Abstract
Background
This retrospective analysis of prospectively collected abdominal aortic aneurysm (AAA) screening data aimed to identify predictors of AAA-related events (surgery or death) with a view to better targeting of screening.
Methods
For the interval 1984–2007, data for 1649 subjects with an AAA were collected prospectively as part of the Chichester AAA screening programme. This included serial aortic size measurements, blood pressure, risk factors for arterial disease and concurrent medications. AAA growth rates were adjusted for risk factor confounders using flexible hierarchical modelling. AAA growth distribution was analysed using Silverman's test of multimodality.
Results
Some 1231 subjects met the inclusion criteria of having more than one scan and a surveillance interval of over 3 months. AAA growth showed a bimodal pattern with nearly 50 per cent of all aneurysms never progressing to surgery or rupture. Adjusted annual AAA growth rates of at least 2 mm significantly predicted AAA-related events.
Conclusion
This analysis identified a bimodal growth pattern for AAA, with a significant association between annual AAA growth rate of at least 2 mm and AAA-related events.
Growth is not linear
Introduction
Abdominal aortic aneurysm (AAA) is a common life-threatening condition predominantly affecting older men. It is generally assumed that the natural history of an AAA is expansion and possible rupture. The optimal management of small AAA (less than 5·5 cm) was clearly defined as surveillance, by the results of the UK Small Aneurysm Trial (UKSAT)1 and the US Veterans Affairs Cooperative Study Group2. Early detection through screening programmes and an increase in medical imaging have led to a massive expansion in AAA surveillance.
The vascular unit in Chichester, UK, was one of the first to implement a screening programme, in 1983. Earlier analysis of AAA size changes over time for the Chichester subjects revealed large variation in aneurysm growth rates, demonstrating that aneurysm expansion does not conform to the simple mechanics of Laplace's law3. This has been confirmed by the surveillance of small AAA in the UKSAT, demonstrating growth rates ranging from −1·0 to 6·1 mm/year (95 per cent range)4. However, it is not yet clear whether this variation in growth rates for smaller AAA reflects a normal distribution or the influence of a spread of pathology with potentially different outcomes.
Data on risk factors for aneurysm expansion are reliant on accurate repeat measurements such as those found in well established screening programmes. Given that the definition of aneurysm formation is arbitrarily based on size (3 cm or greater), unreflective of pathophysiology, it is possible that some of the risk factors described below are associated with AAA expansion and not aneurysm formation.
Several studies have identified factors that influence AAA growth. This department has previously employed a logarithmic model to calculate growth rates, acknowledging this as a ‘best fit’ rather than biological model. This demonstrated that small AAA growth is proportional to diameter, although there was considerable individual variability3. The UKSAT data were analysed using a hierarchical model, identifying associations between peripheral arterial disease and diabetes with slowed AAA growth, and smoking with faster AAA growth4. These findings have also been reported in other data sets; however, associations with hypercholesterolaemia, sex and hypertension are less clear5–11. Several biomarkers have also been associated with AAA expansion, the strongest being serum elastin peptides12. More recently, associations have been made between decreased AAA growth, statin use13,14 and angiotensin-converting enzyme (ACE) inhibitor therapy15, but this has yet to be confirmed in adequately sized prospective trials.
The association between AAA growth and outcomes is less well documented. Utilizing the UKSAT expansion data, Brown and colleagues16 demonstrated an association between increased AAA growth and rupture, using a linear model16. Santilli and co-workers5 were unable to demonstrate any differences in expansion and non-AAA-related mortality in the US Veterans Affairs Cooperative Study Group data5.
The present paper examined the pattern of AAA growth, as well as the relationship between AAA growth and risk factors (mean arterial pressure (MAP), history of hypertension, hypercholesterolaemia, diabetes, smoking, ischaemic heart disease (IHD), sex) and AAA-related events (surgery or death).
