Abstract
OX40 is a member of the tumor necrosis factor (TNF) receptor superfamily and known to be an important costimulatory molecule expressed on activated T cells. To investigate the role of costimulation of OX40 in human immunodeficiency virus type 1 (HIV-1) infection by its natural ligand, gp34, the OX40-transfected ACH-2 cell line, ACH-2/OX40, chronically infected with HIV-1, was cocultured with paraformaldehyde (PFA)-fixed gp34-transfected mouse cell line, SV-T2/gp34. The results showed that HIV-1 production was strongly induced. This was followed by apparent apoptosis, and both processes were specifically inhibited by the gp34-specific neutralizing monoclonal antibody 5A8. Endogenous TNF alpha (TNF-α) and TNF-β production were not involved in the enhanced HIV-1 production. Furthermore, enhanced HIV-1 transcription in gp34-stimulated ACH-2/OX40 cells was dependent on the κB site of the HIV-1 long terminal repeat, and the OX40-gp34 interaction activated NF-κB consisting of p50 and p65 subunits. When primary activated CD4+ T cells acutely infected with HIV-1NL4-3 (CXCR4-using T-cell-line-tropic) were cocultured with PFA-fixed gp34+ human T-cell leukemia virus type 1-bearing MT-2 cells or SV-T2/gp34 cells, HIV-1 production was also markedly enhanced. The enhancement was again significantly inhibited by 5A8. The present study first shows that OX40-gp34 interaction stimulates HIV-1 expression and suggests that OX40 triggering by gp34 may play an important role in enhancing HIV-1 production in both acutely and latently infected CD4+ T cells in vivo.
Interaction between molecules of the tumor necrosis factor receptor (TNFR) superfamily, including TNFRI (CD120a), TNFRII (CD120b), Fas (CD95), CD40, CD30, CD27, 4-1BB (CD137), and OX40 (CD134), and their ligand molecule superfamily has been shown to costimulate a variety of cellular responses, such as cell growth, differentiation, and programmed cell death (apoptosis) (13). Human OX40 was identified as a cell surface type I transmembrane glycoprotein antigen of 50 kDa, whose expression is limited to activated T cells (20). Its natural ligand, gp34/human OX40 ligand (OX40L), belongs to tumor necrosis factor (TNF) superfamily, including TNF, FasL, CD40L, CD30L, CD27L, and 4-1BBL. Gp34 was originally identified as a type II transmembrane glycoprotein of 34 kDa expressed on human T-cell leukemia virus type 1 (HTLV-1)-infected T-cell lines and induced by the transactivator p40tax of HTLV-1 (26, 40). Subsequently, it was shown that gp34 was the ligand for human OX40 (3, 11). The expression of gp34 is limited to activated normal B cells and activated Epstein-Barr virus-transformed cells (11, 28), vascular endothelial cells (VEC) (16), and a subset of blood-derived unstimulated or CD40-stimulated dendritic cells (DC) (30).
The OX40-gp34 interaction has been shown to induce bidirectional signals and thus is implicated in various immunological responses. (i) For example, gp34 on DC and gp34 transfectants provided a costimulatory signal to OX40+ helper T cells, resulting in increased proliferation, cytokine production, and preferential differentiation of naive CD4+ T cells to type 2 helper phenotype. Simultaneously, ligation of gp34 on human DC by OX40 enhanced their maturation and production of cytokines (3, 11, 30, 31): (ii) gp34 expressed on activated B cells can receive a signal from OX40 on helper T cells, which resulted in B-cell proliferation and immunoglobulin secretion (28); and (iii) the OX40/gp34 system directly mediated the adhesion of OX40+ T cells to gp34+ human umbilical vein endothelial cells (HUVEC) (16), and c-jun m-RNA was induced in gp34+ HUVEC (22).
