Abstract
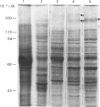
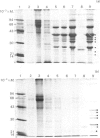
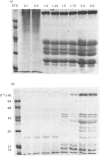
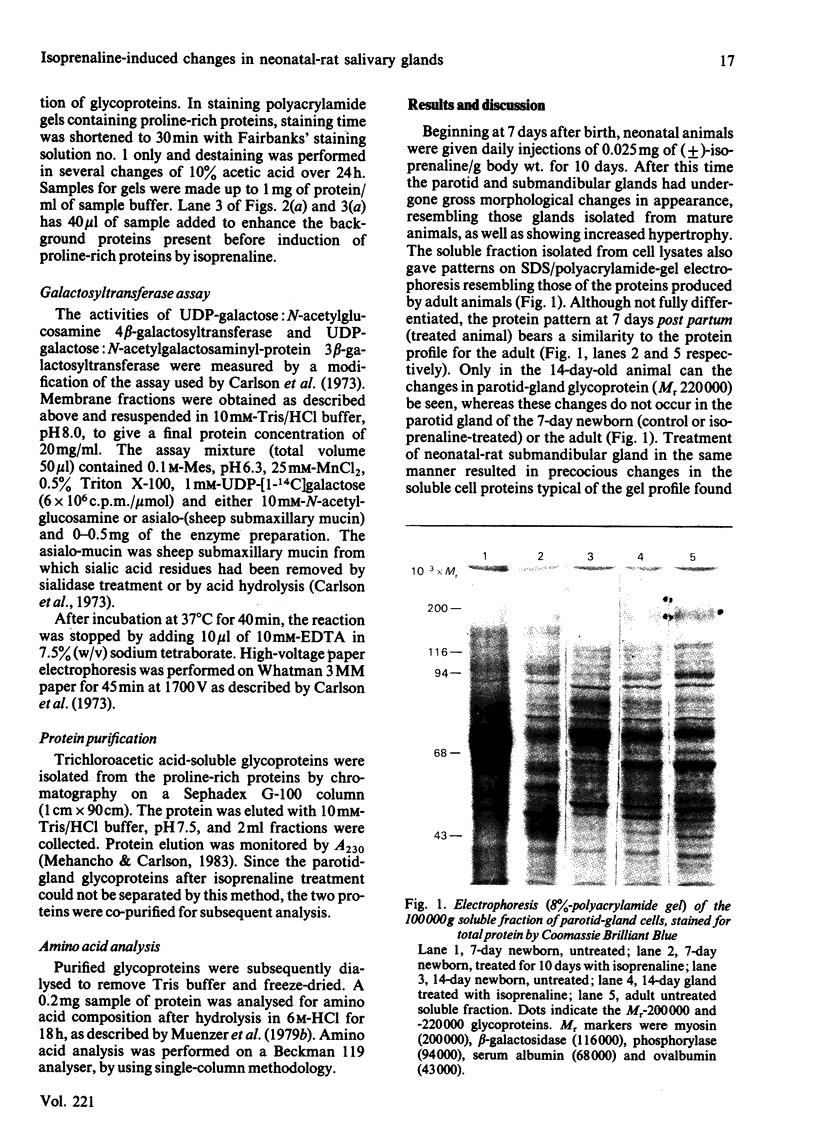
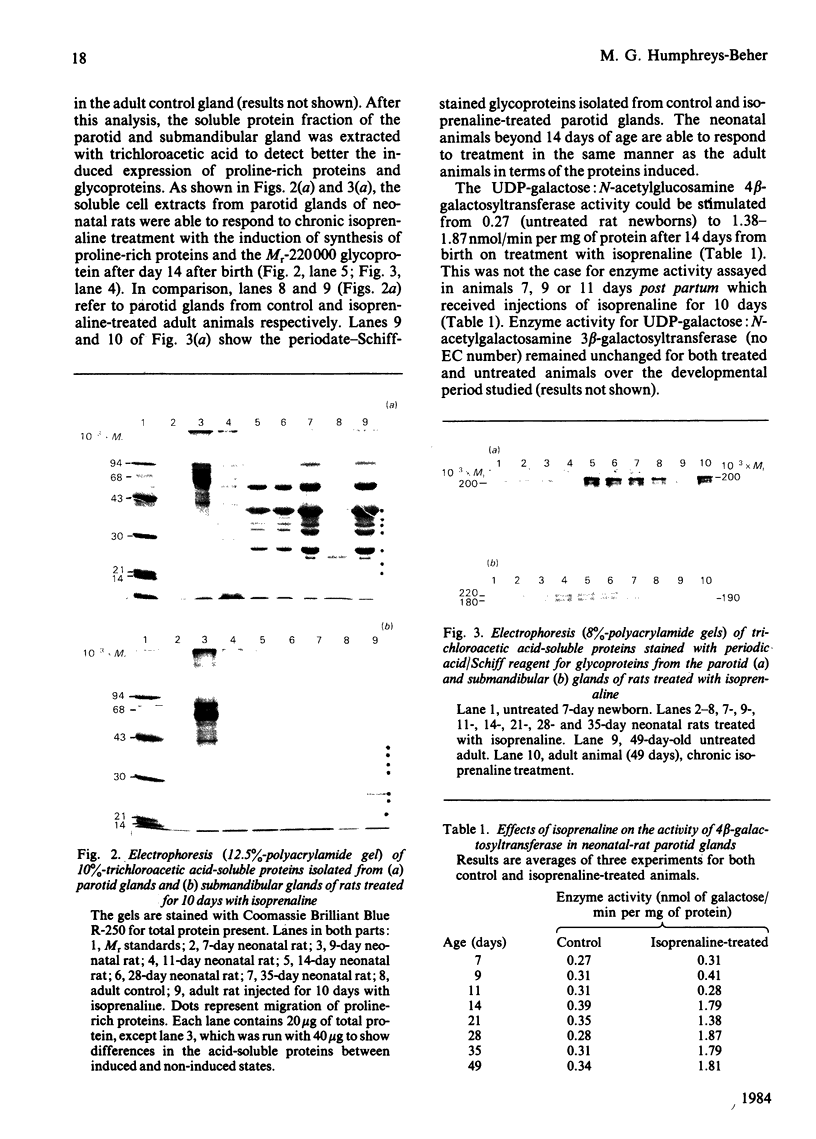
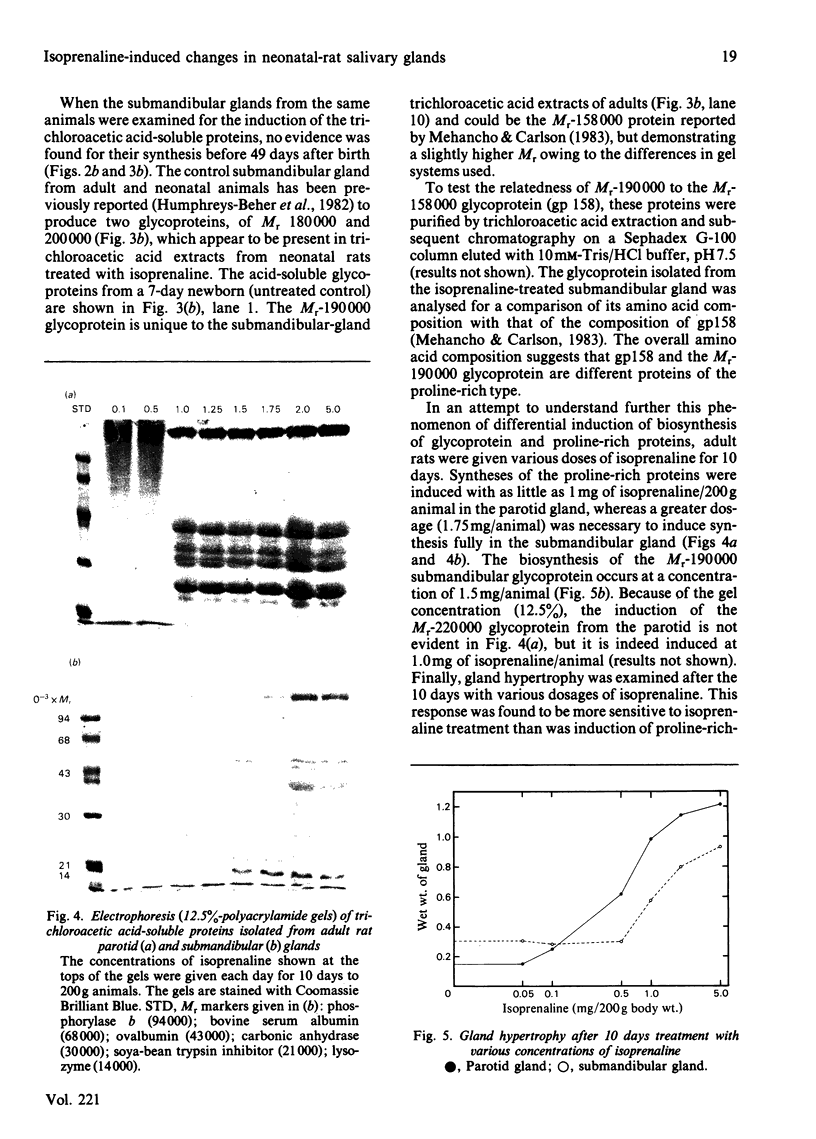
Neonatal rats treated with chronic injections of isoprenaline (isoproterenol) for 10 days revealed differential induction of proline-rich proteins and glycoprotein synthesis between the parotid and submandibular glands. Biosynthesis of proline-rich proteins (Mr 17000-35000) and a Mr-220000 glycoprotein were detectable by solubilization in 10%-trichloroacetic acid extracts from parotid glands 14 days after birth. The enzyme lactose synthase (UDP-galactose: 2-acetamido-2-deoxy-D-glucosamine 4 beta-galactosyltransferase) (EC 2.4.1.22) is also induced 4-7-fold in specific activity compared with control neonatal rats, but again only after 14 days post partum, with isoprenaline treatment. This is in accord with the ability of the parotid gland to respond to beta-receptor stimulation and subsequent increases in intracellular cyclic AMP necessary for induction of protein synthesis [Grand, Chong & Ryan (1975) Am. J. Physiol. 228, 608-612]. Induction of the proline-rich proteins and a Mr-190000 glycoprotein in the soluble fraction from the submandibular gland were not detected until 49 days after birth under identical conditions in the same animal. Cyclic AMP in the submandibular gland undergoes increases on beta-receptor stimulation similar to those achieved in the adult animal, 1 day after birth (Grand et al., 1975). This same differential induction between parotid and submandibular gland was obtained with a range of isoprenaline dosages in adult animals. Trichloroacetic acid-soluble proline-rich proteins were isolated from parotid glands at a dosage of 4.0 mg of