Abstract
Background
The need for an objective method for measuring skin hydration levels is becoming increasingly important. Various devices with different measuring principles for assessing skin hydration have been developed and are widely used.
Objective
This study aimed to investigate the reproducibility and correlation between clinical evaluation and skin hydration measurement devices that are the most widely used in the field.
Methods
A prospective comparative clinical trial was conducted on 184 healthy volunteers. Skin hydration levels were measured using the Corneometer (CM820) and hydration probe (HP: DermaLab Combo) at 3 points: the ventral forearm, the dorsal forearm, and the shin. We used the intraclass correlation coefficient (ICC) to evaluate the reproducibility and Pearson’s correlation coefficient (PCC) to evaluate the correlation of each measurement. Simple linear regression was used to analyze the Corneometer and HP skin hydration value changes according to changes in xerosis severity scale (XSS) values, which were evaluated by clinicians.
Results
Both the Corneometer and HP showed significant, excellent reproducibility (ICC for Corneometer: 0.954–0.971, ICC for HP: 0.980–0.986) and significant high positive correlations (PCC: 0.708–0.737) regardless of the measurement site. Both devices showed negative regression coefficients in all measurement sites in XSS analysis, but this was not statistically significant.
Conclusion
The Corneometer and HP were both accurate and objective skin hydration measuring devices, regardless of the measurement site. Using reliable and objective devices such as the Corneometer or HP can aid in understanding an individual’s skin condition and making more informed decisions for skin care.
Trial Registration
Clinical Research Information Service Identifier: KCT0005146
Keywords: Electric capacitance, Electric conductance, Equipment and supplies, Skin physiological phenomena
INTRODUCTION
The skin can be considered as the interface between the human body and the environment. The most important role of the skin is to act as a physical, biochemical, and immunological barrier. The outermost layer of the skin, the stratum corneum (SC), which has a brick-and-mortar structure, is essential for healthy skin. The water content of the SC is necessary for proper SC maturation and skin desquamation1. An increase in transepidermal water loss can result in dry and flaky skin, which prevents the skin from executing its full range of functions, and may also contribute to skin aging1,2.
Measuring skin hydration, as one of the indicators of skin condition, has been of interest for a considerable amount of time. Traditionally, physicians have utilized a visual examination-based scale, such as the xerosis severity scale (XSS)3. However, these scales are subjective and may not provide an accurate measurement of skin hydration levels. In recent years, various electrical devices have been developed that allow for more objective measurements of skin hydration. These include the Corneometer (Courage+Khazaka, Cologne, Germany), which measures skin capacitance, and the DermaLab Combo hydration probe (HP; Cortex Technology, Hadsund, Denmark), which uses the skin conductance for the evaluation4,5,6. This type of technology is relatively simple to use and yields quick and precise results.
While the Corneometer and HP are both known to be highly reproducible and reliable measurement methods, there are advantages and disadvantages associated with the properties of each device. Because the HP measures hydration using the conductance principle, any applied cosmetics may affect the current that directly passes through the skin4,5,7. In contrast, in the case of the Corneometer, the sensor and the skin are separated by glass. Thus, measurements are less affected by applied substances8,9. In addition, the Corneometer is known to be particularly sensitive to dry skin6,10. Thus, it is useful to compare these 2 widely used devices to address these disparities.
This research was designed to investigate the reproducibility and correlation of the Corneometer and HP, which use different principles, and compare the values with XSS scores, as observed by clinicians. We examined the skin hydration level at 3 points: the ventral forearm, the dorsal forearm, and the shin, to determine if there were any changes in measurements based on location and to determine the optimal measurement site.
MATERIALS AND METHODS
This research was a prospective clinical trial that was approved by the Institutional Review Board of Samsung Medical Center, Sungkyunkwan University School of Medicine in Seoul, Korea (2020-04-028). This study was registered in the Clinical Research Information Service on June 18, 2020 (http://cris.nih.go.kr, KCT0005146) and conducted according to the tenets of the Declaration of Helsinki.
XSS
We used the XSS as a tool to measure the severity of xerosis. This score is based on the physical appearance of the skin, such as the presence of cracks, fissures, or erythema. The XSS by Rogers et al.3 typically assigns a numerical score to the extent of dryness and scaling: normal (0), mild (1–2), moderate (3–4), and severe (5–6).
