Abstract
1. The primary objective of this study was to examine in man, how induced changes in global cerebral blood flow (gCBF) affected a regional cerebral blood flow (rCBF) increase resulting from a neural activation task (opening of eyes). A secondary objective was to quantify how such induced changes in gCBF were distributed between representative regions of either predominantly grey matter or white matter. 2. Positron emission tomography with intravenous infusion of H2(15)O, was used to measure gCBF in six normal males. Concomitant measures of rCBF were obtained in three different regions of interest (ROI): a representative area of predominantly grey matter, a representative area of predominantly white matter and an area of visual cortex. 3. Cerebral blood flow was altered by establishing steady-state changes in PCO2 at a near constant ventilation of approximately 30 l min-1. The mean PET,CO2 (+/- S.D.) levels (mmHg) that resulted were: low, 21.8 +/- 1.8; normal, 39.8 +/- 1.0, and high, 54.8 +/- 1.2. The normal and high levels were obtained by adding appropriate amounts of CO2 to the inspirate. The corresponding mean gCBF levels across all six subjects with eyes closed were: low, 24.2 +/- 4.6; normal, 37.2 +/- 3.9 and high, 66.8 +/- 7.6 ml min-1 dl-1. 4. Blood flow in grey matter (insular cortex) and white matter (centrum semiovale) at normal levels of PCO2 