Abstract
A lactate/albumin ratio (LAR) greater than 0.5 measured early in the course of pediatric critical illness is associated with greater mortality. Whether the elevated LAR can be explained by microcirculation disorders in children with sepsis is not known. In this longitudinal retrospective study (January 2021-January 2024), serum albumin and lactate were measured on admission to the pediatric intensive care unit (PICU), with sublingual video microscopy performed simultaneously to measure microcirculation. A total of 178 children were included, 37% of whom had septic shock measured with the Phoenix Sepsis Score. Patients with remote sepsis had greater odds of an elevated LAR (aOR 6.87: 95% CI 1.98–23.73; p < 0.01). Children with an elevated LAR had more microvascular blood flow abnormalities (aOR 1.31 95% CI 1.08–1.58; p < 0.01), lower 4-6-micron capillary density (aOR 1.03 95% CI 1.01–1.05; p < 0.01) and greater odds of dying (aOR 3.55 95% CI 1.21–10.38; p = 0.02) compared to those with a low LAR. We found no association between LAR and endothelial glycocalyx degradation. A normal LAR is associated with less risk of microcirculatory injury (aOR 0.77 95% CI 0.65–0.93; p < 0.01). In children with sepsis, an elevated LAR is associated with microcirculation abnormalities (microvascular density and flow). The lactate/albumin ratio is a potentially useful biomarker for microcirculatory injury in sepsis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73112-5.
Keywords: Children, Sepsis, Mortality, Septic shock, Fluid bolus
Subject terms: Biomarkers, Cardiology, Medical research, Risk factors, Signs and symptoms
Introduction
Sepsis is a severe critical condition caused by infection leading to organ dysfunction, with cardiovascular problems being the main cause of death in the first 48 h after diagnosis1–4. Traditionally, hemodynamic status has been assessed using macrocirculation outcome variables (e.g., blood pressure, heart rate, pulse pressure, cardiac output, etc.) which have been taken as surrogates for tissue perfusion3. In this regard, serum lactate is a biomarker for tissue hypoxia that indicates abnormal perfusion and is used in adults and children as a marker of severity and a guide for interventions5,6. However, lactate levels may be elevated in circumstances unrelated to tissue hypoxia7. On the other hand, serum albumin is both a nutritional biomarker and an acute phase reactant in inflammatory states. Hypoalbuminemia is associated with higher mortality and worse outcomes in patients with sepsis8,9. Low albumin levels may occur in children with sepsis due to increased microvascular permeability (associated with systemic inflammation), increased capillary leak syndrome and decreased hepatic synthesis10,11.
In children with sepsis vascular endothelial dysfunction occurs as a result of inflammation and tissue hypoxia11. This situation contributes to multiple organ dysfunction syndrome (MODS) secondary to sepsis, and mortality12,13. The serum levels of lactate and albumin in these cases have been evaluated separately and associated with worse outcomes. Wang B et al.14 evaluated these two biomarkers jointly in adults with sepsis. This ratio was termed the “lactate-albumin ratio (LAR).” They found that, in adults with sepsis, a high LAR was associated with higher mortality and MODS14. Since then, this ratio has been used as a prognostic biomarker in critically ill patients15,16. In children, a recent multicenter study found that an LAR greater than 0.5 (IQR 0.3–0.8) was associated with greater odds of MODS and mortality compared with individually evaluated lactate and albumin17.
Microcirculatory dysfunction plays a key role in the pathogenesis of tissue dysoxia and organ failure in sepsis12. Our hypothesis is that an abnormal LAR is an expression of microcirculatory injury. In this study, we aimed to determine the association between LAR and microcirculation changes in children with sepsis.
Method
Study design
This was a longitudinal, analytical, retrospective study of children hospitalized in the pediatric intensive care unit (PICU) at Fundación Cardioinfantil in Bogotá, Colombia, between January 2021 and January 2024. It is part of a secondary analysis of the MICROCOL project11,13 that evaluates microcirculatory changes in children with sepsis, which was approved by the institutional review board (IRB) at Universidad de La Sabana (MED 256–2019). This study was also approved by the IRB ethics committee at Fundación Cardioinfantil-Instituto de Cardiología in Bogotá, Colombia, (IRB-DDI 4911 − 2024). All of the parents/guardians signed informed consent for the sublingual video microscopy, as this is a partially invasive non-routine procedure in children with sepsis. All research procedures followed the ethical standards of the Fundación Cardioinfantil-IRB and were consistent with the Helsinki Declaration of 1975.