Devices
1) Corneometer CM820
The 7×7 mm sensor probe has a resonating system that consists of an interdigital grid of gold electrodes coated with a low-dielectric vitrified material8. This probe sends a small electric current (0.9–1.2 MHz) through the skin and detects the frequency change in the oscillating system related to the capacitance of the tissue11. The total capacitance is converted to arbitrary units (AUs) of skin hydration.
2) DermaLab Combo HP
This probe has a central circular electrode surrounded by 8 small pins to minimize moisture collection caused by occlusion of the skin area under the electrode4,12. The probe is equipped with a spring-loaded function that adjusts the applied pressure throughout the measurement4. A single frequency equal to 300 kHz was emitted by the probe, and the software calculated the average result (μS).
Study population
The participants in this study were registered as healthy volunteers who visited the Department of Dermatology, Samsung Medical Center, Seoul, Korea. Participants who had a diagnosis of eczema or hyperhidrosis or a history of any diseases requiring treatment at the evaluated sites were excluded. All of the included patients signed written informed consent for their participation.
Procedure
The research was conducted between October 2019 and May 2022, regardless of season. All measurements were conducted in a room where the temperature (20°C–22°C) and humidity (45%–55%) were regulated. The participants were instructed to rest for 30 minutes prior to the procedure. Two skilled specialists measured skin hydration using a Corneometer and HP to reduce the impact of operator factors, such as pressure.
Measurements were taken at 3 defined points: left ventral forearm and dorsal forearm 10 cm above the wrist and midpoint of the left shin. These flat skin areas can be commonly used to measure skin hydration levels in clinical settings or researches related with skin hydration. The measured site was gently cleaned with a non-woven tissue prior to all measurements to ensure an accurate measurement using 2 devices. Each measurement was repeated 3 times by the Corneometer and HP. A dermatologist evaluated the XSS prior to measuring the skin hydration level using one of the devices.
Statistical analysis
The data were analyzed by R 4.1.313. We used the intraclass correlation coefficient (ICC) to evaluate the reliability of measurements by the Corneometer and HP. ICC values range from 0 and 1. Higher ICC implies better reliability of the measurements. Measurements are not very reliable for ICC values between 0 and 0.5; moderately reliable for ICC values between 0.50 and 0.75; good for ICC values between 0.75 and 0.90, and excellent for ICC values greater than 0.914.
To evaluate the association between the correlation of Corneometer and HP in each part of the measurement sites, we used Pearson’s correlation coefficient (PCC) with a scatterplot15. PCC values range from −1 to 1. If the PCC value is between 0 and 0.3 (between −0.3 and 0), the association is negligible. For PCC values between 0.30 and 0.50 (−0.50 and −0.30), the positive (negative) association is low. For PCC values between 0.50 and 0.70 (−0.70 and −0.50), the positive (negative) association is moderate. For PCC values between 0.70 and 0.90 (−0.90 and −0.70), the positive (negative) association is high, and for PCC values greater than 0.9 (less than −0.9), the positive (negative) association is very high16.
To evaluate the association between the correlation between XSS and 2 devices, we use simple linear regression to analyze the skin hydration value changes in Corneometer and HP according to the changes in XSS values. The regression coefficients indicated decreases in the values measured by the Corneometer and HP as the XSS increased by one unit.
RESULTS
In total, we assessed 184 participants in this study (Table 1). Sixty-three participants (34.2%) were male, and 121 (65.8%) were female. The participants’ ages ranged from 7 to 88 years, with a mean age of 42.6 years. XSS was scored from 0 to 6 in 171 participants (0 [40, 23.4%], 1 [63, 36.8%], 2 [47, 27.5%], 3 [18, 10.5%], 4 [3, 1.8%], 5 [0], and 6 [0]). Of the participants, 183 were measured at the ventral forearm, 177 at the dorsal forearm, and 168 at the shin. Missing data occurred when a measurement was refused or when the measurer made an error.
