. doi: 10.1086/314797. [DOI] [PubMed] [Google Scholar]
- 10.Delgado P, Fernandez E, Dave V, Kappes D, Alarcon B. CD3delta couples T-cell receptor signalling to ERK activation and thymocyte positive selection. Nature. 2000;406:426–430. doi: 10.1038/35019102. [DOI] [PubMed] [Google Scholar]
- 11.Deora A, Spearman P, Ratner L. The N-terminal matrix domain of HIV-1 Gag is sufficient but not necessary for viral protein U-mediated enhancement of particle release through a membrane-targeting mechanism. Virology. 2000;269:305–312. doi: 10.1006/viro.1999.0094. [DOI] [PubMed] [Google Scholar]
- 12.Du B, Wolf A, Lee S, Terwilliger E. Changes in the host range and growth potential of an HIV-1 clone are conferred by the vpu gene. Virology. 1993;195:260–264. doi: 10.1006/viro.1993.1370. [DOI] [PubMed] [Google Scholar]
- 13.Ewart G D, Sutherland T, Gage P W, Cox G B. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J Virol. 1996;70:7108–7115. doi: 10.1128/jvi.70.10.7108-7115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geraghty R J, Talbot K J, Callahan M, Harper W, Panganiban A T. Cell type-dependence for Vpu function. J Med Primatol. 1994;23:146–150. doi: 10.1111/j.1600-0684.1994.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez M E, Carrasco L. The human immunodeficiency virus type 1 Vpu protein enhances membrane permeability. Biochemistry. 1998;37:13710–13719. doi: 10.1021/bi981527f. [DOI] [PubMed] [Google Scholar]
- 16.Gottlinger H G, Dorfman T, Cohen E A, Haseltine W A. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc Natl Acad Sci USA. 1993;90:7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gummuluru S, Emerman M. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J Virol. 1999;73:5422–5430. doi: 10.1128/jvi.73.7.5422-5430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase A T. Population biology of HIV-1 infection: viral and CD4(+) T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 19.Haase A T, Schacker T W. Potential for the transmission of HIV-1 despite highly active antiretroviral therapy. N Engl J Med. 1998;339:1846–1848. doi: 10.1056/NEJM199812173392510. [DOI] [PubMed] [Google Scholar]
- 20.Hung C S, Pontow S, Ratner L. Relationship between productive HIV-1 infection of macrophages and CCR5 utilization. Virology. 1999;264:278–288. doi: 10.1006/viro.1999.0013. [DOI] [PubMed] [Google Scholar]
- 21.Janes P W, Ley S C, Magee A I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jecht M, Probst C, Gauss-Muller V. Membrane permeability induced by hepatitis A virus proteins 2B and 2BC and proteolytic processing of HAV 2BC. Virology. 1998;252:218–227. doi: 10.1006/viro.1998.9451. [DOI] [PubMed] [Google Scholar]
- 23.Kiernan R E, Ono A, Englund G, Freed E O. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–4126. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z Q, Mukherjee S, Sahni M, McCormick-Davis C, Leung K, Li Z, Gattone II V H, Tian C, Doms R W, Hoffman T L, Raghavan R, Narayan O, Stephens E B. Derivation and biological characterization of a molecular clone of SHIV(KU-2) that causes AIDS, neurological disease, and renal disease in rhesus macaques. Virology. 1999;260:295–307. doi: 10.1006/viro.1999.9812. [DOI] [PubMed] [Google Scholar]
- 25.Luciw P A, Mandell C P, Himathongkham S, Li J L, Low T A, Schmidt K A, Shaw K E S, Cheng-Mayer C. Fatal immunopathogenesis by SIV/HIV-1 (SHIV) containing a variant form of the HIV-1SF33 env gene in juvenile and newborn rhesus macaques. Virology. 1999;263:112–127. doi: 10.1006/viro.1999.9908. [DOI] [PubMed] [Google Scholar]
- 26.McCormick-Davis C, Zhao L J, Mukherjee S, Leung K, Sheffer D, Joag S V, Narayan O, Stephens E B. Chronology of genetic changes in the vpu, env, and nef genes of chimeric simian-human immunodeficiency virus (strain HXB2) during acquisition of virulence for pig-tailed macaques. Virology. 1998;248:275–283. doi: 10.1006/viro.1998.9300. [DOI] [PubMed] [Google Scholar]
- 27.Moran M, Miceli M C. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T cell activation. Immunity. 1998;9:787–796. doi: 10.1016/s1074-7613(00)80644-5. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen D H, Hildreth J E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ometto L, Zanchetta M, Cabrelle A, Esposito G, Mainardi M, Chieco-Bianchi L, De Rossi A. Restriction of HIV type 1 infection in macrophages heterozygous for a deletion in the CC-chemokine receptor 5 gene. AIDS Res Hum Retrovir. 1999;15:1441–1452. doi: 10.1089/088922299309955. [DOI] [PubMed] [Google Scholar]
- 30.Sakai H, Tokunaga K, Kawamura M, Adachi A. Function of human immunodeficiency virus type 1 Vpu protein in various cell types. J Gen Virol. 1995;76:2717–2722. doi: 10.1099/0022-1317-76-11-2717. [DOI] [PubMed] [Google Scholar]
- 31.Sansom M S, Kerr I D, Smith G R, Son H S. The influenza A virus M2 channel: a molecular modeling and simulation study. Virology. 1997;233:163–173. doi: 10.1006/viro.1997.8578. [DOI] [PubMed] [Google Scholar]
- 32.Schubert U, Bour S, Ferrer-Montiel A V, Montal M, Maldarell F, Strebel K. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J Virol. 1996;70:809–819. doi: 10.1128/jvi.70.2.809-819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schubert U, Clouse K A, Strebel K. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J Virol. 1995;69:7699–7711. doi: 10.1128/jvi.69.12.7699-7711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert U, Ferrer-Montiel A V, Oblatt-Montal M, Henklein P, Strebel K, Montal M. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 1996;398:12–18. doi: 10.1016/s0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- 35.Schubert U, Strebel K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J Virol. 1994;68:2260–2271. doi: 10.1128/jvi.68.4.2260-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spearman P, Wang J J, Vander Heyden N, Ratner L. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J Virol. 1994;68:3232–3242. doi: 10.1128/jvi.68.5.3232-3242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swingler S, Mann A, Jacque J M, Brichacek B, Sasseville V G, Williams K, Lackner A A, Janoff E N, Wang R, Fisher D, Stevenson M. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:997–1003. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Kuppeveld F J, Hoenderop J G, Smeets R L, Willems P H, Dijkman H B, Galama J M, Melchers W J. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westervelt P, Henkel T, Trowbridge D B, Orenstein J, Heuser J, Gendelman H E, Ratner L. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J Virol. 1992;66:3925–3931. doi: 10.1128/jvi.66.6.3925-3931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z Q, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4(+) T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]