Sample and data
Patients from one month to 18 years old who were diagnosed with sepsis or septic shock and admitted consecutively to the PICU were included. The sepsis definition recommended by the Surviving Sepsis Campaign Guidelines3 was used during data collection. For patients included in the current analysis, sepsis was defined as a Phoenix Sepsis Score (PSS) equal to or greater than 2, and those with more than one cardiovascular point were considered to have septic shock1,2. In addition, the entire conventional bundle treatment for septic shock was implemented, including antibiotics, fluid boluses, vasopressors and inotropes, as recommended in the recent sepsis guidelines3. The initial arterial lactate and albumin were measured in plasma within six hours of admission to the PICU. The LAR was calculated by dividing the initial lactate value by the initial albumin value (drawn simultaneously). An LAR greater than 0.5 was considered elevated, in line with the previous pediatric multicenter study17. The microcirculation was measured using sublingual video microscopy (Glycocheck System®) at the same time the LAR sample was drawn. The LAR was measured, and video microscopy was performed within six hours of PICU admission and 24 h after admission, with both tests always conducted simultaneously. Patients who had received 5% albumin boluses for fluid resuscitation in the first 24 h after emergency room admission and those with chronic kidney disease (defined as a glomerular filtration rate less than 60 mL/min per 1.73 m2 for more than three months), head trauma, diabetic ketoacidosis or the need for continuous renal replacement therapy were excluded. Patients with a history of heart disease or who had undergone cardiopulmonary bypass surgery during the same hospitalization in which sepsis was diagnosed were also excluded.
Demographic variables as well as clinical macrocirculation (heart rate, blood pressure, pulse pressure) and microcirculation (capillary refill, arteriovenous CO2 gap) data were collected when LAR and video microscopy measurements were taken and 24 h later. Hypoalbuminemia was defined as serum albumin less than 3.0 g/dL13,17, and hyperlactatemia was established in patients with serum lactate greater than 2.0 mmol/L. Severity was evaluated in all patients using the Pediatric Index of Mortality (PIM-2) and the presence of multiple organ failure was evaluated with the Pediatric Logistic Organ Dysfunction-2 (PELOD-2) scale12. The vasoactive-inotropic score (VIS) was calculated using the formula described for critically ill children18. Elevated semiquantitative procalcitonin (PCT) was defined as more than 2.0 gr/dL, elevated C-reactive protein (CRP) as more than 4 mg/dL, elevated ferritin as more than 500 mg/dL and elevated D-dimer as more than 1.5 mg/L. Patients on mechanical ventilation, along with in-hospital mortality, were recorded. Additionally, in line with the new definition of sepsis, when the organ dysfunction occurred in the organ with the sepsis-causing infection, it was considered local sepsis2, and when there was an infectious focus and dysfunction in distant organs, it was considered remote sepsis.
Angiopoietin-2 (Ang-2/Human Angiopoietin 2 ELISA Kit ANG 2; ab99971, Abcam Lab ®, Cambridge, United Kingdom) was used as a biomarker of endothelial activation and increased vascular permeability19. All biomarker measurements were performed according to the manufacturer’s instructions, at the same time as LAR measurement and video microscopy. The samples consisted of 100 µl of citrate plasma centrifuged for 30 min at 1,000 rpm, and then stored at (-) 20ºC for subsequent processing using an enzyme-linked immunosorbent assay (ELISA).
Microcirculation measurements
The microcirculation comprises all blood vessels smaller than 100 microns and capillary vessels smaller than 10 microns with no smooth muscle layer and containing single-file red blood cells20. The microcirculation and endothelial glycocalyx degradation were measured in vivo using dark-field video microscopy (Glycocheck System® - Microvascular Health Solutions Inc. 2014, Salt Lake City, UT, USA). Measurements taken within the first six hours were analyzed (taken as the baseline) and repeated 24 h later.
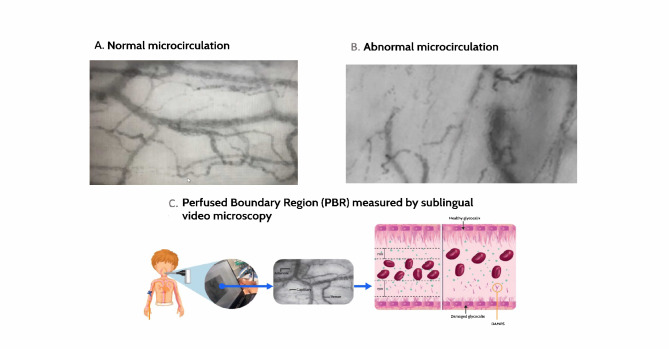
Video microscopes measure blood vessels with a 4–25 μm diameter by emitting a green light that detects reflection from the red blood cells (CapiScope, HVCS, KK Technology United Kingdom). This image is taken with a dark-field camera that amplifies the images and captures 23 frames per second (Fig. 1). This information travels to a software program (Glycocheck System®) that analyzes the data independently from the examiner and investigators21–24. This system reports the perfused boundary region (PBR) in µm, as an indicator of glycocalyx damage. It corresponds to the distance between the red blood cells and the endothelial glycocalyx (Fig. 1C). A value greater than 2.0 μm is considered abnormal in adults and children21,25, indicating glycocalyx degradation and a greater distance between the red blood cells and the capillary wall. This equipment has proven to have low inter and intraobserver variability, with high concordance in different clinical settings (intraclass correlation coefficient of 0.77; 95%CI 0.52–0.87)25.