Table 1. Demographics of the participants.
| Characteristics | No. of patients | |
|---|---|---|
| Total participants | 184 | |
| Sex | ||
| Male | 63 | |
| Female | 121 | |
| Male:Female | 1:1.9 | |
| Mean age (yr) | 42.6 (range: 7–88) | |
| XSS | ||
| Total | 171 | |
| 0 | 40 | |
| 1 | 63 | |
| 2 | 47 | |
| 3 | 18 | |
| 4 | 3 | |
| 5 | 0 | |
| 6 | 0 | |
| Measurement location | ||
| Ventral forearm | 183 | |
| Dorsal forearm | 177 | |
| Shin | 168 | |
XSS: xerosis severity scale.
Skin hydration values measured by the Corneometer and HP
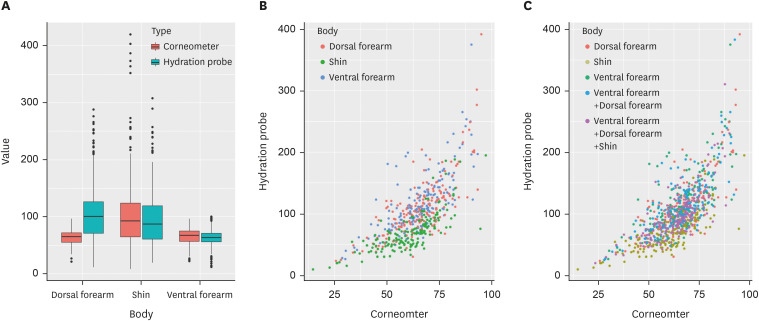
We calculated the average skin hydration values measured 3 times by each device at the ventral forearm, the dorsal forearm, and the shin. The skin hydration values of the ventral forearm measured by the Corneometer and HP ranged from 28.7 to 93.3 AU (mean 65.4, standard deviation [SD] 12.8) and from 26.7 to 374.7 μS (mean 116.7, SD 48.9), respectively. The skin hydration values of the dorsal forearm measured by the Corneometer and HP ranged from 25.7 to 95.0 AU (mean 65.9, SD 13.2) and from 23.7 to 391.7 μS (mean 111.2, SD 50.8), respectively. In the case of the shin, skin hydration values measured by the Corneometer and HP ranged from 14.7 to 97.3 AU (mean 60.4, SD 13.5) and from 10.0 to 194.7 μS (mean 70.2, SD 32.4), respectively. Fig. 1A displays a boxplot of skin hydration values at each site where measurements were taken using each device.
Fig. 1. Comparisons of the skin hydration values from the Corneometer and the hydration probe, and the relationship between the devices. (A) Boxplot of skin hydration values. (B) Scatter plots of correlations for forearm and shin. (C) Scatter plots of correlations for forearms and shin with entire arm and body.
Test-retest reliability
The ICC determined the test-retest reliability of the skin hydration measurements. This analysis evaluated each measurement at 3 sites as well as either ventral or dorsal forearm measurements and the whole measurement, including the forearms and shin area. The Corneometer and HP showed excellent agreement, which ranged from 0.954 to 0.971 and from 0.980 to 0.986, respectively (Table 2). The ICC calculation showed that the consistency between each measurement of the Corneometer and the HP was extremely high. The order of the ICC for the Corneometer was, in descending order from the nearest to 1: the ventral forearm, the entire arm (ICC=0.971), the dorsal forearm (ICC=0.970), the entire body (ICC=0.966) and the shin (ICC=0.954). The order of ICC for the HP was quite similar: the entire body (ICC=0.986), the dorsal forearm (ICC=0.984), the ventral forearm, the entire arm (ICC=0.983), and the shin (ICC=0.980). The ICC of the shin was the lowest for both devices.
Table 2. Test-retest reliability of the Corneometer and hydration probe.
| Measurement location | Device | ICC | 95% confidence interval |
|---|---|---|---|
| Ventral forearm | Corneometer | 0.971 | 0.963–0.978 |
| Hydration probe | 0.983 | 0.979–0.987 | |
| Dorsal forearm | Corneometer | 0.970 | 0.962–0.977 |
| Hydration probe | 0.984 | 0.980–0.988 | |
| Shin | Corneometer | 0.954 | 0.941–0.965 |
| Hydration probe | 0.980 | 0.974–0.984 | |
| Entire arm | Corneometer | 0.971 | 0.965–0.975 |
| Hydration probe | 0.984 | 0.981–0.986 | |
| Entire body | Corneometer | 0.966 | 0.961–0.971 |
| Hydration probe | 0.986 | 0.984–0.988 |
Entire arm means ventral and dorsal forearm, and entire body means all 3 sites measured.