Methods
All subjects with an AAA, identified through the Chichester screening programme between January 1984 and January 2007, were considered for this analysis. Subjects with an AAA of 5·5 cm or smaller were entered into the surveillance arm of the screening programme, with the intention to treat once they fulfilled the criteria for intervention. The protocols for this programme have been described previously17. Serial AAA measurements, together with demographic data, were entered prospectively into a comprehensive screening database. Subjects with no more than one ultrasonographic measurement or with follow-up of less than 3 months were excluded. As part of their screening and surveillance visits, subjects were asked to fill out a questionnaire concerning current and previous illnesses and treatment, current medications, smoking habits and family history of AAA. Three separate automated blood pressure readings were then taken and recorded. Ultrasonographic measurements of the infrarenal aorta (anteroposterior and transverse) were then taken, and the images saved for future reference.
Patient demographics and outcomes
Cardiovascular risk factors for each subject, including MAP, history of hypertension, hypercholesterolaemia, diabetes, smoking, IHD and sex, were recorded. Outcomes and mortality data (including AAA rupture, elective AAA repair, continued surveillance, in-hospital AAA-related mortality, community AAA-related mortality and non-AAA-related mortality) were obtained for each subject in the study. Surgical outcomes were collected from hospital records and mortality data from death certificates.
Statistical analysis
Median AAA growth rates were calculated using flexible hierarchical modelling as described by Brady and colleagues4. Quadratic growth models were fitted using Markov chain Monte Carlo methods as implemented in MLwiN software18. Non-informative priors were used. Intercept, slope and curvature terms were assumed to follow a multivariable normal distribution and a normal distribution was assumed for residuals. In this model, groups of subjects were compared by combining all the follow-up points for each group (anteroposterior AAA diameter and time scale), adjusting for a common initial AAA diameter of 35 mm and quantifying the difference in median growth rates between the comparison groups. By adjusting to a common initial diameter, a direct comparison of growth rates from groups of AAA of differing size was possible. (More details of this model can be found at http://www.mrcbsu.cam.ac.uk/BSUsite/Publications/Preprints/grow1_p3.pdf.) Demographic confounders (MAP, history of hypertension, hypercholesterolaemia, diabetes, smoking, IHD and sex) were included as fixed-effect co-variables in these models. The cross-level interactions of each risk factor and outcome variable over time were tested in order to compare growth rates for these groups. Observations censored owing to surgery were considered missing at random. Kernal density plots were used to display distributions of AAA diameter. To allow for variable surveillance intervals, the distributions of final diameters were extrapolated to a fixed time interval (using initial diameters and calculated growth rates for each subject). The Silverman test was used to demonstrate multimodality19,20. All analysis was performed using Stata® software (StataCorp LP, College Station, Texas, USA).
Results
Of 1649 subjects with an AAA in the Chichester database, 418 did not meet the inclusion criteria and were excluded, leaving a total of 1231 subjects with an AAA (Fig. 1). Of the 418 excluded subjects, 282 had undergone only one ultrasonographic scan (making an estimate of growth impossible), 53 had borderline initial measurements and were subsequently found to have an aortic diameter of less than 30 mm, and 83 had not been subject to surveillance for long enough to estimate growth (less than 3 months). Of the 1231 subjects included in this study, 158 were lost to follow-up and outcomes could not be accounted for in 14.
Fig. 1.