averaged 56.8 +/- 10.1 and 20.3 +/- 3.4 ml min dl-1 respectively. As PCO2 rose, the increase in rCBF to grey matter was approximately three times greater than that to white matter. 5. An activation state of eyes open in a brightly lit room was compared to a baseline state of eyes closed in a darkened room at the three levels of PCO2 (and hence at three levels of gCBF). Over the whole gCBF range a significant (P = 0.028) effect of increasing rCBF in the visual cortex ROI was found in response to opening the eyes; the effect of this activation on rCBF was not significantly dependent (P = 0.34) on the PCO2 (and hence gCBF) level. The effect of the activation on the rCBF was apparently 'additive' to the rise of rCBF associated with PCO2-related gCBF increase. 6. The results confirm the need to normalize for changes in gCBF during studies of rCBF in response to an activation protocol. They also provide support for the use of an 'additive' model to achieve such normalization provided that other cortical areas behave in a similar manner to that of the visual cortex.
Full text
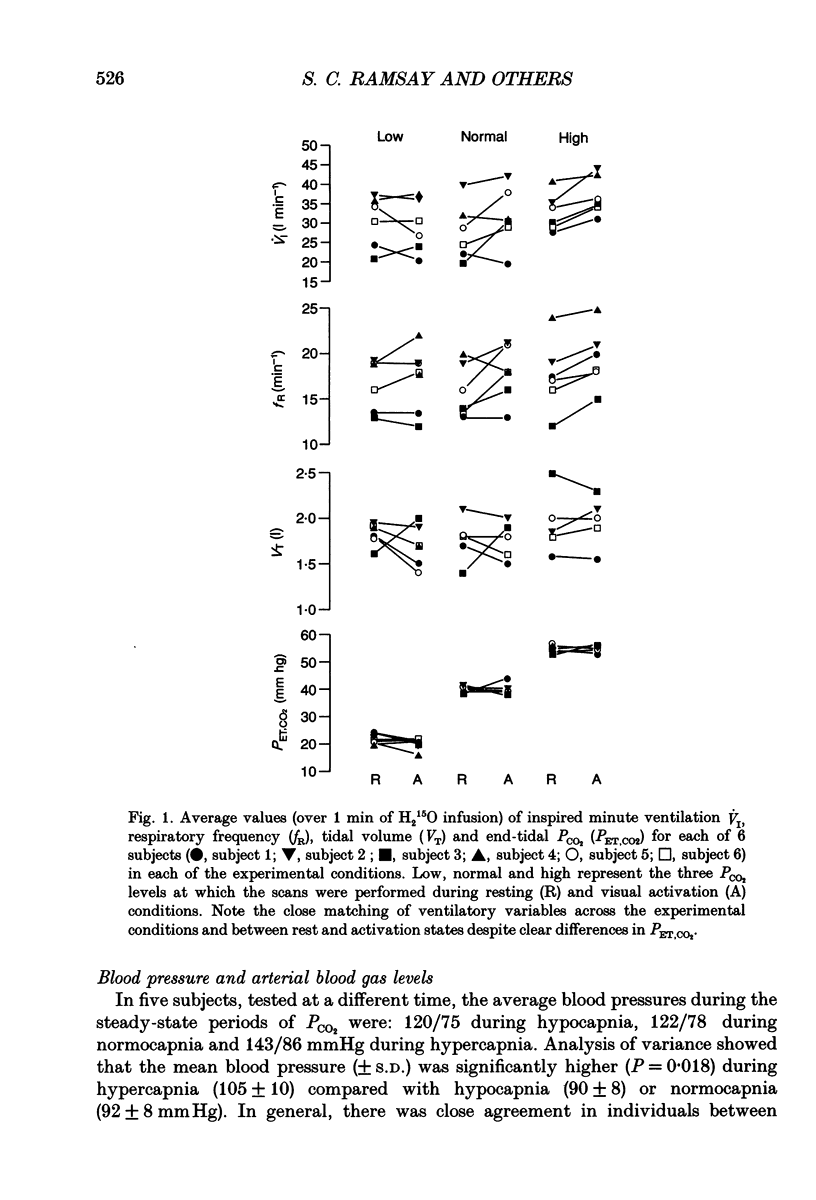
PDF





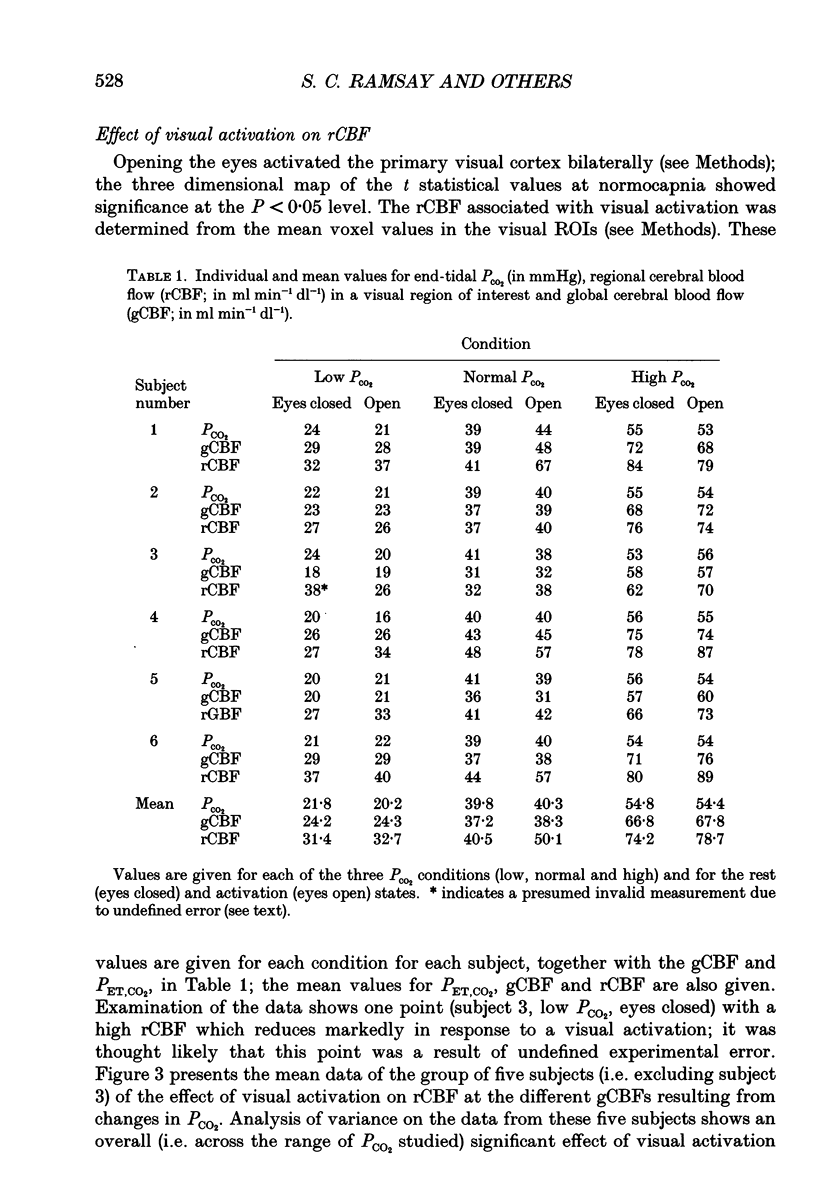
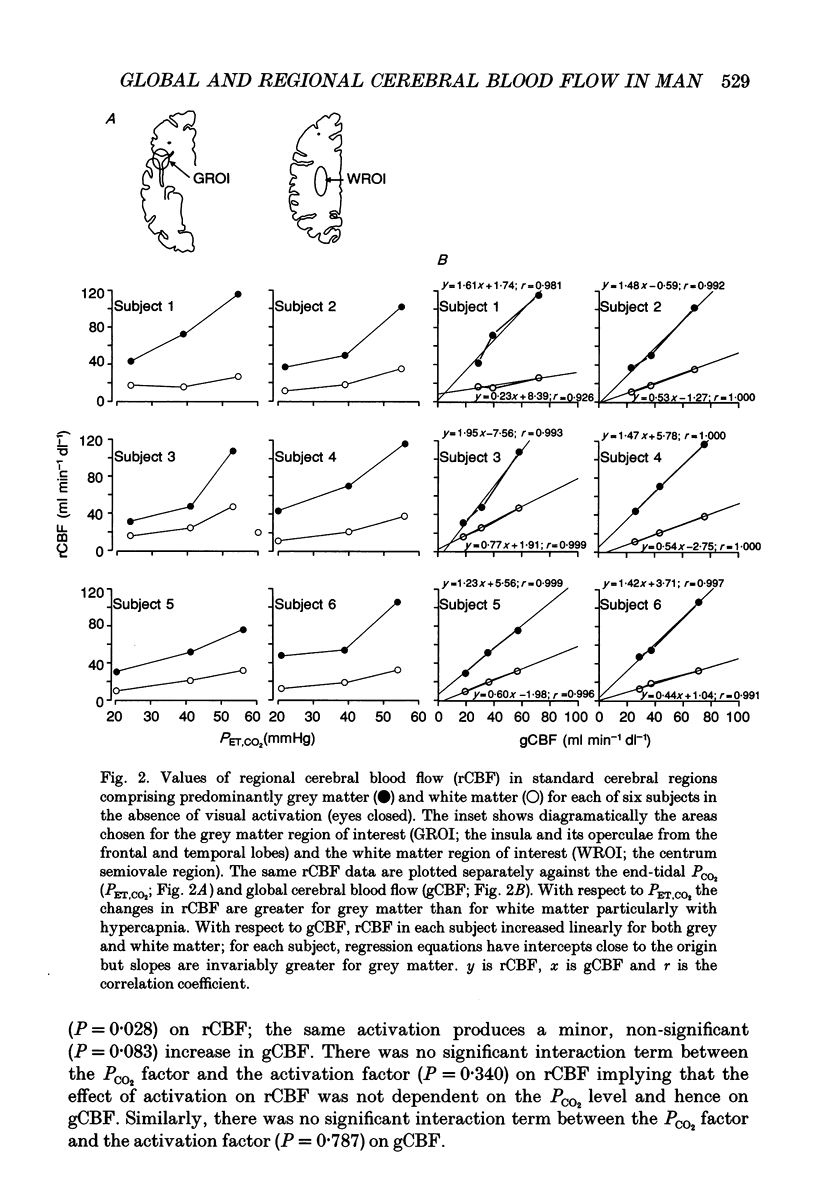




Selected References
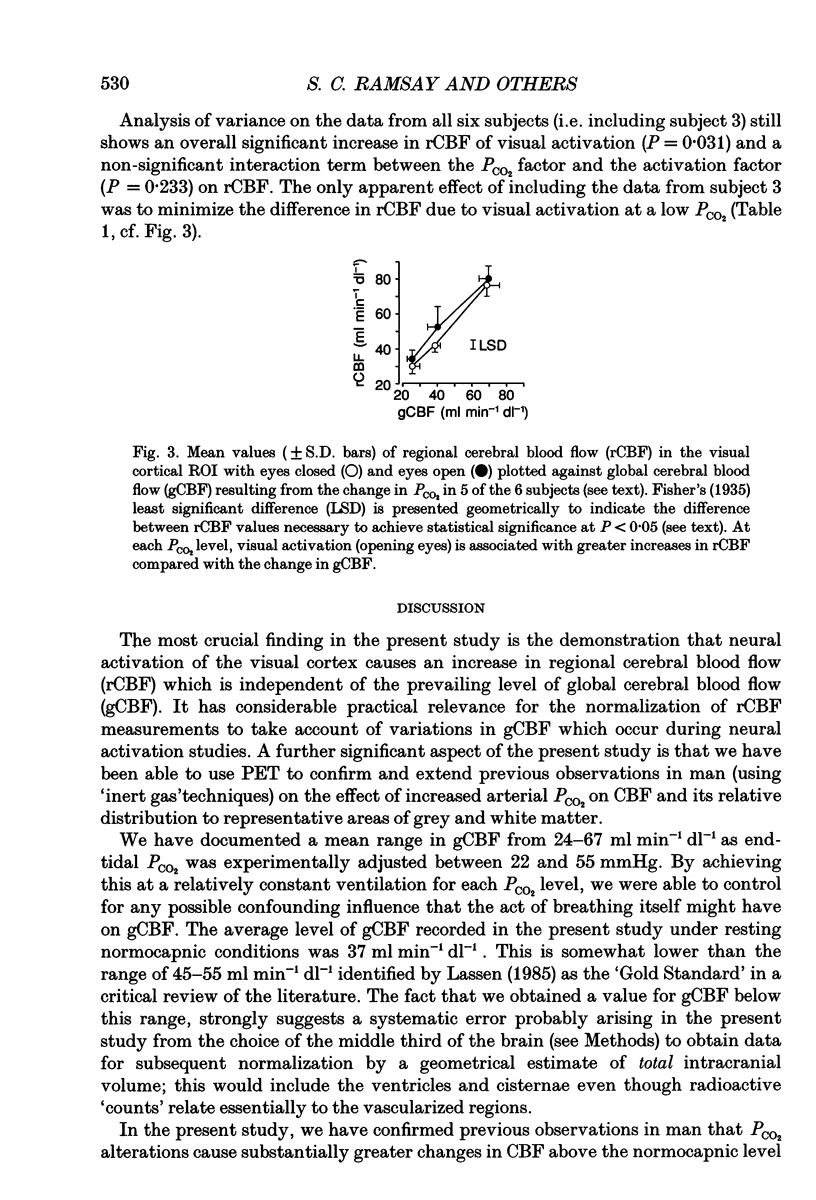
These references are in PubMed. This may not be the complete list of references from this article.