ICC: intraclass correlation coefficient.
Correlation between Corneometer and HP
The PCC showed that there was a significant correlation between the Corneomter and the HP (Table 3). A high positive correlation was observed (PCC >0.7) at all measuring points. Fig. 1B and C show the scatter plot between the Corneometer and the HP for each measurement site.
Table 3. Correlation between the Corneometer and hydration probe with subgroup analysis of the XSS.
| Measurement location | PCC (95% confidence interval) | ||
|---|---|---|---|
| Overall | Group I (XSS=0) | Group II (XSS ≥1) | |
| Ventral forearm | 0.72 (0.64–0.78) | 0.65 (0.42–0.80) | 0.79 (0.64–0.80) |
| Dorsal forearm | 0.74 (0.66–0.80) | 0.74 (0.56–0.86) | 0.75 (0.66–0.81) |
| Shin | 0.75 (0.68–0.81) | 0.79 (0.63–0.88) | 0.74 (0.65–0.81) |
| Entire arm | 0.74 (0.67–0.80) | 0.71 (0.52–0.84) | 0.75 (0.66–0.82) |
| Entire body | 0.75 (0.67–0.81) | 0.74 (0.55–0.85) | 0.76 (0.67–0.82) |
Entire arm means ventral and dorsal forearm, and entire body means all 3 sites measured.
PCC: Pearson’s correlation coefficient, XSS: xerosis severity scale.
To analyze the correlation between the Corneometer and the HP in visually normal skin and visually dry skin, we divided XSS scores into 2 groups: Group I (XSS=0) and Group II (XSS ≥1) (Table 3). The correlation coefficients of all subgroups showed a high positive correlation except for the ventral forearm in Group I (PCC=0.65, moderate positive). Group I correlation values were lower than those of Group II, with the exception of the shin. In addition, the 95% confidence intervals for PCC indicated that the interval for Group I was wider than the interval for Group II, with the exception of measurements at the shin area.
Correlation between 2 devices and XSS scores
We compared the average skin hydration values measured by each device for each XSS group (Supplementary Tables 1 and 2). Only in the XSS=4 group did the Corneometer and HP show notably low average hydration levels.
Both devices showed negative regression coefficients in the ventral and the dorsal forearm, the shin, the entire arm, and the entire body. However, they were not statistically significant (p-value >0.05) (Table 4).
Table 4. Correlation of xerosis severity scale with the Corneometer and hydration probe by simple linear regression (entire arm means ventral and dorsal forearm, and entire body means all 3 sites measured).
| Measurement location | Device | Regression coefficient | p-value |
|---|---|---|---|
| Ventral forearm | Corneometer | −0.355 | 0.727 |
| Hydration_probe | −3.874 | 0.338 | |
| Dorsal forearm | Corneometer | −1.028 | 0.335 |
| Hydration probe | −5.073 | 0.223 | |
| Shin | Corneometer | −1.672 | 0.125 |
| Hydration probe | −4.980 | 0.064 | |
| Entire arm | Corneometer | −0.692 | 0.488 |
| Hydration probe | −4.474 | 0.259 | |
| Entire body | Corneometer | −0.838 | 0.384 |
| Hydration robe | −3.519 | 0.300 |
DISCUSSION
Skin hydration is an important aspect of skin health. Dry skin not only causes various skin problems, such as itching, flaking, cracking, premature aging, and barrier dysfunction but can also be related to other chronic diseases, including atopic dermatitis17,18. Using a skin hydration measuring device is the starting point for evaluating and maintaining proper skin hydration.
This study was conducted to investigate the reproducibility of 2 widely used skin hydration measurement devices, the Corneometer (capacitance) and the HP (conductance), and to examine the correlation between the 2 devices. There is no standard for which area to measure when using the Corneometer or HP, so the measuring site varied based on the clinician’s preference. Therefore, we also investigated whether there were differences in reproducibility and correlation depending on the measurement site.