Several lines of evidence showed that some members of the TNF/TNFR superfamily play important roles in enhancing human immunodeficiency virus type 1 (HIV-1) infection: (i) TNF alpha (TNF-α) enhances HIV-1 replication by directly increasing its transcription level through induction of NF-κB in T-cell lines (8, 32); (ii) CD30 triggering stimulated HIV-1 expression through an NF-κB-dependent pathway in the chronically HIV-1-infected T-cell line ACH-2 (4), and CD4+ T cells from HIV-1-positive individuals also demonstrated enhanced HIV-1 production by CD30 stimulation in the presence of anti-CD3 monoclonal antibody (MAb) (21); (iii) cross-linking of 4-1BB with agonistic MAb significantly enhanced HIV-1 replication in CD4+ T cells from HIV-1+ individuals in the presence of anti-CD3 MAb (46); and (iv) CD40 stimulation in the presence of interleukin-4 (IL-4) and IL-2 upregulated HIV replication in B cells (12). Recent studies have shown that activation of TNFR superfamily proteins, including TNFRI, TNFRII, CD30, 4-1BB, CD40, and OX40, recruited several members of signal transducers called TNFR-associated factors (TRAFs), and some members of the TRAF family are responsible for the activation of NF-κB (1, 17, 19).
In the present study, we explored the effect of OX40 costimulation by gp34. We show here that stimulation of acutely and chronically HIV-1-infected T cells through OX40 by gp34+ HTLV-1+ T cells or gp34-transfected cells, respectively, significantly enhances HIV-1 expression in vitro. Our findings suggest that OX40-gp34 interaction in vivo in HIV-1-infected patients may be involved in the enhancement of productive infection of HIV-1.
MATERIALS AND METHODS
Cells.
ACH-2 cell line (7) was cultured in RPM1 1640 medium (Nikken Biolaboratory, Kyoto, Japan) containing 10% heat-inactivated fetal bovine serum (FBS; JRH Biosciences, Lenexa, Kans.), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (hereinafter called RPM1-CM) at 37°C and 5% CO2. The SV-T2 cell line that originated from murine embryo musculus was obtained from the Human Science Research Resources Bank (Osaka, Japan), and cultured in Dulbecco modified Eagle medium (DMEM; Nikken Biolaboratory) containing 10% FBS, 4 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (hereinafter called DME-CM). To establish transfectants of ACH-2 cells expressing OX40, ACH-2/OX40, 20 μg of the OX40 plasmids (pCAGIPuro/OX40) were linearized by digestion with PvuI, suspended in K-PBS (120 mM KCl, 30 mM NaCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 10 mM MgCl2), and introduced into 5 × 106 cells of the ACH-2 cell line by an electroporation method using the Gene Pulser II (Bio-Rad Laboratories, Hercules, Calif.). ACH-2/mock cells were also produced by electroporation of lineared pCAGIPuro vector without an insert. These transfectants were suspended at 1 × 105 to 2 × 105 cells/ml in RPMI-CM, dispensed at 5 × 104 to 10 × 104 cells/well in 24-well plates, and cultured for 4 days, selected by RPMI-CM containing 0.2 μg of puromycin (Clontech Laboratories, Palo Alto, Calif.) per ml, and then cultured in 1 μg of puromycin per ml thereafter. Selected ACH-2/OX40 cells were examined for spontaneous release of HIV-1 by measuring the p24 in the culture supernatants. Ten cells producing low levels of p24 were selected for further studies. Their OX40 and gp34 expression were examined by flow cytometric analysis. Similarly, gp34-expressing SV-T2 cells, SV-T2/gp34 cells, and SV-T2/mock cells were established and cultured in DME-CM containing 2 μg of puromycin per ml. The levels of gp34 expression were determined by flow cytometric analysis.