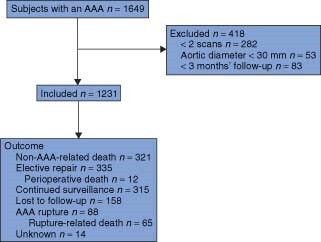
Description of subjects with an abdominal aortic aneurysm (AAA) in the present study
Outcomes and mortality
The overall median AAA diameter at baseline was 35 (interquartile range (i.q.r.) 31–42) mm. AAA of all subjects grew by a median of 9 mm over 3·2 years (linear growth rate 2·81 mm/year, adjusted growth rate 1·43 (95 per cent confidence interval (c.i.) −2·26 to 6·06) mm/year). Subjects who had an AAA-related event (elective surgery or rupture) had growth rates of 2·99 (2·80 to 3·18) and 2·85 (2·59 to 3·11) mm/year respectively, whereas other subjects under surveillance had a growth rate of 1·08 (0·89 to 1·27) mm/year (P < 0·001). There was no significant difference between growth rates for subjects who died from non-AAA-related causes during surveillance (1·01 (95 per cent c.i. 0·82 to 1·20) mm/year) and those who continued under surveillance. Subjects who were lost to follow-up had a significantly slower AAA growth rate (−0·06 (−0·27 to 0·15) mm/year). The 77 subjects who had an AAA-related death had a significantly faster AAA growth than subjects who died from non-AAA-related causes during surveillance (2·81 (2·48 to 3·14) versus 1·01 (0·82 to 1·20) mm/year; P < 0·001). The AAA size prompting referral to a vascular surgeon changed during the study from 6 cm (up until 2002) to 5·5 cm, in accordance with the UKSAT. The median final diameter recorded at the last surveillance visit in subjects who had a ruptured AAA was 5·5 cm.
There were significant differences in the length of follow-up between patients according to outcome. Follow-up was longer for dropouts (median 5·03 (i.q.r. 1·99–8·95) years) than for non-AAA-related deaths (3·63 (2·00–6·38) years; P = 0·005), elective repairs (3·16 (1·42–5·80) years; P < 0·001) and continued surveillance (3·09 (1·97–6·05) years; P = 0·007). In addition, non-AAA-related deaths had longer surveillance than elective repairs (P = 0·024). No other groups differed significantly.
Risk factors
The distribution of age and sex within the study group reflected both the variety of screening strategies undertaken during the early period of screening research, and a later more established screening protocol for men. Table 1 details the range of subject characteristics by tertiles of final AAA diameter.
Table 1.
Subject characteristics by tertiles of final abdominal aortic aneurysm diameter
| Overall (n = 1231) | Tertiles based on final AAA diameter | ||||
|---|---|---|---|---|---|
| Tertile 1 (n = 437) | Tertile 2 (n = 397) | Tertile 3 (n = 397) | P ‡ | ||
| Initial AAA diameter mm) | 35 (31–42) | 32 (31–34) | 37 (33–42) | 44 (37–50) | < 0·001§ |
| Final AAA diameter (mm) | 44 (35–57) | 32 (29–35) | 45 (42–50) | 60 (57–63) | |
| Follow-up (years) | 3·2 (1·7–6·0) | 3·1 (1·1–5·3) | 3·4 (2·0–6·0) | 3·4 (1·7–6·1) | 0·112§ |
| Age (years) | 67 (65–71) | 66 (65–71) | 67 (65–71) | 68 (65–71) | 0·456§ |
| Women* | 73 (5·9) | 37 (8·5) | 20 (5·0) | 16 (4·0) | 0·017 |
| IHD* | 286 (23·2) | 78 (17·8) | 97 (24·4) | 111 (28·0) | 0·028 |
| Hypertension* | 696 (56·5) | 231 (52·9) | 233 (58·7) | 232 (58·4) | 0·154 |
| Diabetes* | 79 (6·4) | 29 (6·6) | 34 (8·6) | 16 (4·0) | 0·033 |
| Statin use* | 383 (31·1) | 119 (27·2) | 155 (39·0) | 109 (27·5) | < 0·001 |
| ACE inhibitor use* | 294 (23·9) | 96 (22·0) | 104 (26·2) | 94 (23·7) | 0·357 |
| Current smoker* | 390 (31·7) | 129 (29·5) | 123 (31·0) | 138 (34·8) | 0·259 |
| Growth rate† | 1·43 (0·09) | −0·46 (0·10) | 1·62 (0·10) | 3·21 (0·11) | < 0·001¶ |
| (−2·26 to 6·06) | (−3·67 to 2·75) | (−1·59 to 4·83) | (0 to 6·42) | ||
Values are median (interquartile range) unless indicated otherwise;
values in parentheses are percentages.