The reproducibility of repeated measurements can strengthen the credibility of the application of these devices and indicate that these devices can be used for follow-up observations. The measurement of skin hydration using the Corneometer and HP showed excellent test-retest reliability, regardless of the measurement site. Thus, from the aspect of reliability, both devices have no restriction on the measurement site, and this can be determined according to the medical situation or the preference of the patient or clinician.
The relationship between these 2 devices was demonstrated to be a statistically significant, high positive correlation, regardless of the measurement site. This shows that despite the differences in measurement principles (capacitance and conductance), the measurement values of the 2 devices were very similar, whether measured on the arm or leg.
Comparison of the correlation between the 2 devices by dividing patients into subgroups according to XSS values (non-dry vs. dry skin: Group I: XSS=0, Group II: XSS ≥1) showed a moderate positive correlation in the ventral forearm in Group I, instead of the high positive correlation seen in other groups. Additionally, Group II (XSS ≥1) data show higher correlation values and a narrower confidence interval. These could have been expected as the Corneometer is known to be more accurate on dry skin, whereas HP is known to be similar regardless of dryness4,6,10.
We also compared the correlation between XSS scores measured visually by a clinician and the 2 devices. Both devices showed negative regression coefficients, indicating that the skin hydration value decreased as the XSS score increased by one unit, but it was not statistically significant. Skin measurements reflect the condition of the measured spot, while XSS scores are determined based on the whole skin condition. Therefore, the objective measurement of skin hydration supported by XSS scores by a subjective evaluation of the whole skin condition can be useful for better understanding and assessment, although XSS scores and skin measurements using the devices did not show statistically significant correlations in this study.
Several previous studies compared the accuracy of skin hydration measuring instruments (Table 5)8,10,12,19,20,21. In those studies, both the Corneometer and HP demonstrated high repeatability, as revealed in Fluhr et al.10 and Hua et al.21 These findings can strengthen the validity of this study. In addition, several devices utilizing different measuring principles were compared for correlation in previous studies, and the results were all statistically significant. However, no sub-analysis according to the measurement sites was performed except for the study by Westermann et al.20
Table 5. Representative research results of skin hydration measurement devices.
| Study | Measurement location | Device | Principle | Correlation coefficient |
|---|---|---|---|---|
| Fluhr et al. (1999)10 | Ventral forearm | Corneometer CM820 | Capacitance | CM820/CM825 SCC: 0.8901 |
| Dorsal forearm | Corneometer CM825 | Capacitance | CM820/Skicon 200 SCC: 0.8996 | |
| Dorsal hand | Skicon 200 | Conductance | CM820/Nova DPM SCC: 0.8165 | |
| Front | Nova DPM | Impedance | CM820/DermaLab SCC: 0.8760 | |
| Cheek | DermaLab | Conductance | CM825/Skicon 200 SCC: 0.8927 | |
| Back (C4) | CM825/Nova DPM SCC: 0.7928 | |||
| Leg | CM825/DermaLab SCC: 0.8964 | |||
| Skicon 200/Nova DPM SCC: 0.8719 | ||||
| Skicon 200/DermaLab SCC: 0.9358 | ||||
| Nova DPM/DermaLab SCC: 0.8876 | ||||
| Alanen et al. (2004)19 | Ventral forearm | Corneometer CM820 | Capacitance | CM820/SC-2 SCC: 0.75 (Ventral forearm) |
| MoistureMeter SC-2 | Capacitance | |||
| Clarys et al. (2012)8 | Sole of foot | Corneometer CM825 | Capacitance | CM825/Skicon 200 PCC: 0.97 |
| Calf | Skicon 200 | Conductance | ||
| Knee | ||||
| Upper leg | ||||
| Palm | ||||
| Dorsal hand | ||||
| Ventral forearm | ||||
| Dorsal forearm | ||||
| Upper arm | ||||
| Forehead | ||||
| Cheek | ||||
| Abdomen | ||||
| Hua et al. (2017)21 | Face | Corneometer CM825 | Capacitance | CM825/HP PCC: 0.808 |
| Forearm | HP | Conductance | ||
| Westermann et al. (2020)20 | Forearm | Corneometer CM825 | Capacitance | CM825/SkinUP PCC: 0.804 (Forearm) |
| Forehead | SkinUp | Impedance | CM825/SkinUP PCC: 0.730 (Forehead) | |
| Cheeks | CM825/SkinUP PCC: 0.696 (Cheeks) | |||
| Choi et al. (2021)12 | Ventral forearm | HP | Conductance | HP/Biodisplay PCC: 0.601 (Ventral forearm) |
| Biodisplay | Capacitance | |||
| Present study | Ventral forearm | Corneometer CM820 | Capacitance | CM820/HP PCC: 0.72 (Ventral forearm) |
| Dorsal forearm | HP | Conductance | CM820/HP PCC: 0.74 (Dorsal forearm) | |
| Shin | CM820/HP PCC: 0.75 (Shin) | |||
| CM820/HP PCC: 0.74 (Entire arm) | ||||
| CM820/HP PCC: 0.75 (Entire body) |
SCC: Spearman’s correlation coefficient, HP: hydration probe, PCC: Pearson’s correlation coefficient.