Preparation of primary CD4+ T cells was as described previously (38, 43). Human peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation with Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) from the heparinized peripheral blood of healthy donors. CD4+ T cells were isolated from adherent-cell-depleted PBMC by positive selection with immunomagnetic beads conjugated to anti-CD4 antibody (Ab; Dynal, Oslo, Norway). The purified CD4+ T cells (106 cells/ml) in RPMI-CM with 50 U of recombinant human IL-2 (rhIL-2; Shionogi Pharmaceutical, Osaka, Japan) and 20 ng of rhIL-4 (R&D Systems, Minneapolis, Minn.) per ml were plated in 12-well plates coated with anti-human CD3 MAb (OKT-3; American Type Culture Collection, Rockville, Md.) and then incubated at 37°C in a 5% CO2 incubator. On day 3, cells in the cultures were harvested, washed, and resuspended in fresh RPMI-CM with rhIL-2 and rhIL-4 (2 × 105 cells/ml) and then restimulated by immobilized anti-CD3 MAb. On day 6, cells were harvested, washed, and resuspended in fresh RPMI-CM with rhIL-2 and rhIL-4 at (5 × 105 cells/ml) and then were plated without immobilized anti-human CD3 MAb. These primary CD4+ T-cell cultures were harvested on day 8 and then used as targets for HIV-1 infection. The HTLV-1-bearing MT-2 cell line (27) was cultured in RPMI-CM at 37°C and 5% CO2.
Sf9 insect cells (Invitrogen, Carlsbad, Calif.) were cultured in TC-100 insect medium (GIBCO-BRL, Gaithersburg, Md.) including 10% FBS, 100 μg of kanamycin per ml, and Tryptose Phosphate Broth (GIBCO-BRL) (hereinafter called TC-100-CM) at 26.5°C. High Five insect cells were cultured in Sf-900 II SFM (GIBCO-BRL) at 26.5°C. A myeloma cell line, SP2/0-Ag14 (Riken Cell Bank, Saitama, Japan), and a MOLT-4 cell line (25) were cultured in RPMI-CM at 37°C and 5% CO2.
Preparation of soluble OX40.
Recombinant soluble OX40 was generated by a baculovirus expression system. The Bac-N-Blue Transfection Kit (Invitrogen), as follows. The extracellular portion of OX40 (nucleotides 85 to 648 tagged with BamHI and HindIII sites) was amplified by PCR and ligated into the pcDNA3.1(−)/Myc-His A vector (Invitrogen); after that, the portion of OX40-Myc-His was ligated into the baculovirus vector, pMclBac A vector (Invitrogen). Bac-N-Blue DNA and pMelBac A/OX40-Myc-His vector were then cotransfected into Sf9 cells (Invitrogen) using SuperFect transfection reagent (Qiagen, Hilden, Germany). A cloned OX40-Myc-His (OX40-MH)-expressing recombinant baculovirus (P1 viral stock) was infected into Sf9 cells at a multiplicity of infection (MOI) of 0.1 in TC-100-CM. After 5 days, the cells and supernatants were harvested (P2 viral stock). Thereafter, this viral stock was used to infect High Five cells (Invitrogen) at an MOI of 50 in Sf-900 II SFM and, after 5 days, the supernatants were harvested. The recombinant fusion protein was purified by Ni-nitrilotriacetic acid–agarose (Qiagen) and was confirmed by silver staining and Western blotting.
Generation of MAb against human OX40 and other Abs.
BALB/c mice were immunized subcutaneously and intramuscularly with 100 μg of recombinant fusion protein, OX40-MH, emulsified with Freund complete adjuvant (Wako, Osaka, Japan), and with Freund's incomplete adjuvant (Wako) two more times at 2-week intervals. The sensitized spleen cells were fused with a mouse myeloma cell line, SP2/0-Ag14, using polyethylene glycol 1500 (Roche Diagnostics, Mannheim, Germany) 3 days after a final booster immunization of OX40-MH alone by the intravenous or the intraperitoneal route. After selection in RPMI-CM containing hypoxanthine, aminopterin, and thymidine (Roche Diagnostics), hybridomas producing MAb reactive with human OX40 were screened by flow cytometric analysis and Western blotting using the OX40+ MT-2 cell line and the OX40− MOLT-4 cell line (16) and then cloned by limiting dilution. B-7B5, newly generated in this study, was typed as immunoglobulin G1 (IgG1) and κ by the Mouse MonoAb ID/SP Kit (Zymed, San Francisco, Calif.) and was also labeled by Cy5 Ab labeled kit (Amersham Pharmacia Biotech) for flow cytometric analysis.
