Abstract
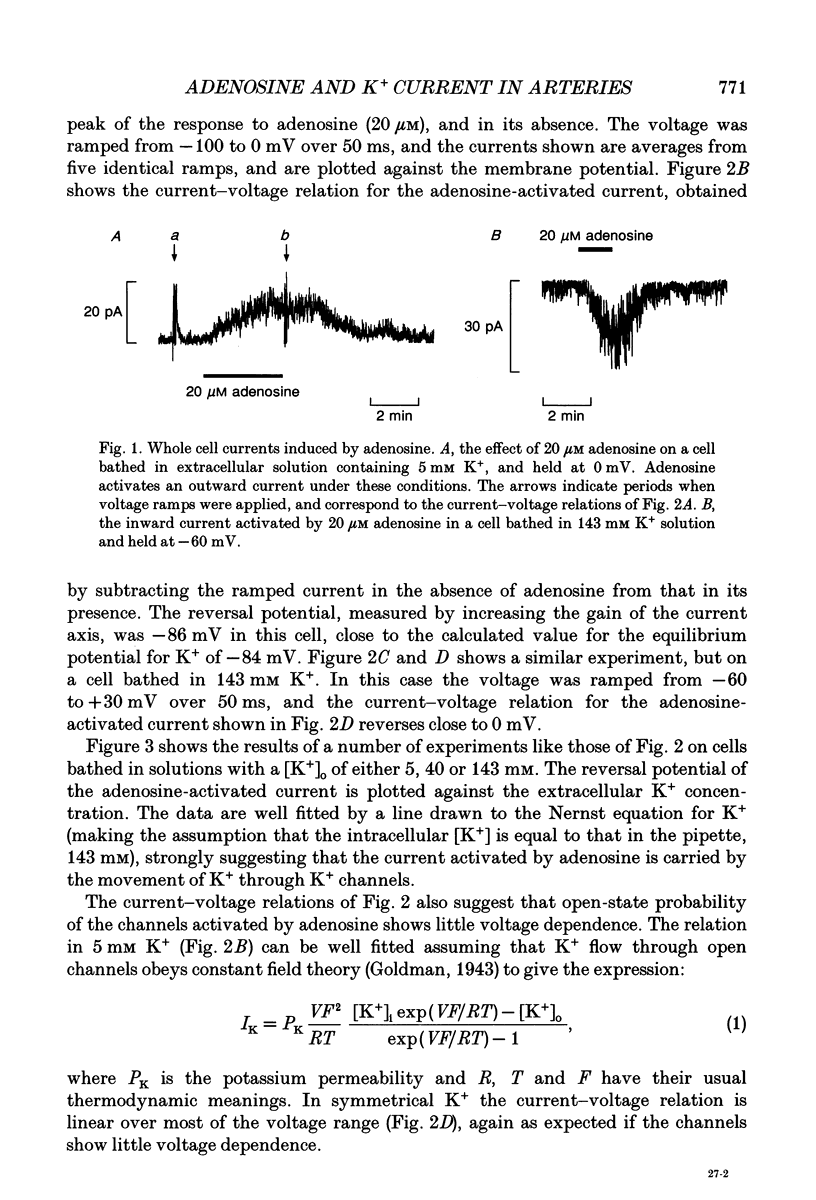
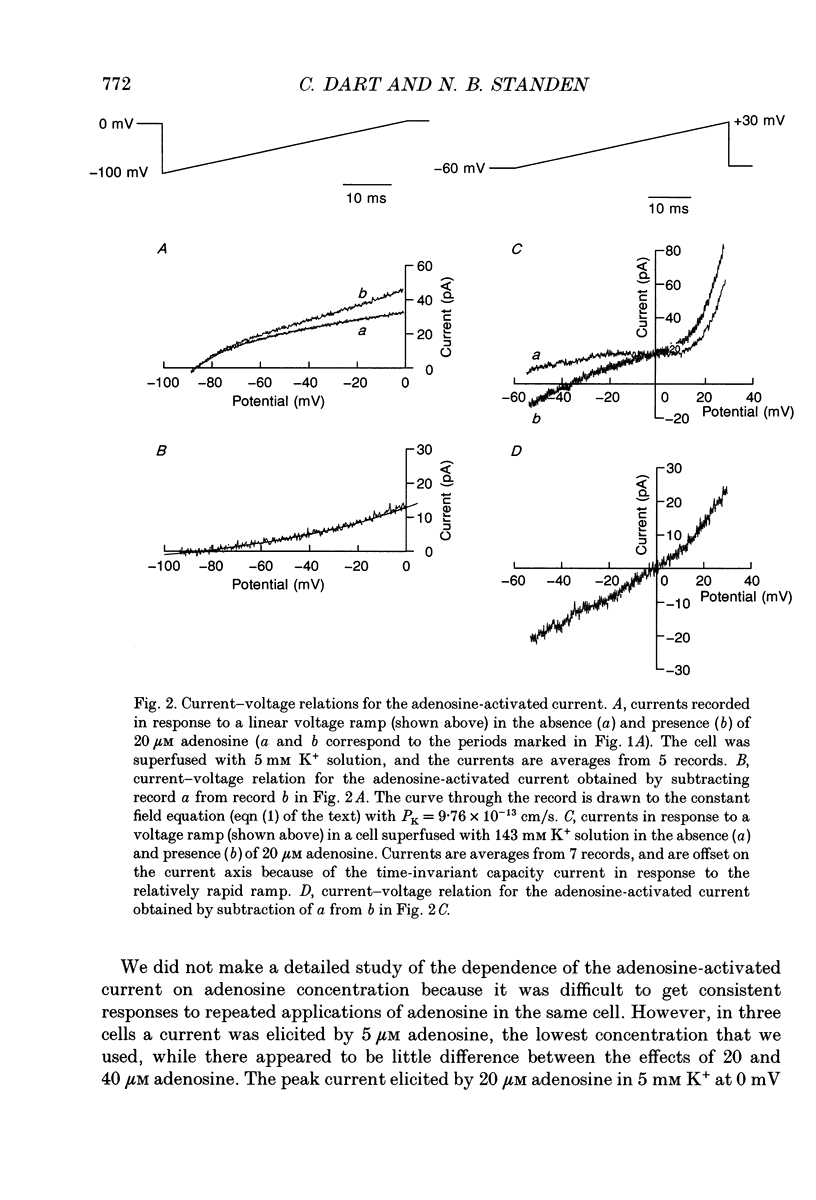
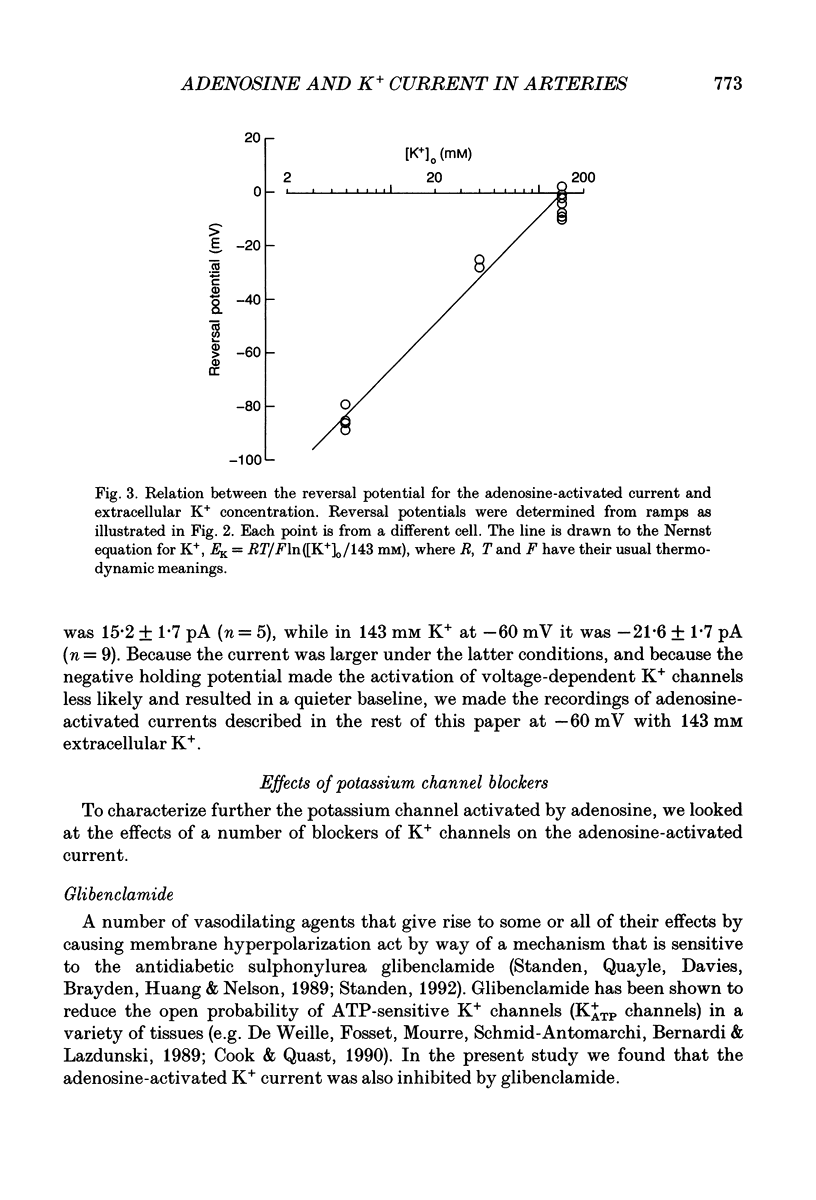
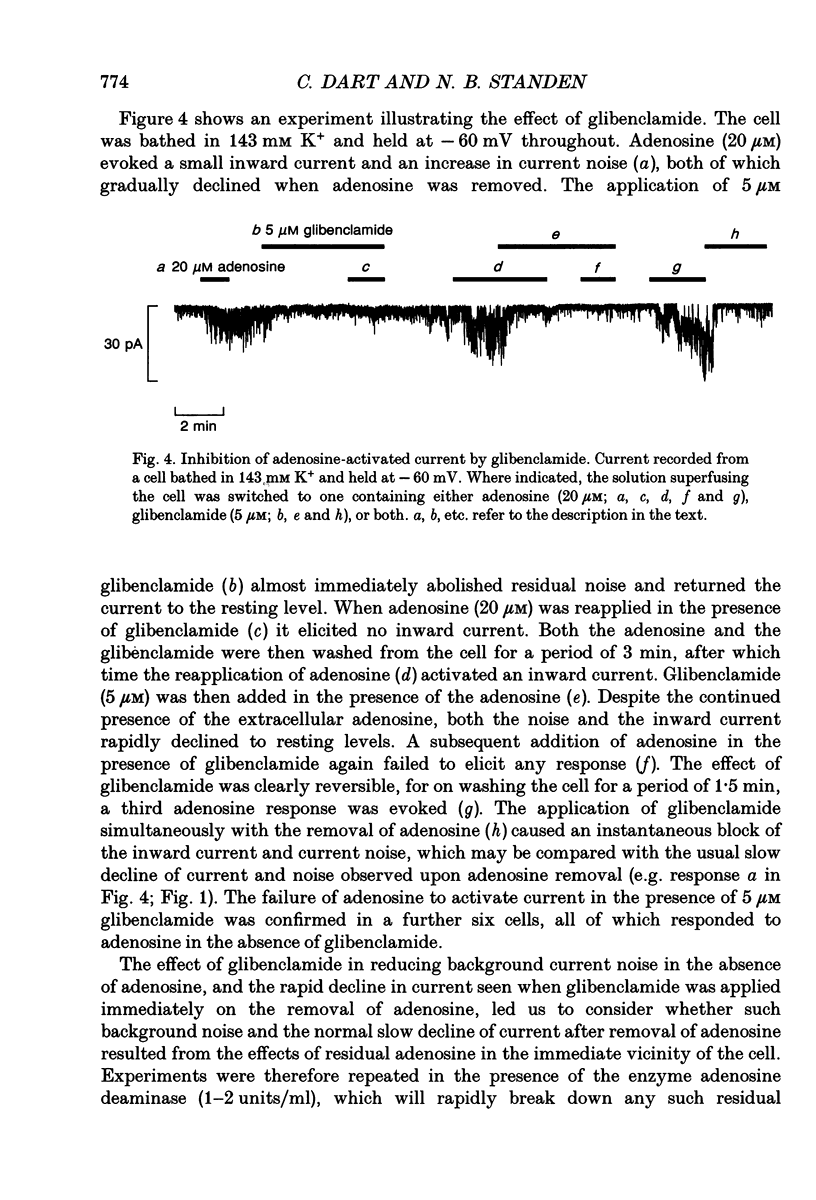
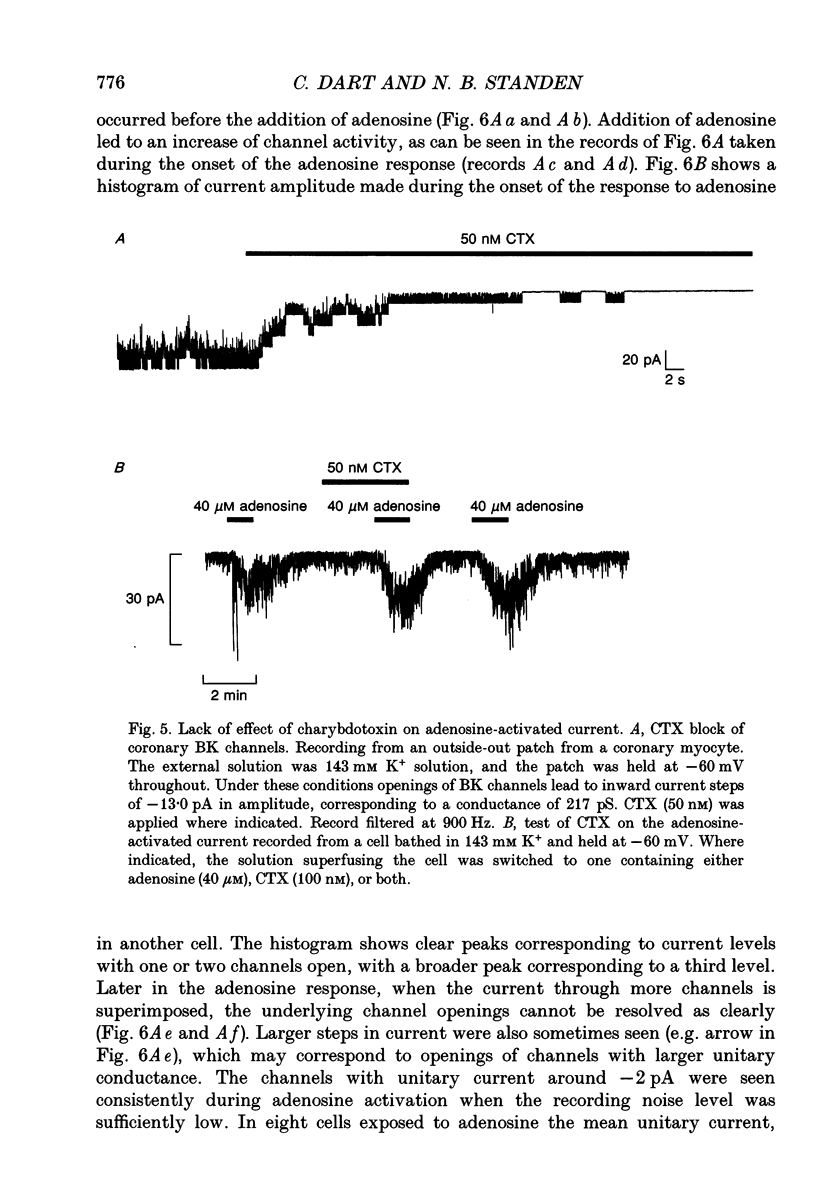
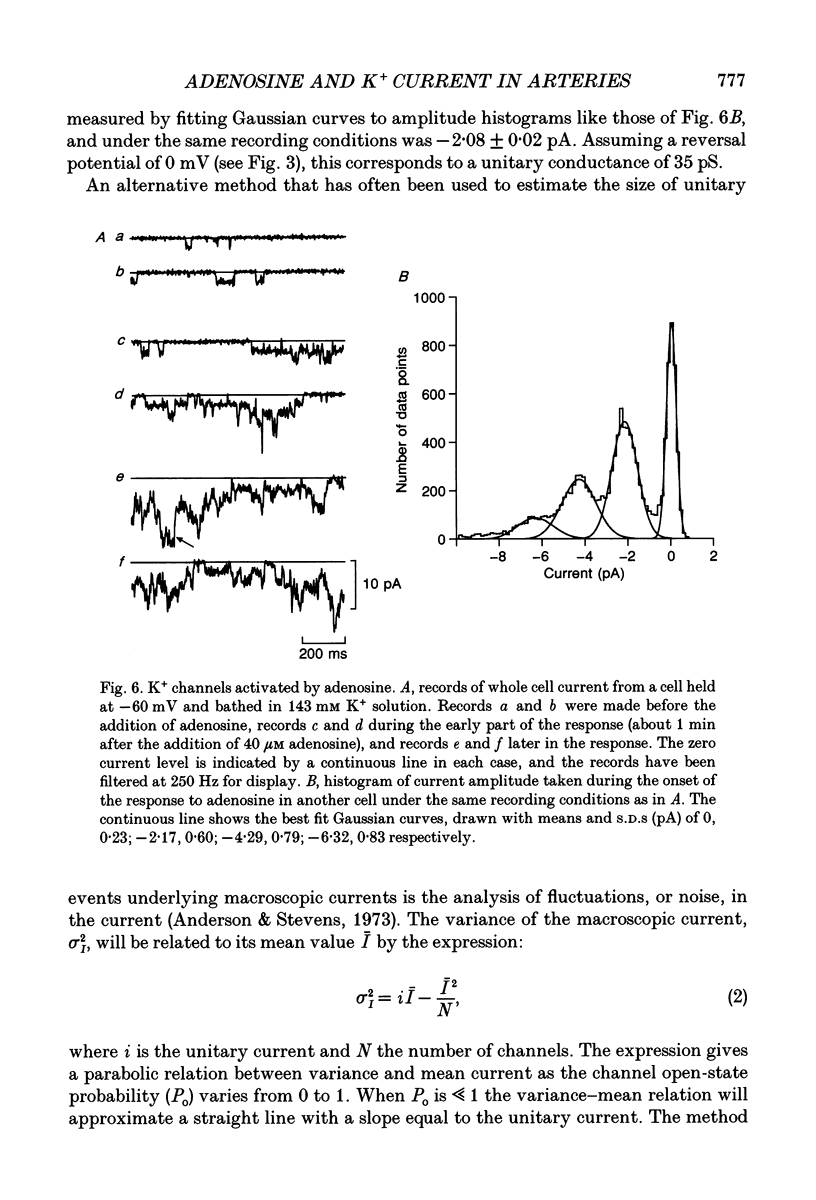
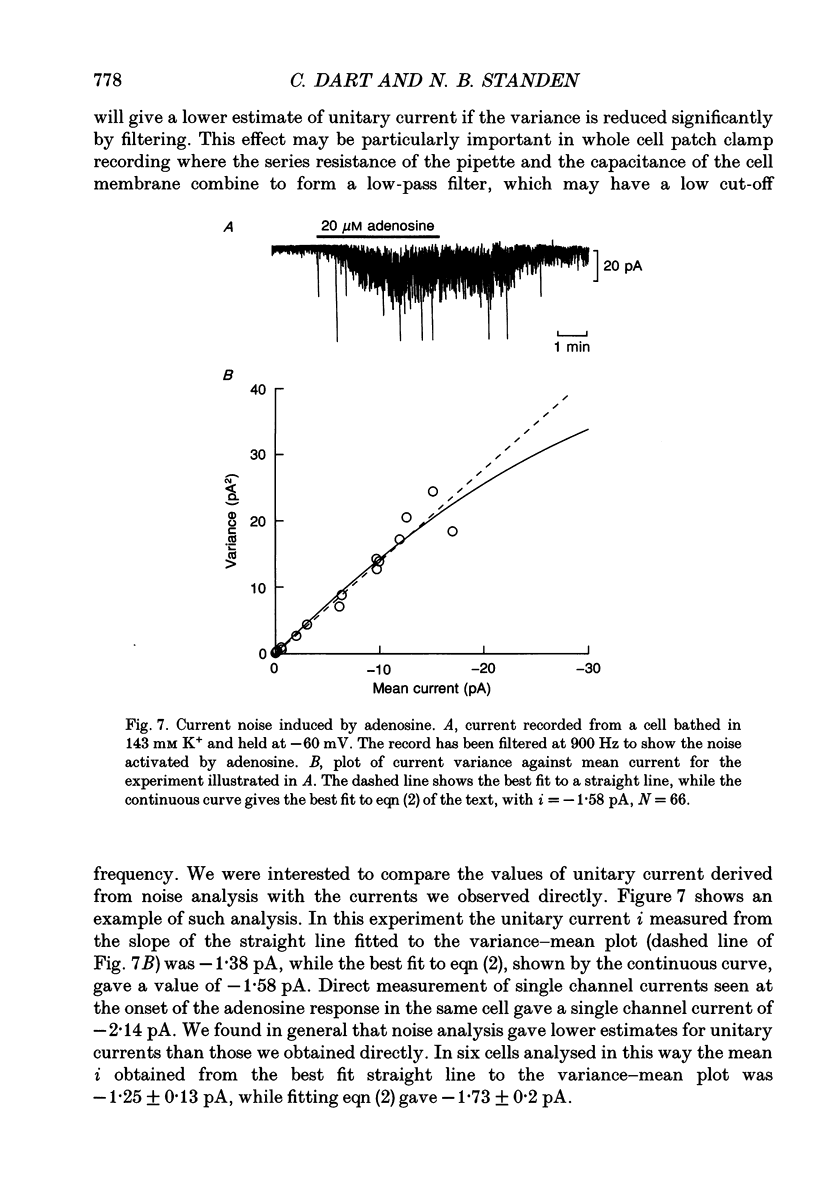
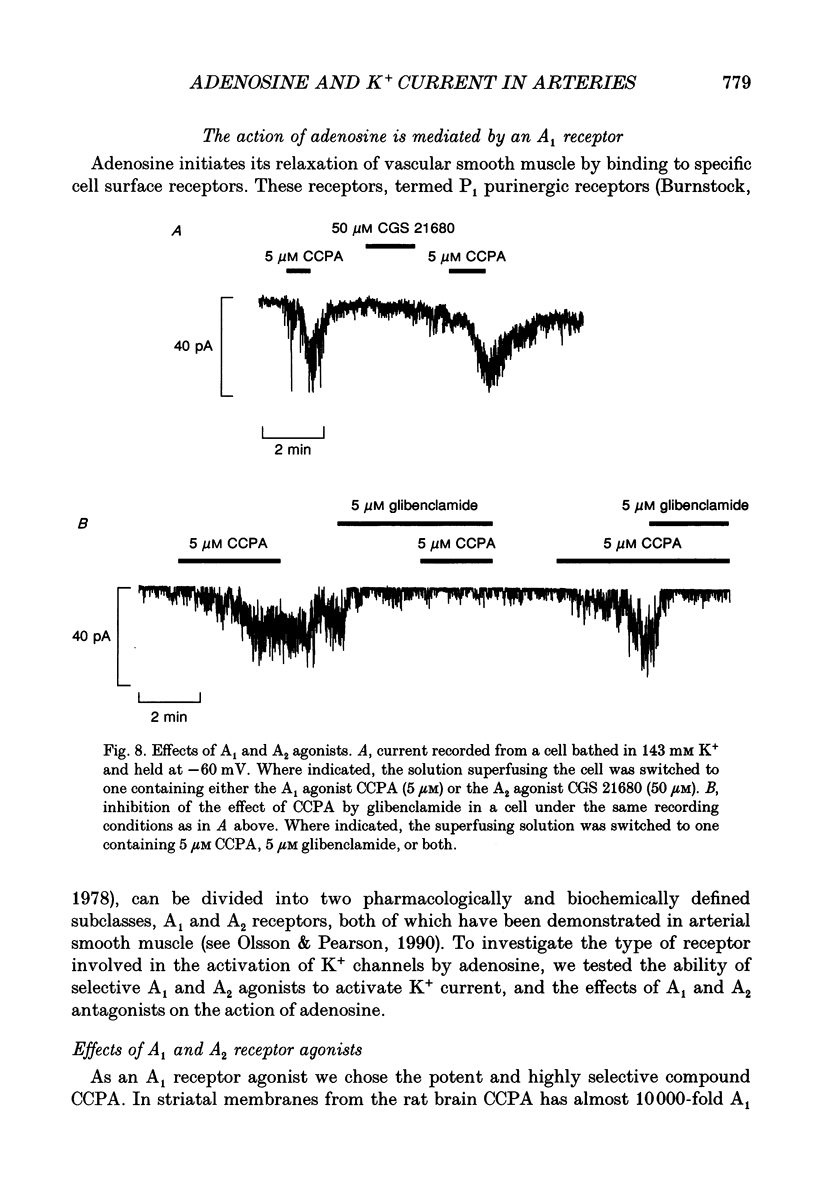
1. The perforated patch technique with nystatin or amphotericin was used to record whole cell currents activated by adenosine in smooth muscle cells isolated enzymatically from pig coronary arteries. 2. Adenosine (5-40 microM) activated an outward current at a holding potential of 0 mV in 5 mM [K+]o and an inward current at -60 mV in 143 mM [K+]o. The dependence of the reversal potential for the adenosine-activated current on [K+]o suggests that it flows through K+ channels, while its current-voltage relation is consistent with the channels showing little voltage dependence. 