Although this study analyzed the skin measurement data based on normal and dry skin, the number of participants with normal skin determined by XSS scores of 0 was rather small (n=40) compared to those with dry skin (XSS ≥1), which was a limitation of this study. Additional studies could be performed to compare the Corneometer and the HP by modifying the measurement conditions. Not only could the measurement values be compared on dry skin but also on more water-rich skin, regardless of whether the skin is healthy or diseased. Measurement of skin hydration should follow European group on Efficacy Measurements of COsmetcis and other topical products (EEMCO) guideline22, which says that application of any topical products in the intended measuring area should be avoided 12 hours prior to participation measurement in the barrier study. The volunteers enrolled in this study were recruited from the outpatient clinic and therefore, we could not restrict prior application of moisturizer, which is another limitation of this study. While it is true that not applying any cosmetics or products within 12 hours for hydration measurement could lead to more precise results for the study to evaluate the efficacy of topical agents, especially moisturizers, this study was not designed to evaluate the efficacy of topical agents, but to compare the devices which are commonly used for the skin hydration. And we followed EEMCO guideline of temperature, hydration and acclimatization period for the exact measurement. In addition, to minimize any possible effect or hindrance of remnant moisturizers volunteers previously used, the measured site was gently cleaned with non-woven tissue prior to all measurements.
In this study, we found that the Corneometer and the HP were reliable and reproducible regardless of the measurement site, consistent with previous studies. However, to make better assessments, visual scales such as the XSS can play a role, supporting the objective assessment of skin hydration levels using devices.
Footnotes
FUNDING SOURCE: None.
CONFLICTS OF INTEREST: The authors have nothing to disclose.
DATA SHARING STATEMENT: The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Average skin hydration values measured by the Corneometer for each xerosis severity scale group
Average skin hydration values measured by the hydration probe for each xerosis severity scale group
References
- 1.Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6:75–82. doi: 10.1111/j.1473-2165.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm KP, Cua AB, Maibach HI. Skin aging. Effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch Dermatol. 1991;127:1806–1809. doi: 10.1001/archderm.127.12.1806. [DOI] [PubMed] [Google Scholar]
- 3.Rogers RS, 3rd, Callen J, Wehr R, Krochmal L. Comparative efficacy of 12% ammonium lactate lotion and 5% lactic acid lotion in the treatment of moderate to severe xerosis. J Am Acad Dermatol. 1989;21:714–716. doi: 10.1016/s0190-9622(89)70242-5. [DOI] [PubMed] [Google Scholar]
- 4.Morin M, Ruzgas T, Svedenhag P, Anderson CD, Ollmar S, Engblom J, et al. Skin hydration dynamics investigated by electrical impedance techniques in vivo and in vitro. Sci Rep. 2020;10:17218. doi: 10.1038/s41598-020-73684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagami H. Electrical measurement of the hydration state of the skin surface in vivo. Br J Dermatol. 2014;171(Suppl 3):29–33. doi: 10.1111/bjd.13245. [DOI] [PubMed] [Google Scholar]
- 6.Van Neste D. Comparative study of normal and rough human skin hydration in vivo: evaluation with four different instruments. J Dermatol Sci. 1991;2:119–124. doi: 10.1016/0923-1811(91)90021-o. [DOI] [PubMed] [Google Scholar]
- 7.Hadi H, Awadh AI, Hanif NM, Md Sidik NF, Mohd Rani MR, Suhaimi MS. The investigation of the skin biophysical measurements focusing on daily activities, skin care habits, and gender differences. Skin Res Technol. 2016;22:247–254. doi: 10.1111/srt.12257. [DOI] [PubMed] [Google Scholar]