- Borgström L., Jóhannsson H., Siesjö B. K. The relationship between arterial po2 and cerebral blood flow in hypoxic hypoxia. Acta Physiol Scand. 1975 Mar;93(3):423–432. doi: 10.1111/j.1748-1716.1975.tb05832.x. [DOI] [PubMed] [Google Scholar]
- Colebatch J. G., Adams L., Murphy K., Martin A. J., Lammertsma A. A., Tochon-Danguy H. J., Clark J. C., Friston K. J., Guz A. Regional cerebral blood flow during volitional breathing in man. J Physiol. 1991 Nov;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford I. Confounded correlations: statistical limitations in the analysis of interregional relationships of cerebral metabolic activity. J Cereb Blood Flow Metab. 1986 Jun;6(3):385–388. doi: 10.1038/jcbfm.1986.63. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A., Reiman E. M., Raichle M. E. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J Cereb Blood Flow Metab. 1988 Oct;8(5):642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Dolan R. J., Lammertsma A. A., Frackowiak R. S. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990 Jul;10(4):458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Frackowiak R. S. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991 Jul;11(4):690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Horwitz B., Duara R., Rapoport S. I. Intercorrelations of glucose metabolic rates between brain regions: application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab. 1984 Dec;4(4):484–499. doi: 10.1038/jcbfm.1984.73. [DOI] [PubMed] [Google Scholar]
- James I. M., Millar R. A., Purves M. J. Observations on the extrinsic neural control of cerebral blood flow in the baboon. Circ Res. 1969 Jul;25(1):77–93. doi: 10.1161/01.res.25.1.77. [DOI] [PubMed] [Google Scholar]
- Kety S. S., Schmidt C. F. THE NITROUS OXIDE METHOD FOR THE QUANTITATIVE DETERMINATION OF CEREBRAL BLOOD FLOW IN MAN: THEORY, PROCEDURE AND NORMAL VALUES. J Clin Invest. 1948 Jul;27(4):476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky W., Wahl M. Local chemical and neurogenic regulation of cerebral vascular resistance. Physiol Rev. 1978 Jul;58(3):656–689. doi: 10.1152/physrev.1978.58.3.656. [DOI] [PubMed] [Google Scholar]
- LASSEN N. A. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959 Apr;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Lammertsma A. A., Cunningham V. J., Deiber M. P., Heather J. D., Bloomfield P. M., Nutt J., Frackowiak R. S., Jones T. Combination of dynamic and integral methods for generating reproducible functional CBF images. J Cereb Blood Flow Metab. 1990 Sep;10(5):675–686. doi: 10.1038/jcbfm.1990.121. [DOI] [PubMed] [Google Scholar]
- Lassen N. A. Normal average value of cerebral blood flow in younger adults is 50 ml/100 g/min. J Cereb Blood Flow Metab. 1985 Sep;5(3):347–349. doi: 10.1038/jcbfm.1985.48. [DOI] [PubMed] [Google Scholar]
- Metter E. J., Riege W. H., Kuhl D. E., Phelps M. E. Cerebral metabolic relationships for selected brain regions in healthy adults. J Cereb Blood Flow Metab. 1984 Mar;4(1):1–7. doi: 10.1038/jcbfm.1984.1. [DOI] [PubMed] [Google Scholar]
- Moeller J. R., Strother S. C., Sidtis J. J., Rottenberg D. A. Scaled subprofile model: a statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab. 1987 Oct;7(5):649–658. doi: 10.1038/jcbfm.1987.118. [DOI] [PubMed] [Google Scholar]
- Murphy K., Mier A., Adams L., Guz A. Putative cerebral cortical involvement in the ventilatory response to inhaled CO2 in conscious man. J Physiol. 1990 Jan;420:1–18. doi: 10.1113/jphysiol.1990.sp017898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVACK P., SHENKIN H. A., BORTIN L., GOLUBOFF B., SOFFE A. M. The effects of carbon dioxide inhalation upon the cerebral blood flow and cerebral oxygen consumption in vascular disease. J Clin Invest. 1953 Aug;32(8):696–702. doi: 10.1172/JCI102783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J. Contralateral focal increase of cerebral blood flow in man during arm work. Brain. 1971;94(4):635–646. doi: 10.1093/brain/94.4.635. [DOI] [PubMed] [Google Scholar]
- REIVICH M. ARTERIAL PCO2 AND CEREBRAL HEMODYNAMICS. Am J Physiol. 1964 Jan;206:25–35. doi: 10.1152/ajplegacy.1964.206.1.25. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Cerebral circulation and metabolism. J Neurosurg. 1984 May;60(5):883–908. doi: 10.3171/jns.1984.60.5.0883. [DOI] [PubMed] [Google Scholar]
- Spinks T. J., Jones T., Bailey D. L., Townsend D. W., Grootoonk S., Bloomfield P. M., Gilardi M. C., Casey M. E., Sipe B., Reed J. Physical performance of a positron tomograph for brain imaging with retractable septa. Phys Med Biol. 1992 Aug;37(8):1637–1655. doi: 10.1088/0031-9155/37/8/002. [DOI] [PubMed] [Google Scholar]