Other MAbs used were fluorescein isothiocyanate (FITC)-conjugated anti-gp34 MAb (TAG-34) (40), Cy5-conjugated negative control mouse IgG1 (TAXY-8) (42), FITC-conjugated negative control mouse IgG1 (DAK-GO1; Dako, Copenhagen, Denmark), anti-human OX40 MAb (Ber Act 35; Ancell, Bayport, Minn.), anti-myc MAb (Invitrogen), anti-Fas MAb (CH-11; MBL, Nagoya, Japan), gp34-neutralizing MAb (5A8) (16, 44), TNF-α-neutralizing MAb (1825.121; R&D Systems), TNF-β-neutralizing MAb (5802.21; R&D Systems), FasL-neutralizing MAb (NOK2; PharMingen, San Diego, Calif.), and negative control IgG1 (MET-3) (41). Alkaline phosphatase-conjugated anti-mouse IgG Ab and rabbit Abs to p50 (sc-7178 X), p52 (sc-298 X), p65 (sc-372 X), c-Rel (sc-70 X), and Rel-B (sc-226 X) were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Control rabbit Ab was purchased from Diaclone Research (Besaçn Cedex, France).
Plasmids.
Expression vectors of human OX40 (pCAGIPuro/OX40) and gp34 (pCAGIPuro/gp34) were constructed as follows. The coding sequences of human OX40 (20) or gp34 (26) were amplified by the PCR method. For human OX40 we used the primers (forward, nucleotides 6 to 35 of human OX40 cDNA tagged with an XhoI site; reverse, nucleotides 808 to 839 tagged with an NotI site) and pKU2-OX40-11 vector for template (15). For gp34 we used the primers (forward, nucleotides 37 to 69 of gp34 cDNA tagged with an XhoI site; reverse, nucleotides 554 to 588 tagged with an NotI site) and pSGP34-1 vector for a template (26). Each PCR product was ligated into pGEM-T vector (Promega, Madison, Wis.). The XhoI/NotI fragments of human OX40 and gp34 were each subcloned into pCAGIPuro expression vector (kindly provided by H. Niwa, Osaka University Medical School, Osaka, Japan).
A series of reporter plasmids of HIV-1 long-terminal-repeat (LTR) activity were constructed as follows. The 3′ LTR fragment of the HIV-1NL4-3 (XhoI-HindIII, nucleotides 8887 to 9663) was inserted into the XhoI and HindIII sites of pGL3-Basic vector (Promega). The deletion mutant of NF-κB binding sites in HIV-1NL4-3 plasmid (36) was also inserted. These plasmids were designated as pLTR-luc and pLTR-lucΔNF-κB.
HIV-1 infection and p24 assay.
Five ACH-2/OX40 cell groups (cell groups 3, 4, 5, 6, and 10), four ACH-2/mock cell groups (cell groups 2, 7, 8, and 10), and ACH-2 cells were suspended at 4 × 105 cells/ml in RPMI-CM and dispensed at 105 cells/well in 48-well plates. Some of these cultures were stimulated with SV-T2/gp34 or SV-T2/mock cells (5 × 104 cells/well) pretreated with 4% PFA for 15 min at room temperature. TNF-α (2 ng/ml; R&D Systems) and TNF-β (10 ng/ml; R&D Systems) were added as controls for positive stimulation for enhanced HIV-1 expression in ACH-2 (4), ACH-2/mock, and ACH-2/OX40 cells. After cultivation for 3 days, cell-free culture supernatants were collected for measuring HIV-1 p24 by a Lumipulse (Fujirebio, Tokyo, Japan). For blocking gp34-OX40 interaction, PFA-fixed SV-T2/gp34 cells and SV-T2/mock cells were preincubated with or without 5, 1, 0.2, or 0.04 μg of anti-gp34 neutralizing MAb (5A8) or control MAb (MET-3) per ml for 1 h at 37°C. For kinetic studies of HIV-1 p24 expression, culture supernatants were harvested at various time points. To exclude an involvement of TNF in enhanced HIV-1 expression in ACH-2/OX40 cells, stimulator cells, PFA-fixed SV-T2/gp34 cells, and SV-T2/mock cells, exogeneously added TNF-α and TNF-β were pretreated with or without anti-human TNF-α neutralizing MAb (50 μg/ml), anti-human TNF-β neutralizing MAb (5 μg/ml), or control MAb (MET-3, 50 μg/ml) for 1 h at 37°C before stimulation. The culture supernatants were harvested after cultivation for 3 days.