- 8.Clarys P, Clijsen R, Taeymans J, Barel AO. Hydration measurements of the stratum corneum: comparison between the capacitance method (digital version of the Corneometer CM 825®) and the impedance method (Skicon-200EX®) Skin Res Technol. 2012;18:316–323. doi: 10.1111/j.1600-0846.2011.00573.x. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich U, Koop U, Leneveu-Duchemin MC, Osterrieder K, Bielfeldt S, Chkarnat C, et al. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825) Int J Cosmet Sci. 2003;25:45–53. doi: 10.1046/j.1467-2494.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Fluhr JW, Gloor M, Lazzerini S, Kleesz P, Grieshaber R, Berardesca E. Comparative study of five instruments measuring stratum corneum hydration (Corneometer CM 820 and CM 825, Skicon 200, Nova DPM 9003, DermaLab). Part II. In vivo. Skin Res Technol. 1999;5:171–178. [Google Scholar]
- 11.Hashmi F, Wright C, Nester C, Lam S. The reliability of non-invasive biophysical outcome measures for evaluating normal and hyperkeratotic foot skin. J Foot Ankle Res. 2015;8:28. doi: 10.1186/s13047-015-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi Y, Oh SJ, Lee JH. Novel technology at hand to measure skin hydration by Biodisplay smartphone touch screen panel. Sci Rep. 2021;11:19410. doi: 10.1038/s41598-021-98784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team. R: a language and environment for statistical computing [Internet] 2013. [cited 2023 March 1]. Available from: https://www.r-project.org/
- 14.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsitz SR, Leong T, Ibrahim J, Lipshultz S. A partial correlation coefficient and coefficient of determination for multivariate normal repeated measures data. Statistician. 2001;50:87–95. [Google Scholar]
- 16.Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5th ed. Boston: Houghton Mifflin College Division; 2003. [Google Scholar]
- 17.Sator PG, Schmidt JB, Hönigsmann H. Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with atopic dermatitis. J Am Acad Dermatol. 2003;48:352–358. doi: 10.1067/mjd.2003.105. [DOI] [PubMed] [Google Scholar]
- 18.Shim JH, Park JH, Lee JH, Lee DY, Lee JH, Yang JM. Moisturizers are effective in the treatment of xerosis irrespectively from their particular formulation: results from a prospective, randomized, double-blind controlled trial. J Eur Acad Dermatol Venereol. 2016;30:276–281. doi: 10.1111/jdv.13472. [DOI] [PubMed] [Google Scholar]
- 19.Alanen E, Nuutinen J, Nicklén K, Lahtinen T, Mönkkönen J. Measurement of hydration in the stratum corneum with the MoistureMeter and comparison with the Corneometer. Skin Res Technol. 2004;10:32–37. doi: 10.1111/j.1600-0846.2004.00050.x. [DOI] [PubMed] [Google Scholar]
- 20.Westermann TV, Viana VR, Berto Junior C, Detoni da Silva CB, Carvalho EL, Pupe CG. Measurement of skin hydration with a portable device (SkinUp® Beauty Device) and comparison with the Corneometer®. Skin Res Technol. 2020;26:571–576. doi: 10.1111/srt.12833. [DOI] [PubMed] [Google Scholar]
- 21.Hua W, Fan LM, Dai R, Luan M, Xie H, Li AQ, et al. Comparison of two series of non-invasive instruments used for the skin physiological properties measurements: the DermaLab® from Cortex Technology vs. the series of detectors from Courage & Khazaka. Skin Res Technol. 2017;23:70–78. doi: 10.1111/srt.12303. [DOI] [PubMed] [Google Scholar]
- 22.Berardesca E, Loden M, Serup J, Masson P, Rodrigues LM. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res Technol. 2018;24:351–358. doi: 10.1111/srt.12599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Average skin hydration values measured by the Corneometer for each xerosis severity scale group
Average skin hydration values measured by the hydration probe for each xerosis severity scale group