Growth rates adjusted for a common initial diameter for tertiles (35 mm); values in parentheses are s.e. and 95 per cent reference range. AAA, abdominal aortic aneurysm; IHD, ischaemic heart disease; ACE, angiotensin-converting enzyme.
χ2 test unless indicated otherwise;
Kruskal–Wallis test;
from hierarchical model.
Current smoking was associated with a 24 per cent increased growth rate 0·56, 95 per cent c.i. 0·29 to 0·83 mm/year (P < 0·001). Female sex was associated with a 42 per cent decreased AAA growth rate −0·84, −1·37 to −0·31 mm/year (P = 0·002). Diabetes was associated with a 56 per cent decreased AAA growth rate −0·95, −1·66 to −0·25 mm/year (P = 0·008). Age (P = 0·230), MAP (P = 0·782), a history of hypertension (P = 0·083) or IHD (P = 0·471) did not affect AAA growth rates in this study. ACE inhibitors and statin use were not associated with differences in growth rates: −0·28 (−0·67 to 0·12) mm/year (P = 0·170) and −0·29 (−0·66 to 0·08) mm/year (P = 0·122) respectively.
Nature of aneurysm expansion
Adjusted AAA growth rates followed a normal distribution (Fig. 2a). The distribution of initial AAA diameters was skewed to the left (Fig. 2b). Yet, there was evidence that the number of modes for the end-diameter distribution was greater than one (P < 0·001) and not more than two (P = 0·430) (Fig. 3c), that was a bimodal pattern. Fig. 3 demonstrates the distribution of initial and estimated final diameters for the entire group and each outcome group after 3·5, 5 and 10 years of surveillance. The mean growth rate least well described the dropouts and the elective repair group.
Fig. 2.
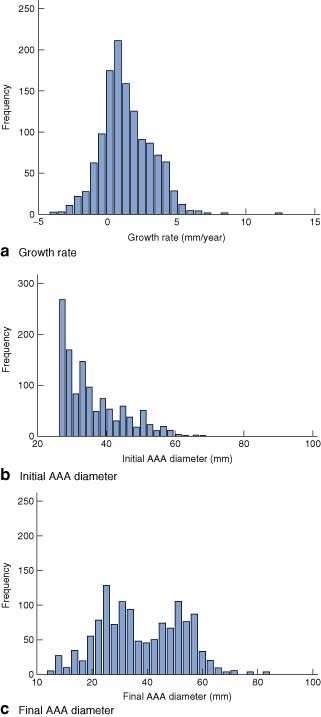
Distribution of a abdominal aortic aneurysm (AAA) growth rates, b initial surveillance diameters and c final surveillance diameters
Fig. 3.
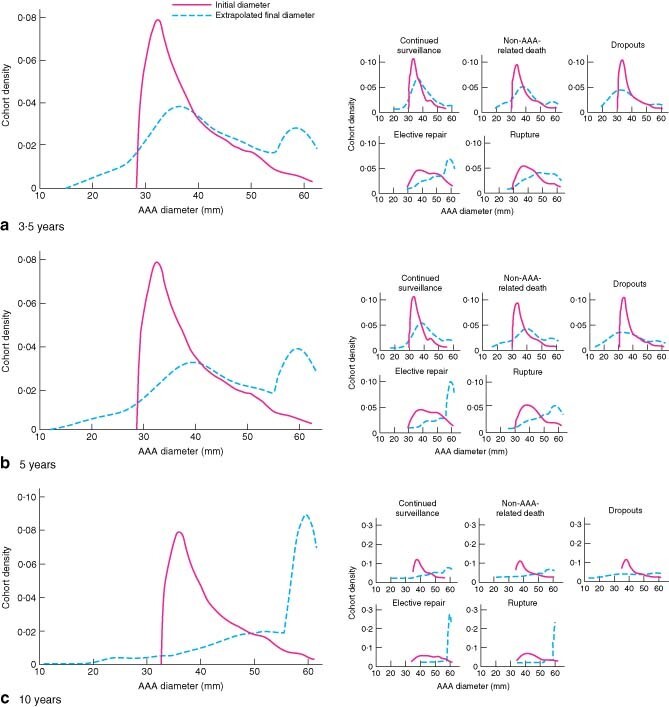
Distribution of initial abdominal aortic aneurysm (AAA) diameters and final diameters, extrapolated from individual AAA growth rates, at a 3·5 years, b 5 years and c 10 years. Data are shown for the entire cohort and for subjects grouped according to outcome
Discussion
This analysis demonstrated that, for subjects with an AAA under surveillance with intention to treat, an adjusted annual growth rate of at least 2 mm was significantly associated with clinical events. Aneurysms with an adjusted annual growth rate of less than 1·5 mm appeared to be of little clinical relevance. These data were based on 25 years of AAA surveillance in a single centre, with a similar number of AAA surveillance years as undertaken in the Multicentre Aneurysm Screening Study (MASS) (4247 versus 4448·5 years respectively)21.