Fig. 1.
Sublingual video microscopy images taken in patients with and without sepsis. Panel A shows a video microscopy image of a patient without sepsis in our database. It shows an appropriate distribution of venules, capillaries and arterioles. Panel B shows an image from a patient included in the study with septic arthritis of the knee caused by S. aureus. It shows few recruited capillaries, poor red blood cell distribution and tissue hypoperfusion. Images published with parental permission. Panel C shows sublingual microcirculation measurement using video microscopy, software analysis of the data obtained, and PBR measurement as an indicator of glycocalyx thickness. A greater distance between the red blood cells and the endothelial wall (and therefore a greater PBR) can be seen in the damaged glycocalyx. DAMPs: damage-associated molecular patterns. PBR: perfused boundary region.
At the same time, the operator captures images with the camera to evaluate microvascular blood flow and functional capillary density (the number of perfused capillaries per area of tissue). This density is evaluated according to the size of each capillary. Generally, it is reported for the smallest capillaries, through which nutrients and oxygen are delivered to the tissues (4–6 μm capillary density), as well as for larger (10 to 25 μm) blood vessels in which blood flows in meta-arterioles that do have a smooth muscle layer. A low 4–6 μm capillary density (CD 4–6) indicates poor additional capillary recruitment capacity, which is related to tissue hypoxia and organ dysfunction22,24,26. This absolute capillary blood volume (CBV-Abs) corresponds to the blood volume entering the smallest capillaries for exchange; in sepsis, this has been found to depend greatly on the larger blood vessels25,26. A higher figure indicates better blood volume for exchange from the larger to the smaller capillaries. It is the main compensatory variable in terms of volume in sepsis, rather than reduced blood flow in small capillaries26. In addition, the MicroVascular Health Score (MVHS) was analyzed, which is the CBV/PBR ratio, indicating the association between microvascular blood flow and endothelial glycocalyx damage.
Outcomes
The primary outcome was the association between the LAR and microcirculatory changes in terms of flow, density and glycocalyx damage. The secondary outcomes were the association between an elevated LAR and mortality, length of hospital stay and other clinical variables of interest.
Statistical methods
Descriptive statistics were derived according to the nature of the variables, reporting each cohort with an LAR greater than or less than 0.5. This value was selected as the cut-off point based on prior studies in critically ill children17. Qualitative variables were reported as percentages with their respective confidence intervals. Quantitative variables underwent the Kolmogorov-Smirnov test to determine their distribution. Normally distributed variables were reported as means with their respective standard deviations, and variables with a non-normal distribution as medians with interquartile range (IQR). A bivariate analysis was run according to the distribution of the variables, using Student’s t-test. In line with the new Phoenix definition of sepsis in children, each local and remote sepsis cohort was analyzed for changes in the microcirculation variables1,2. The Mann-Whitney test was used for variables with a non-normal distribution. Pearson’s or Spearman’s correlation was used for quantitative variables, according to the variables’ normal or non-normal distribution. A receiver operating characteristic (ROC) curve was used to evaluate the predictive capacity of LAR and microcirculation changes. Univariate logistic regression was run to evaluate the raw odds ratios of LAR abnormalities related to each of the microcirculation variables. For the primary outcome, a binary logistic regression was run taking an LAR greater than 0.5 as the dependent variable, and microcirculation abnormalities as the independent variable, including confounding variables (age, disease severity and vasoactive support evaluated with the VIS) in the model. For the secondary outcomes, the independent variables were mortality, hospital stay, and inflammatory response measured by ferritin and C-reactive protein levels. These variables were selected using the Hosmer-Lemeshow criteria and according to biological plausibility, based on prior studies conducted by our group13. The forward method was used, and the model was evaluated with an omnibus test. Microcirculation predictor variables with multi-collinearity, assessed by the Variance Inflation Factor (VIF) technique, were excluded from the logistic regression model. The model’s performance was assessed by the Hosmer-Lemeshow test for calibration and the Nagelkerke R2 statistic for assessing predictive strength of the logistic regression model. Two-sided analyses were performed, with a p value less than or equal to 0.05 considered to be statistically significant. The analyses were run using SPSS (IBM® version 26 statistical package).
Results
A total of 178 children with sepsis or septic shock were enrolled during the study period. The median age was 23 (IQR 6.1-120.1) months. Of the enrolled patients, 47.2% (84/178) were females. The main reasons for PICU admission were pneumonia and gastrointestinal tract diseases (Table 1). Altogether, 25% had hypoalbuminemia (44/178) and 20.3% (11/178) had elevated serum lactate on admission (Supplemental Material, eFigures 1 and 2). A total of 37.8% had an LAR greater than 0.5 (50/178), 22.9% of whom did not survive (11/50). During the study period, 2.2% of the children were admitted to intensive care for SARS-CoV2 infection. 37% (66/178) had septic shock according to the Phoenix Criteria, with a median score of 4 (IQR 3–5), and 63% (112/178) had a Phoenix score equal to or greater than 2 (Table 1). Children with an elevated LAR had a higher total Phoenix score (p < 0.01), with a high Phoenix cardiovascular (p < 0.01) and respiratory (p = 0.04) score, compared with the normal LAR group.