isoprenaline/kg body wt., but 7.0 mg/kg was required to induce also biosynthesis of these proteins in the submandibular gland. Gland hypertrophy showed the same differential dosage kinetics, based on gland weight, between the two glands; however, hypertrophy could be accomplished at a lower dosage of isoprenaline than that used to induce proline-rich-protein biosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barka T., Van Der Noen H. Stimulated growth of submandibular gland. Lab Invest. 1976 Nov;35(5):507–514. [PubMed] [Google Scholar]
- Bressler R. S. Fine structure of the differentiating acini in submandibular glands of isoproterenol-treated rats. Am J Anat. 1973 Dec;138(4):431–447. doi: 10.1002/aja.1001380403. [DOI] [PubMed] [Google Scholar]
- Carlson D. M., David J., Rutter W. J. Galactosyltransferase activities in pancreas, liver and gut of the developing rat. Arch Biochem Biophys. 1973 Aug;157(2):605–612. doi: 10.1016/0003-9861(73)90680-2. [DOI] [PubMed] [Google Scholar]
- Ekfors T., Chang W. W., Bressler R. S., Barka T. Isoproterenol accelerates the postnatal differentiation of rat submandibular gland. Dev Biol. 1972 Sep;29(1):38–47. doi: 10.1016/0012-1606(72)90041-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Chong D. A., Ryan S. J. Postnatal development of adenylate cyclase in rat salivary glands: patterns of hormonal sensitivity. Am J Physiol. 1975 Feb;228(2):608–612. doi: 10.1152/ajplegacy.1975.228.2.608. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Schay M. I. Development of secretory function in rat parotid gland. Pediatr Res. 1978 Feb;12(2):100–104. doi: 10.1203/00006450-197802000-00007. [DOI] [PubMed] [Google Scholar]