Primary CD4+ T cells were infected with wild-type or NF-κB mutant HIV-1NL4-3 (45) at an MOI of 0.002 and were cultured at 5 × 105 cells/ml in RPMI-CM containing 50 U of rhIL-2 per ml in a 12-well plate, with or without the addition of a half number of PFA-fixed gp34+ HTLV-1-bearing MT-2 cells (40), SV-T2/gp34 cells, or SV-T2/mock cells pretreated with either 10 μg of anti-gp34 neutralizing MAb (5A8) or a control MAb (MET-3) per ml. On day 3, 4, 5, 6, and 7 after HIV-1 infection, the culture supernatants were collected for p24 assay.
Silver staining and Western blotting.
The fusion protein was mixed with an equal volume of twofold-concentrated sample buffer (125 mM Tris-HCl, pH 6.8; 4% sodium dodecyl sulfate [SDS]; 20% glycerol; 0.1% bromophenol blue), including 8% 2-mercaptoethanol, and boiled for 5 min.
Cell lysates were obtained by lysis of 2 × 107 cells in 1 ml of a lysis buffer (10 mM Tris-HCl [pH 8.0] containing 140 mM NaCl, 3 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride [PMSF], and 0.5% Nonidet P-40) on ice for 20 min, followed by centrifugation at 13,000 × g for 10 min at 4°C. The cell lysates were mixed with an equal volume of twofold-concentrated sample buffer without 2-mercaptoethanol and treated for 30 min at room temperature.
Samples were fractionated by SDS-polyacrylamide gel electrophoresis using a 12.5% gel. The fusion protein in gel was stained by a silver staining kit (Daiichi Pure Chemicals, Tokyo, Japan). A part of the sample were transferred to Clear Blot Membrane-P (Atto, Tokyo, Japan) in transfer buffer (25 mM Tris, 192 mM glycine, 1% ethanol), blocked with Block Ace (Dainippon Pharmaceutical, Osaka, Japan), and incubated with the primary Abs of 1 μg of anti-OX40 MAb (Ber Act 35 or B-7B5), 0.2 μg of anti-myc MAb, or 1 μg of negative control MAb per ml. These were incubated with alkaline phosphatase-conjugated anti-mouse immunoglobulin G (IgG) Ab (1:5,000). The reaction was detected by using Attophos Substrate Set (Roche Diagnostics) and an image analyzer, the FLA-2000 G (Fujifilm, Tokyo, Japan).
FACS.
Sample cells (2 × 105) were resuspended in 100 μl of cold phosphate-buffered saline (PBS) containing 2% FBS and 0.1% sodium azide (hereinafter called fluorescence-activated cell sorter[FACS] buffer), preincubated with 20 μg of normal human IgG solution per ml for 15 min on ice to block the crystallizable fragment receptor (FcR), and then incubated with each fluorescence-conjugated MAbc for 30 min on ice. After being washed with FACS buffer, the cells were fixed with 1% PFA solution in PBS and subsequently analyzed on a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Data were analyzed using the CellQuest program (Becton Dickinson Immunocytometry Systems).
Detection of apoptosis.