Table 1.
Clinical and laboratory characteristics of the analyzed patients.
| Characteristic | Total n = 178 | Elevated LAR n = 50 | Normal LAR n = 128 | P value1 |
|---|---|---|---|---|
| Age, months (IQR) | 23.1 (6.1-120.1) | 52.5 (7.2-144.7) | 12.0 (6.1-102.8) | 0.1 |
| Weight, kg (IQR) | 10.8 (6.4–29.9) | 17.0 (6.8–31.0) | 10 (6.4–28.4) | 0.35 |
| Female sex (%) | 84 (47.2) | 25 (50) | 59 (46.1) | 0.76 |
| Days in PICU (IQR) | 12.0 (7.0–22.0) | 14.0 (6.5–22.5) | 12 (7.0–19.0) | 0.91 |
| Focus of infection (%) | 0.34 | |||
| Respiratory | 61 (35.3) | 20 (40.8) | 41 (33.1) | |
| Gastrointestinal tract | 42 (24.3) | 14 (28.6) | 28 (22.6) | |
| Central nervous system | 9 (5.2) | 1.0 (2.0) | 8.0 (6.5) | |
| Other | 61 (35.3) | 14 (28.6) | 47.0 (37.9) | |
| Local sepsis (%) | 61 (34.2) | 9 (18) | 52 (40.6) | 0.53 |
| Remote sepsis (%) | 117 (65.7) | 41 (82) | 76 (59.4) | 0.46 |
| PIM-2 (IQR) | 15 (6.5–26) | 18.7 (6.7–26.5) | 14 (6.3–25.6) | 0.57 |
| PELOD-2 score (IQR) | 6.0 (3.0–9.0) | 6.0 (3–8.0) | 7.0 (3.0–9.0) | 0.29 |
| Phoenix Sepsis Score | 4.0 (3.0–5.0) | 4.0 (4.0–5.0) | 4.0 (2.5-4.0) | 0.01 |
| Lactate, mmol/L | 2.45 (1.9–3.7) | 2.8 (2.4–3.2) | 2.2 (1.9–2.3) | 0.08 |
| Albumin, g/dL | 3.0 (2.2–3.6) | 2.8 (2.5-3.0) | 3.2 (2.9–3.4) | 0.12 |
| Glucose mg/dL (IQR) | 114.0 (94.6-139.5) | 121.0 (99.4–149.0) | 108.6 (91.6-132.5) | 0.04 |
| Ferritin mg/dL (IQR) | 424 (190-1586.4) | 583.2 (298-2130.5) | 373.5 (160-1265.8) | 0.02 |
| C-reactive protein, mg/dL (IQR) | 4.7 (1.6–11.4) | 5.3 (1.6–12.6) | 4.4 (1.6–9.1) | 0.61 |
| D-dimer, mg/L (IQR) | 3.6 (1.6–8.3) | 4.3 (2.1–8.3) | 3.5 (1.2–8.4) | 0.48 |
| Procalcitonin, g/dL (IQR) | 1.4 (0.4-5.0) | 2.1 (0.9–13.3) | 1.1 (0.3–4.3) | < 0.01 |
| Creatinine, mg/dL (IQR) | 0.4 (0.3–0.6) | 0.5 (0.3–0.8) | 0.4 (0.3–0.6) | 0.08 |
| Vasoactive score (IQR) | 10 (5.0-28.4) | 19 (6.2–31.9) | 7.5 (4.0-24.1) | 0.01 |
| Mechanical ventilation (%) | 152 (87.9) | 44 (89.8) | 108 (87.1) | 0.81 |
| Mortality (%) | 21.0 (12.1) | 11 (22.9) | 10 (8.0) | 0.01 |
An LAR (lactate-albumin ratio) greater than 0.5 is considered elevated, and equal to or less than 0.49 is considered normal1. Statistically significant p values are in bold (p < 0.05). P-values by Student’s t test, Chi-square test, or Mann–Whitney U test, as applicable. Those not labeled were analyzed using the Chi-square test. PIM-2: Pediatric Index of Mortality-2; PELOD-2: Pediatric Logistic Organ Dysfunction-2. PICU: pediatric intensive care unit. IQR: interquartile range.
The descriptive statistics for microvascular and inflammatory parameters are shown in Table 2. There were no differences in PICU length of stay (10.12 [6.01–16.52] days vs. 12.01 [7.12–22.13] days), arteriovenous CO2 gap, or Ang-2 levels (p = 0.31) between the groups. Patients with remote sepsis had higher odds of an elevated LAR compared to patients with local sepsis, regardless of age or disease severity on admission (aOR 6.87 95% CI 1.98–23.73; p < 0.01), as measured with the PIM-2 scale.