AAA remains one of the main causes of death in the Western world. Although the MASS trial demonstrated that screening for AAA in men aged 65 years and over reduced AAA-related mortality by 54 per cent, most of those identified with an AAA will suffer no consequence from the condition. Only 32·6 per cent in the MASS cohort and 27·2 per cent of the present cohort ended up requiring AAA repair during follow-up. These figures suggest that considerable potential exists to improve the cost-effectiveness of AAA screening and surveillance.
In the present study it was noted that subjects who had an AAA-related event (surgery or rupture) had significantly increased adjusted growth rates. Although this may seem intuitive, it does suggest that adjusted AAA growth rates could be used to predict the natural history of individual aneurysms. This has the potential to allow the redistribution of screening resources to target higher-risk AAA.
In the present cohort, a natural selection process took place whereby subjects with slow AAA growth regularly dropped out of surveillance. These subjects were not actively sought by the programme organizers with a view to discharge. They tended to cease attending owing to advancing age or ailing health after many years of surveillance (median 5·03 years).
Of particular importance in this study was the 7·1 per cent of subjects whose aneurysm ruptured while under surveillance, and for whom simple diameter measurement failed to predict this event. The existence of this group raises the question as to whether AAA growth rates, or a combination of diameter measurement and AAA growth rates, could be superior to diameter alone for predicting AAA-related events. Interestingly, subjects who died from a non-AAA-related condition had a similar aneurysm growth rate to subjects currently under surveillance. However, there was an expectation to observe faster AAA growth rates, in keeping with a higher rupture risk, in this high morbidity subgroup, as reported by the Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators22. The small difference in follow-up duration between these groups (3·63 versus 3·09 years) is unlikely to have influenced this observation.
Studying the effect of risk factors, it was observed that smoking was associated with an increased AAA growth rate. Similar observations were not true for serial arterial pressure measurements or a history of hypertension, as reported previously4. Nor did ACE inhibitor or statin use influence overall growth rate, as suggested in other cohorts13–15. Unexpectedly, female sex was associated with a slower adjusted AAA growth, in contrast with other studies10,11. This may reflect the small numbers of women within this cohort, as well as a bias towards having smaller AAA (Table 1). Diabetes seemed to have the greatest effect on AAA growth, proving to be protective, as reported in other studies4,5,23.
In an attempt to understand AAA growth patterns further, cohorts were divided by tertiles of final diameter; subjects in the third tertile were at highest risk of having an AAA-related event median final size 60 (i.q.r. 57–63) mm. This analysis demonstrated a different clinical relevance to the above risk factors. For example, although smoking was associated with an accelerated overall growth rate, it did not appear to predict those AAA requiring repair in the third tertile (P = 0·536). This apparent contradiction may be explained by the increased co-morbidity of smoking resulting in relatively early non-AAA-related deaths in subjects with small aneurysms. It was also observed that, instead of AAA (grouped by tertiles of final diameter) demonstrating follow-up times relative to the diameter, as might be expected, there was no difference in median follow-up between the tertiles (P = 0·112). This provided evidence that the fate of any particular aneurysm is determined early in its life.