3. The adenosine-activated current was inhibited by the sulphonylurea glibenclamide (5 microM) and by phencyclidine (5 microM). It was unaffected by charybdotoxin (50 nM) or apamin (100 nM), blockers of large and small conductance Ca(2+)-activated K+ channels respectively. 4. At -60 mV in 143 mM K+ solution, openings of single channels passing a current of just over -2 pA could sometimes be detected in the absence of adenosine. Openings became more frequent after the application of adenosine, with several levels then being detected. Openings of channels with a larger conductance were sometimes also seen in the presence of adenosine. Fluctuation analysis gave somewhat lower estimates of unitary current than did direct measurements. 5. The effect of adenosine could be mimicked by the A1 receptor agonist CCPA (2-chloro-N6-cyclopentyladenosine), while the A2 agonist CGS 21680 (2-p-(2-carboxethyl)phenethylamino-5'-N-ethylcarboxamido adenosine hydrochloride) was without effect. The response to adenosine was inhibited by the A1 antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine), but was unaffected by the A2 antagonist CGS 15943A (5-amino-9-chloro-2-(2-furanyl)-1,2,4- triazolo[1,5-C]quinazoline monomethanesulphonate). 6. Our results suggest that adenosine acts at an A1 receptor to activate K+ channels. We consider it most likely that these are ATP-dependent K+ channels. We discuss the mechanism by which K+ channel activation may lead to hyperpolarization and so vasorelaxation.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Linden J., Berne R. M. The cardiac effects of adenosine. Prog Cardiovasc Dis. 1989 Jul-Aug;32(1):73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Böhm M., Brückner R., Hackbarth I., Haubitz B., Linhart R., Meyer W., Schmidt B., Schmitz W., Scholz H. Adenosine