- Humphreys-Beher M. G., Hollis D. L., Carlson D. M. Comparative developmental analysis of the parotid, submandibular and sublingual glands in the neonatal rat. Biochem J. 1982 Jun 15;204(3):673–679. doi: 10.1042/bj2040673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Nagatsu T., Yajima T., Maeda N., Kumegawa M., Kato T. Developmental changes in the activities of prolinase and prolidase in rat salivary glands, and the effect of thyroxine administration. Mol Cell Biochem. 1982 Jan 16;42(1):31–36. doi: 10.1007/BF00223536. [DOI] [PubMed] [Google Scholar]
- Kumegawa M., Takuma T., Takagi Y. Precocious induction of secretory granules by hormones in convoluted tubules of mouse submandibular glands. Am J Anat. 1977 May;149(1):111–114. doi: 10.1002/aja.1001490108. [DOI] [PubMed] [Google Scholar]
- Ludford J. M., Talamo B. R. Independent regulation of beta-adrenergic receptor and nucleotide binding proteins of adenylate cyclase. Developmental and denervation-dependent responses in rat parotid. J Biol Chem. 1983 Apr 25;258(8):4831–4838. [PubMed] [Google Scholar]
- Ludford J. M., Talamo B. R. beta-Adrenergic and muscarinic receptors in developing rat parotid glands. Selective effect of neonatal sympathetic denervation. J Biol Chem. 1980 May 25;255(10):4619–4627. [PubMed] [Google Scholar]
- Mehansho H., Carlson D. M. Induction of protein and glycoprotein synthesis in rat submandibular glands by isoproterenol. J Biol Chem. 1983 May 25;258(10):6616–6620. [PubMed] [Google Scholar]
- Muenzer J., Bildstein C., Gleason M., Carlson D. M. Properties of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979 Jul 10;254(13):5629–5634. [PubMed] [Google Scholar]
- Muenzer J., Bildstein C., Gleason M., Carlson D. M. Purification of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979 Jul 10;254(13):5623–5628. [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Factors affecting the electrophoretic mobility of the major outer membrane proteins of Escherichia coli in polyacrylamide