From Fig. 3 it was apparent that, although initial AAA diameter follows a unimodal distribution, this has changed into a bimodal distribution by the end of follow-up. Although this observation can be attributed partly to the truncation of further AAA expansion by clinical events, it demonstrated for all practical purposes a bimodal pattern of AAA expansion. This suggests that for any given AAA the processes of aneurysm initiation and aneurysm progression may be distinct, as only half of all AAA have the required set of conditions for both processes. Indeed, within the distribution of adjusted AAA growth rates (Fig. 2a), there was considerable scope for static or negative growth, raising the question of whether these subjects do indeed have AAA disease in its broader clinical sense.
These observations question the commonly held notion that all small aneurysms increase in size over time, albeit at different rates of growth. Instead, they suggest that half of small AAA remain quiescent with little growth, whereas the other half continue to expand and develop the potential for undergoing surgery or rupture. These findings offer a considerable scope for combining AAA growth and risk factors for AAA progression in a formula that improves the efficiency of AAA surveillance within large-scale screening programmes.
To illustrate this further and without the influence of variable surveillance intervals, the data have been extrapolated to three further fixed points in time (Fig. 3). At 5 years the distribution of AAA is still dominated by the left-hand side (non-treatment) peak, with only 29·3 per cent reaching 5·5 cm. It is not until after a hypothetical 10 years of surveillance that a small majority (58·8 per cent) of AAA reach 5·5 cm.
Twenty-five years of surveillance experience in the Chichester cohort have demonstrated that it is unrealistic to expect a median surveillance time of more than 3·5 years for any particular aneurysm requiring intervention. This makes the current surveillance protocol an inefficient and costly way of identifying subjects for AAA intervention.
Extrapolation of AAA growth rates within subgroups of outcome (Fig. 3) revealed the importance of initial diameter in identifying patients who go on to sustain an AAA-related event. Within the subjects under current surveillance there is confirmation of the long surveillance interval needed to detect an AAA requiring treatment. With hindsight, initial diameters and growth rates within the dropout group make intervention at any point unlikely. Identifying this group early could improve the efficiency of surveillance.
Acknowledgements
The authors acknowledge the pioneering work of Mr Alan Scott in the field of AAA screening in general and in establishing the Chichester AAA screening programme in particular. They thank Mrs Stephanie Druce for her help in the day-to-day running of the aneurysm screening centre, Dr Michael Fabricius for compiling data for the study, and Professor Steve Humphries for his support in facilitating the data analysis.
British Heart Foundation funding contributed to this analysis (PG 2005/014). Additional funding for this work and for the collected data came from Western Sussex NHS Trust (previously Royal West Sussex NHS Trust) and West Sussex Primary Care Trust. The authors declare no conflict of interest.
Contributor Information
A R Thompson, Department of Vascular Surgery, Western Sussex Hospital NHS Trust, Chichester, UK; Centre for Cardiovascular Genetics, British Heart Foundation Laboratories, University College London, London, UK.
J A Cooper, Centre for Cardiovascular Genetics, British Heart Foundation Laboratories, University College London, London, UK.
Mr H A Ashton, Department of Vascular Surgery, Western Sussex Hospital NHS Trust, Chichester, UK.
H Hafez, Department of Vascular Surgery, Western Sussex Hospital NHS Trust, Chichester, UK.