Table 2.
Microcirculatory characteristics and inflammatory biomarkers in children with local vs. remote sepsis on PICU admission.
| Microcirculation evaluation variables | Total n = 178 | Local sepsis n = 61 | Remote sepsis n = 117 | P value1 |
|---|---|---|---|---|
| PBR, µm (SD) | 2.11 (0.28) | 2.08 (0.28) | 2.11 (0.29) | 0.37 |
| PBR flow corrected µm (IQR) | 2.06 (1.79–2.38) | 2.14 (1.89–2.25) | 2.03 (1.76–2.37) | 0.41 |
| Worst PBR, µm (IQR) | 3.24 (2.95–3.73) | 3.21 (2.96–3.76) | 3.26 (2.92–3.62) | 0.82 |
| 4–6 μm capillary density (IQR) | 24.95 (15.8-42.91) | 27.72 (18.9–41.3) | 23.51 (15.2-50.32) | 0.64 |
| Absolute capillary blood volume (IQR) | 5.9 (4.1–9.4) | 5.3 (4.4–8.6) | 6.1 (4.1–10.7) | 0.57 |
| Angiopoietin-2 ng/mL (IQR) | 11.1 (7.4–24.5) | 23.3 (10.3–24.7) | 10.6 (7.1–23.6) | 0.31 |
| CO2 delta (IQR) | 5.14 (3.21–7.05) | 5.06 (2.41–7.14) | 5.12 (3.22–7.15) | 0.69 |
| Ferritin, mg/dL (IQR) | 439.64 (185.08-1772.31) | 313.38 (159.17-557.17) | 514.21 (222.41–2280) | < 0.001 |
| Procalcitonin, g/dL (IQR) | 1.50 (0.41–6.28) | 1.09 (0.28–2.95) | 1.89 (0.53–7.46) | < 0.001 |
| D-dimer, mg/L (IQR) | 3.26 (1.21–7.52) | 1.63 (0.88–3.4) | 3.58 (2.34–9.91) | < 0.001 |
| Capillary refill time in seconds (IQR) | 1.43 (1.1–2.14) | 1.31 (0.82–172) | 1.51 (1.11–2.15) | 0.01 |
1Statistically significant p values are in bold (p < 0.05). P-values from the Student’s t test, Chi-square test, or Mann–Whitney U test, as applicable. PBR: perfused boundary region. CO2 delta: CO2 gap. IQR: interquartile range.
Primary outcome
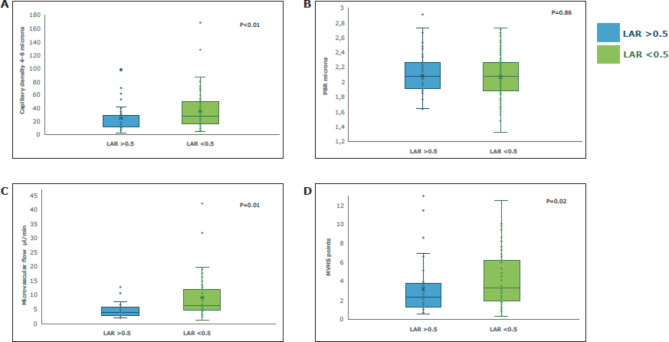
Microcirculation was evaluated in terms of microvascular flow (CBV) in both groups of patients. There was a weak inverse correlation between the LAR level and the number of 4-6-micron capillaries recruited (Spearman’s correlation, rho − 0.25; p < 0.01) as well as the 4-25-micron capillary recruitment capacity (rho − 0.23; p = 0.04). Recruitment capacity and functional capillary density were also evaluated. Patients with an LAR greater than 0.5 were found to mainly have altered recruitment capacity of small capillaries (4-6-micron CD) compared to larger vessels (10-25-micron CD) (p < 0.01). (Fig. 2).
Fig. 2.
Microcirculatory and lactate-albumin ratio changes on admission in children with sepsis. LAR: lactate-albumin ratio. PBR: perfused boundary region. CBV: capillary blood volume. MVHS: Microvascular Health Score (relationship between the CBV/PBR). The Mann-Whitney U test was performed on the analyzed variables.
Children with an elevated LAR had higher odds of altered microvascular blood flow (aOR 1.31 95% CI 1.08–1.58; p < 0.01) regardless of disease severity and the need for vasoactive drugs, compared to the normal LAR group (Table 3). The assessed model had good calibration (p = 0.58 for the Hosmer-Lemeshow test) and good predictive strength (Nagelkerke test R2 = 0.82). The LAR on admission predicted a change between the concurrently measured 4-6-micron capillary density and that measured 24 h later (AUC 0.72 95% CI 0.70–0.84; p < 0.01). We found no association between LAR and PBR on admission (p = 0.86).