gels. Biochim Biophys Acta. 1979 Nov 23;581(1):163–178. doi: 10.1016/0005-2795(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Sreebny L. M. Proliferative behavior of differentiating cells in the developing rat parotid gland. J Cell Biol. 1970 Jul;46(1):81–87. doi: 10.1083/jcb.46.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEYER C. A. Salivary gland changes after isoproterenol-induced enlargement. Am J Physiol. 1962 Aug;203:232–236. doi: 10.1152/ajplegacy.1962.203.2.232. [DOI] [PubMed] [Google Scholar]
- SELYE H., CANTIN M., VEILLEUX R. Abnormal growth and sclerosis of the salivary glands induced by chronic treatment with isoproterenol. Growth. 1961 Sep;25:243–248. [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Hall H. D. Growth pattern of postnatally developing rat parotid gland. Proc Soc Exp Biol Med. 1969 Feb;130(2):603–607. doi: 10.3181/00379727-130-33617. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A. Retardation of secretory capacity of immature rat parotid gland by chronic administration of isoproterenol. Pediatr Res. 1978 Jun;12(6):726–728. doi: 10.1203/00006450-197806000-00010. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. The function of alpha- and beta-adrenergic receptors and a cholinergic receptor in the secretory cell of rat parotid gland. Adv Cytopharmacol. 1974;2:29–32. [PubMed] [Google Scholar]
- Sheetz J. H., Menaker L. Morphological and functional study of the effect of isoproterenol on salivary gland cells. Cell Tissue Res. 1979;203(2):321–329. doi: 10.1007/BF00237246. [DOI] [PubMed] [Google Scholar]
- Takuma T., Nakanishi M., Takagi Y., Tanemura T., Kumegawa M. Precocious differentiation of mouse parotid glands and pancreas induced by hormones. Biochim Biophys Acta. 1978 Jan 18;538(2):376–383. doi: 10.1016/0304-4165(78)90365-3. [DOI] [PubMed] [Google Scholar]