References
- 1. UK Small Aneurysm Trial Participants . Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352: 1649–1655. [PubMed] [Google Scholar]
- 2. Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW et al. ; Aneurysm Detection and Management Veterans Affairs Cooperative Study Group . Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002; 346: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 3. Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, Quick CR et al. Growth rates and risk of rupture of abdominal aortic aneurysms. Br J Surg 1998; 85: 1674–1680. [DOI] [PubMed] [Google Scholar]
- 4. Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT; UK Small Aneurysm Trial Participants . Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004; 110: 16–21. [DOI] [PubMed] [Google Scholar]
- 5. Santilli SM, Littooy FN, Cambria RA, Rapp JH, Tretinyak AS, d'Audiffret AC et al. Expansion rates and outcomes for the 3·0-cm to the 3·9-cm infrarenal abdominal aortic aneurysm. J Vasc Surg 2002; 35: 666–671. [DOI] [PubMed] [Google Scholar]
- 6. MacSweeney ST, Ellis M, Worrell PC, Greenhalgh RM, Powell JT. Smoking and growth rate of small abdominal aortic aneurysms. Lancet 1994; 344: 651–652. [DOI] [PubMed] [Google Scholar]
- 7. Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg 2000; 87: 195–200. [DOI] [PubMed] [Google Scholar]
- 8. Englund R, Hudson P, Hanel K, Stanton A. Expansion rates of small abdominal aortic aneurysms. Aust N Z J Surg 1998; 68: 21–24. [DOI] [PubMed] [Google Scholar]
- 9. Lindholt JS, Heegaard NH, Vammen S, Fasting H, Henneberg EW, Heickendorff L. Smoking, but not lipids, lipoprotein(a) and antibodies against oxidised LDL, is correlated to the expansion of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2001; 21: 51–56. [DOI] [PubMed] [Google Scholar]
- 10. Mofidi R, Goldie VJ, Kelman J, Dawson AR, Murie JA, Chalmers RT. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg 2007; 94: 310–314. [DOI] [PubMed] [Google Scholar]
- 11. Solberg S, Singh K, Wilsgaard T, Jacobsen BK. Increased growth rate of abdominal aortic aneurysms in women. The Tromso Study. Eur J Vasc Endovasc Surg 2005; 29: 145–149. [DOI] [PubMed] [Google Scholar]
- 12. Urbonavicius S, Urbonaviciene G, Honoré B, Henneberg EW, Vorum H, Lindholt JS et al. Potential circulating biomarkers for abdominal aortic aneurysm expansion and rupture—a systematic review. Eur J Vasc Endovasc Surg 2008; 36: 273–280. [DOI] [PubMed] [Google Scholar]
- 13. Schouten O, van Laanen JH, Boersma E, Vidakovic R, Feringa HH, Dunkelgrün M et al. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg 2006; 32: 21–26. [DOI] [PubMed] [Google Scholar]
- 14. Sukhija R, Aronow WS, Sandhu R, Kakar P, Babu S. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. Am J Cardiol 2006; 97: 279–280. [DOI] [PubMed] [Google Scholar]
- 15. Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case–control study. Lancet 2006; 368: 659–665. [DOI] [PubMed] [Google Scholar]
- 16. Brown PM, Zelt DT, Sobolev B. The risk of rupture in untreated aneurysms: the impact of size, gender, and expansion rate. J Vasc Surg 2003; 37: 280–284. [DOI] [PubMed] [Google Scholar]
- 17. Hafez H, Druce PS, Ashton HA. Abdominal aortic aneurysm development in men following a ‘normal’ aortic ultrasound Scan. Eur J Vasu Endovasc Surg 2008; 36: 553–558. [DOI] [PubMed] [Google Scholar]
- 18. Browne WJ. MCMC Estimation in MLwiN, v2·10. Centre for Multilevel Modelling, University of Bristol: Bristol, 2009. [Google Scholar]
- 19. Silverman BW. Using Kernal density estimates to investigate multilodality. J R Stat Soc Series B 1981; 43: 97–99. [Google Scholar]
- 20. Izenman AJ, Sommer C. Philatelic mixtures and multimodal densities. J Am Stat Assoc 1988; 83: 941–953. [Google Scholar]
- 21. Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA et al. ; Multicentre Aneurysm Screening Study Group . The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 22. Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD Jr, Blebea J et al. ; Veterans Affairs Cooperative Study #417 Investigators . Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002; 287: 2968–2972. [DOI] [PubMed] [Google Scholar]
- 23. Vega de Céniga M, Gómez R, Estallo L, Rodríguez L, Baquer M, Barba A. Growth rate and associated factors in small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2006; 31: 231–236. [DOI] [PubMed] [Google Scholar]