Table 3.
Univariate and multivariate logistic regression of microcirculatory changes and an elevated lactate-albumin ratio on PICU admission.
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | aOR (95% CI) | p value* | |
| Capillary density 4–6 microns | 1.03 (1.01–1.06) | < 0.01 | 1.03 (1-01-1.05) | < 0.01 |
| Capillary density 10–20 microns | 1.01 (1.00-1.01) | 0.06 | 1.01 (0.99–1.02) | 0.08 |
| Absolute vascular flow | 1.25 (1.10–1.49) | < 0.01 | 1.31 (1.08–1.58) | < 0.01 |
| MVHS | 0.97 (0.84–1.13) | 0.77 | 0.98 (0.84–1.14) | 0.82 |
| PBR | 1.25 (0.63–2.48) | 0.52 | 1.20 (0.57–2.51) | 0.86 |
| PBR flow corrected | 1.11 (0.55–2.22) | 0.77 | 1.13 (0.53–2.35) | 0.74 |
| Worst PBR | 1.28 (0.79–2.09) | 0.31 | 1.18 (0.72–1.94) | 0.51 |
MVHS: MicroVascular Health Score (the ratio between the PBR and relative vascular flow). PBR: perfused boundary region. CI: confidence interval. OR odds ratio. The adjusted odds ratio (aOR) was calculated for confounding variables (PIM-2, age and vasoactive index score). A lactate-albumin ratio greater than 0.5 was considered elevated. *Logistic regression using the forward method and omnibus test.
Secondary outcome
Patients with an elevated LAR on admission had higher odds of dying (aOR 3.55 95% CI 1.21–10.38; p = 0.02) regardless of disease severity evaluated with the PIM-2 score and the need for vasoactives (evaluated with the vasoactive score), compared to children with a normal LAR. If the LAR was less than 0.5, the patients who survived had a higher 4-6-micron capillary recruitment capacity (aOR 0.97; 95% CI 0.95–0.99; p < 0.01). We found a weak correlation between the LAR level and elevated ferritin (rho 0.18; p = 0.04) and D-dimer abnormalities (rho 0.19; p = 0.04), but not C-reactive protein (rho 0.07; p = 0.33). Patients with a normal LAR on admission had lower odds of requiring vasopressor support based on the VIS (aOR 0.22 95% CI 0.14–0.74; p = 0.01), regardless of disease severity or age. We were unable to show an association between prolonged capillary refill time on admission and an elevated LAR (p = 0.62). The relationship between the use of inotropes, VIS and the LAR are shown in the supplementary material. We found that an LAR greater than 0.5 on admission had a good predictive capacity for elevated D-dimer (AUC 0.68, 95% CI 0.58–0.76; p = 0.04), at least 1 cardiovascular point on the PSS (AUC 0.67, 95% CI 0.58–0.75; p < 0.01) and mortality (AUC 0.71, 95% CI 0.61–0.81; p = 0.01).
Discussion
In this study of children with sepsis admitted to the PICU, we found that an elevated LAR on admission was associated with abnormal microvascular blood flow and functional small capillary density (4-6-micron CD) but not with endothelial glycocalyx degradation. Children with an elevated LAR have a higher mortality, inflammatory response and need for vasoactive support. Our study is the first to evaluate an association between the LAR and microcirculatory abnormalities.
In children and adults, lactate has been considered to be a late indicator of tissue hypoperfusion5–7. Albumin is the main plasma protein, and low levels have been associated with more inflammation and a higher risk of dying in adults and children with sepsis8,10. It is also acutely reduced by hemodilution, increased capillary permeability and hepatocellular dysfunction. The combination of the two biomarkers in our study was associated with lower microcirculatory blood flow (hypoperfusion) and less capacity to recruit additional small capillaries. This abnormality indicates that an elevated LAR may be a good surrogate of microcirculatory damage in children with sepsis and, although other conditions may affect it, microcirculatory damage could be biologically plausible with respect to the disease’s pathophysiology.
Following the original description of the LAR in adults with sepsis14, this elevated biomarker has been associated with multiple organ dysfunction27 and mortality28–31 in patients with trauma, burns, myocardial infarction and pancreatitis15,17,27,32,33. Both lactate and albumin abnormalities are related to cardiovascular system involvement. Using them together in the LAR has proven to be a good prognostic and predictive biomarker in critically ill patients28,32,33. This could be a good reflection of tissue oxygenation in inflammation, reflecting the underlying pathophysiological phenomenon associated with the disease. In fact, in our cohort, patients who were admitted with an elevated LAR had higher levels of procalcitonin and required more vasoactive support. These findings would be congruent with compromised microvascular flow, increased endothelial permeability due to inflammation and lower tissue oxygenation, with greater need for vasopressor support.