inhibition of catecholamine-induced increase in force of contraction in guinea-pig atrial and ventricular heart preparations. Evidence against a cyclic AMP- and cyclic GMP-dependent effect. J Pharmacol Exp Ther. 1984 Aug;230(2):483–492. [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991 Sep;76(5):677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Clayton F. C., Hess T. A., Smith M. A., Grover G. J. Coronary reactive hyperemia and adenosine-induced vasodilation are mediated partially by a glyburide-sensitive mechanism. Pharmacology. 1992;44(2):92–100. doi: 10.1159/000138877. [DOI] [PubMed] [Google Scholar]
- Crépel V., Krnjević K., Ben-Ari Y. Glibenclamide depresses the slowly inactivating outward current (ID) in hippocampal neurons. Can J Physiol Pharmacol. 1992 Feb;70(2):306–307. doi: 10.1139/y92-038. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Ghai G., Francis J. E., Williams M., Dotson R. A., Hopkins M. F., Cote D. T., Goodman F. R., Zimmerman M. B. Pharmacological characterization of CGS 15943A: a novel nonxanthine adenosine antagonist. J Pharmacol Exp Ther. 1987 Sep;242(3):784–790. [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- Haleen S. J., Steffen R. P., Hamilton H. W. PD 116,948, a highly selective A1 adenosine receptor antagonist. Life Sci. 1987 Feb 9;40(6):555–561. doi: 10.1016/0024-3205(87)90369-9. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues M., Romey G., Duval D., Vincent J. P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison A. J., Webb R. L., Oei H. H., Ghai G. R., Zimmerman M. B., Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989 Oct;251(1):47–55. [PubMed] [Google Scholar]
- Itoh T., Seki N., Suzuki S., Ito S., Kajikuri J., Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajioka S., Kitamura K., Kuriyama H. Guanosine diphosphate activates an adenosine 5'-triphosphate-sensitive K+ channel in the rabbit portal vein. J Physiol. 1991 Dec;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Codina J., Birnbaumer L., Brown A. M. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H820–H826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- Kovacs R. J., Nelson M. T. ATP-sensitive K+ channels from aortic smooth muscle incorporated into planar lipid bilayers. Am J Physiol. 1991 Aug;261(2 Pt 2):H604–H609. doi: 10.1152/ajpheart.1991.261.2.H604. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Schwabe U., Cristalli G., Vittori S., Grifantini M. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- Merkel L. A., Lappe R. W., Rivera L. M., Cox B. F., Perrone M. H. Demonstration of vasorelaxant activity with an A1-selective adenosine agonist in porcine coronary artery: involvement of potassium channels. J Pharmacol Exp Ther. 1992 Feb;260(2):437–443. [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Mills I., Gewirtz H. Cultured vascular smooth muscle cells from porcine coronary artery possess A1 and A2 adenosine receptor activity. Biochem Biophys Res Commun. 1990 May 16;168(3):1297–1302. doi: 10.1016/0006-291x(90)91170-w. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Nakaya Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1991 Dec 16;181(2):700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Nakaya Y., Wakatsuki T., Nakaya S., Fujino K., Saito K., Inoue I. Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res. 1992 Mar;70(3):612–616. doi: 10.1161/01.res.70.3.612. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sabouni M. H., Hargittai P. T., Lieberman E. M., Mustafa S. J. Evidence for adenosine receptor-mediated hyperpolarization in coronary smooth muscle. Am J Physiol. 1989 Nov;257(5 Pt 2):H1750–H1752. doi: 10.1152/ajpheart.1989.257.5.H1750. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A. Properties of single potassium channels in vesicles formed from the sarcolemma of frog skeletal muscle. J Physiol. 1985 Jul;364:339–358. doi: 10.1113/jphysiol.1985.sp015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B. The G. L. Brown Lecture. Potassium channels, metabolism and muscle. Exp Physiol. 1992 Jan;77(1):1–25. doi: 10.1113/expphysiol.1992.sp003564. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Hsu L. C., Tansey M. G., Kamm K. E. Myosin light chain kinase phosphorylation in tracheal smooth muscle. J Biol Chem. 1990 Sep 25;265(27):16683–16690. [PubMed] [Google Scholar]
- von Beckerath N., Cyrys S., Dischner A., Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: an analysis of the underlying mechanisms. J Physiol. 1991 Oct;442:297–319. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]