Furthermore, our study analyzed microcirculatory changes in children with sepsis with local or remote organ dysfunction. Patients with remote sepsis had higher systemic inflammation biomarkers with no changes in microvascular flow, capillary recruitment capacity and glycocalyx damage on admission to the PICU, compared to children with local sepsis. These changes persisted 24 h after admission, with no further deterioration. The PSS validation cohort showed a high frequency of sepsis with a remote focus of infection (85%) in countries with both high and low availability of resources1,2. Our hypothesis to explain these findings is that the intrinsic microcirculatory compensatory mechanisms in sepsis may be progressive and directly related to the severity of inflammation. Since LAR is a binary variable, we cannot adequately differentiate the evolution of this biomarker. However, in both adults and children, normalization of the macrocirculation targets with persistent microcirculation damage (due to loss of hemodynamic coherence) has been associated with greater mortality, worse outcomes and late phases of the disease11,24,34,35. Likewise, patients with an elevated LAR had a higher PSS and more cardiovascular impairment. This finding has physiological plausibility, because patients with impaired perfusion and more inflammation (which is associated with a high LAR), would have more microcirculation damage, with high lactate levels and therefore a higher PSS.
The focus of fluid and hemodynamic resuscitation in children with sepsis is changing. It has gone from a mechanistic approach considering only macrocirculatory targets (cardiac output, blood pressure, heart rate, etc.) to the inclusion of microcirculatory normalization targets36. Sublingual video microscopy is one of the best ways to evaluate endothelial and microcirculatory damage in sepsis20,21,25. Unfortunately, it is expensive and not available in many facilities. Therefore, clinical tools have been sought to identify microcirculatory damage in sepsis. One of these tools has been prolonged capillary refill time, determining that it should be interpreted not just as an indicator of tissue hypoperfusion in adults and children, but also as a marker of microcirculatory damage24,37. Serum biomarkers that evaluate microcirculatory damage have similar limitations. Many are costly or nonspecific for glycocalyx damage or increased capillary permeability26. With our study’s findings, we believe that the LAR could be an accessible and inexpensive alternative available in many facilities to identify microcirculatory damage in children with sepsis.
We consider that our study has several limitations. First, it is the experience of a single referral center in a country with limited resources where children are admitted with serious diseases and the need for advanced interventions. This could limit the extrapolation of our findings to this population. However, our patients received the care recommended in the most recent sepsis guidelines. In addition, due to the observational nature of our study, a causal relationship between an abnormal LAR and microcirculatory damage cannot be established. However, there is biological and physiological plausibility and a temporal association between an elevated LAR and microvascular abnormalities in our patients38. Furthermore, we used rigorous inclusion criteria and advanced statistical analyses controlling for confounding variables, among others, which allow causal interpretations of the outcomes of an observational study, because solid and well-interpreted assumptions are maintained39. Finally, we were unable to follow the LAR changes associated with microcirculatory modifications up to hospital discharge. Prospective studies are needed to follow the LAR for a longer period of time and determine the relationship between treatment interventions in children with sepsis and changes in the LAR.
Conclusion
In children with sepsis, an elevated LAR is associated with microcirculatory abnormalities in terms of lower microvascular blood flow, lower functional capillary density and compromised capillary recruitment capacity. We found no relationship between this index and endothelial glycocalyx degradation. An elevated LAR on admission was associated with a greater inflammatory response and need for vasoactive support, as well as higher odds of dying. Further investigation is warranted to clarify the potential usefulness of the LAR as a biomarker for microcirculatory injury in children with sepsis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
I would like to thank the PICU physicians and nurses at Fundación Cardioinfantil for their constant help with our patients.
Author contributions
MCP, JFS contributed to the concept or design of the study, data collection, analysis or interpretation of the data, drafting of the manuscript, and critical review of the manuscript for important intellectual content. All authors (MCP, JFS, JDB, SFJ, PRC, AN, NL, SF, JMF, CC, MAG, JPFS, ILR, AC, JP, LA, JDS, CDA) had full access to the data, contributed to the data collection, approved the final version for publication, and take responsibility for its accuracy and integrity.
Funding
This study was supported by the MED256-2019 project at Universidad de La Sabana.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schlapbach, L. J. et al. International consensus criteria for pediatric sepsis and septic shock. JAMA331(8), 665–674 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez-Pinto, L. N. et al. Development and validation of the phoenix criteria for pediatric sepsis and septic shock. JAMA331(8), 675–686 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss, S. L. et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr. Crit. Care Med.21(2), e52–e106 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Souza, D. C. et al. Epidemiology of sepsis in children admitted to PICUs in South America. Pediatr. Crit. Care Med.17(8), 727–734 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Scott, H. F. et al. Association between early lactate levels and 30-Day mortality in clinically suspected sepsis in children. JAMA Pediatr.171(3), 249–255 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Hernández, G. et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-Day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA321(7), 654–664 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinowitz, J. D. et al. Lactate: The ugly duckling of energy metabolism. Nat. Metab.2(7), 566–571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu, W. et al. Interpretable machine learning prediction of all-cause mortality. Commun. Med. (Lond). 3 (2), 125 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saucedo-Moreno, E. M. et al. Hypoalbuminemia as a predictor of mortality in abdominal sepsis. Cir. Cir.88 (4), 481–484 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Pinto, L. N. et al. Derivation, validation, and clinical relevance of a pediatric sepsis phenotype with persistent hypoxemia, encephalopathy, and shock. Pediatr. Crit. Care Med.24(10), 795–806 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Sarmiento, J. et al. The association between hypoalbuminemia and microcirculation, endothelium, and glycocalyx disorders in children with sepsis. Microcirculation30(8), 1–10 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Shen, Y. et al. Meta-analysis for the prediction of mortality rates in a pediatric intensive care unit using different scores: PRISM-III/IV, PIM-2, and PELOD-2. Front. Pediatr.24, 712276 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarta-Mantilla, M. et al. Microcirculation, endothelium and glycocalyx changes associated with the use of milrinone in children with septic shock. Transl. Pediatr.31(5), 727–737 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, B. et al. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J. Crit. Care. 30, 271–275 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Gharipour, A. et al. Lactate/albumin ratio: An early prognostic marker in critically ill patients. Am. J. Emerg. Med.38(10), 2088–2095 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Bou Chebl, R. et al. Lactate/albumin ratio as a predictor of In-Hospital mortality in septic patients presenting to the emergency department. Front. Med. (Lausanne)22(7), 550182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray, C. C. The association of the lactate-albumin ratio with mortality and multiple organ dysfunction in PICU patients. Pediatr. Crit. Care Med.24(9), 760–766 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perizes, E. N. et al. Derivation and validation of vasoactive Inotrope score trajectory groups in critically Ill children with shock. Pediatr. Crit. Care Med.23(12), 1017–1026 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smadja, D. M. et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 23 (4), 611–620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ince, C. et al. Cardiovascular dynamics section of the ESICM. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med.44(3), 281–299 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Rovas, A. et al. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: The NOSTRADAMUS study. Crit. Care25, 112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nussbaum, C. et al. Perturbation of the microvascular glycocalyx and perfusion in infants after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg.150, 1474–1481 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Sarmiento, J. et al. Endothelial and glycocalyx biomarkers in children with sepsis after one Bolus of unbalanced or balanced crystalloids. Pediatr. Crit. Care Med.24(3), 213–221 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Sarmiento, J. et al. The association between prolonged capillary refill time and microcirculation changes in children with sepsis. BMC Pediatr.24 (1), 68 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovas, A. et al. Bedside analysis of the sublingual microvascular glycocalyx in the emergency room and intensive care unit - the GlycoNurse study. Scand. J. Trauma. Resusc. Emerg. Med.26 (1), 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rovas, A. et al. Microvascular and proteomic signatures overlap in COVID-19 and bacterial sepsis: The MICROCODE study. Angiogenesis25(4), 503–515 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo, W. et al. The value of lactate/albumin ratio for predicting the clinical outcomes of critically ill patients with heart failure. Ann. Transl. Med.9, 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, Y. et al. Association between lactate/albumin ratio and all-cause mortality in patients with acute respiratory failure: A retrospective analysis. PLoS One16, e0255744 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, R. et al. Lactate albumin ratio is associated with mortality in patients with moderate to severe traumatic brain Injury. Front. Neurol.1(13), 662385 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudoignon, E. et al. Usefulness of lactate albumin ratio at admission to predict 28-day mortality in critically ill severely burned patients: A retrospective cohort study. Burns48, 1836–1844 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Lau, K. K. et al. Utility of the Lactate/Albumin ratio as a predictor for mortality in necrotizing Fasciitis patients. Emerg. Med. Int.13, 3530298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, G. et al. Lactate/albumin ratio as a predictor of in-hospital mortality in critically ill children. BMC Pediatr.22 (1), 725 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, D. et al. Association between lactate/albumin ratio and all-cause mortality in critical patients with acute myocardial infarction. Sci. Rep.13 (1), 15561 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Santis, P. et al. Incoherence between systemic hemodynamic and microcirculatory response to fluid challenge in critically ill patients. J. Clin. Med.10(3), 507 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallat, J. et al. Pathophysiology, mechanisms, and managements of tissue hypoxia. Anaesth. Crit. Care Pain Med.41 (4), 101087 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Duranteau, J. et al. The future of intensive care: The study of the microcirculation will help to guide our therapies. Crit. Care27(1), 190 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez, G. et al. Monitoring capillary refill time in septic shock. Intensive Care Med.50 (4), 580–582 (2024). [DOI] [PubMed]
- 38.Horvat, C. Statistical note: Confounding and causality in observational studies. Pediatr. Crit. Care Med.22(5), 496–498 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahabreh, I. J. & Bibbins-Domingo, K. Causal inference about the effects of interventions from observational studies in medical journals. JAMA May9, 10.1001/jama.2024.7741 (2024). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.