As described above, 105 ACH-2/OX40 (cell groups 4 and 10), ACH-2/mock (cell group 7), and ACH-2 cells per well were stimulated with each stimulus for 5 days. The numbers of live cells were monitored every day by staining with trypan blue (GIBCO-BRL). Morphological changes of the cells were visualized by a microscope 3 days after the introduction of the stimuli. DNA fragmentation was then determined as described previously (18). Briefly, 106 cells were harvested 3 days after each stimuli, including anti-Fas MAb (500 ng/ml), inducing apoptosis, washed in cold PBS, and lysed in 0.5 ml of lysis buffer (10 mM Tris-HCl, 10 mM EDTA, 0.2% Triton X-100) for 10 min on ice. The lysates were centrifuged (13,000 × g) for 10 min at 4°C to separate high-molecular-weight DNA (pellet) from cleaved low-molecular-weight DNA (supernatant). The supernatants were incubated with RNase A (0.2 mg/ml) for 1 h at 37°C and then with proteinase K (0.4 mg/ml) for 2 h at 55°C. The samples were extracted with phenol-chloroform-isoamyl alcohol and then precipitated by ethanol. The pellets were air dried, resuspended in dissolving buffer (10 mM Tris-HCl, 1 mM EDTA, 1% SDS), and then incubated at 60°C for 10 min. The samples were subjected by electrophoresis in a 2% agarose gel containing ethidium bromide and then photographed on an UV transilluminator. Low-molecular-weight apoptotic DNA levels (103 cells/well) were also measured by using Cell Death Detection ELISA Plus (Roche Diagnostics).
TNF-α and TNF-β assays.
For the determination of TNF-α and TNF-β production, supernatants of ACH-2/OX40 cells stimulated by PFA-fixed SV-T2/gp34 cells were collected at each time point (0, 1, 2, 3, 4, 6, 8, 12, 15, 18, 24, 48, and 72 h), and the concentrations of TNF-α and TNF-β were assayed using a Quantikine TNF-α and TNF-β Kit (R&D Systems).
Luciferase assay.
Plasmid DNAs were introduced into each cell by a modification of the DEAE-dextran transfection procedure (6). The 5 × 106 cells were washed once in plain RPMI and resuspended in 5 ml of transfection solution (3.35 ml of plain RPMI, 0.9 ml of H2O, 0.25 ml of 2 M Tris-HCl [pH 7.4], 0.5 ml of a 10-mg/ml concentration of DEAE-dextran [Amersham Pharmacia Biotech]) containing 10 μg of pLTR-luc or pLTR-lucΔNF-κB plus 1 μg of a pRL-TK plasmid (Toyo Ink, Tokyo, Japan) as internal controls. After incubation at 37°C for 30 min, the cells were washed twice in plain RPMI and then resuspended at 4 × 105 cells/ml with RPMI-CM, dispensed at 4 × 105 cells/well in 12-well plates, and cultured for 40 h. After incubation, PFA-fixed SV-T2/mock cells (2:1), PFA-fixed SV-T2/gp34 cells (2:1), TNF-α (2 ng/ml), or medium was added to these cells. The stimulated cells were harvested after 8 h, and lysed with 40 μl of lysis buffer per ∼7 × 105 cells. Assays for luciferase activity were performed by Dual-Luciferase Reporter Assay System (Promega) and Luminescencer JNR (ATTO).
Electrophoretic mobility shift assay (EMSA).
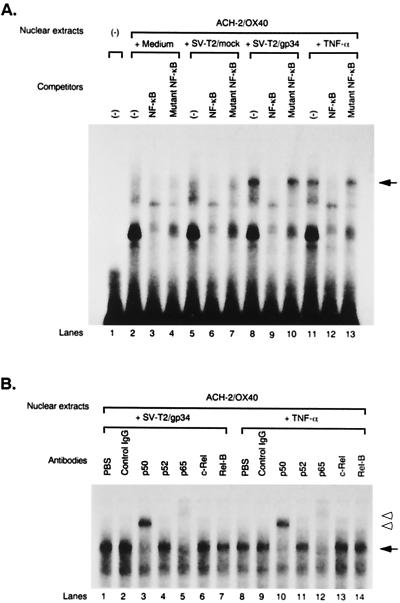
ACH-2/OX40 cells, ACH-2/mock cells, and ACH-2 cells cocultured with PFA-fixed SV-T2/gp34 cells (2:1) or SV-T2/mock cells (2:1) for 3 h or cultured in the presence or absence of TNF-α (2 ng/ml) for 1 h were harvested, and their nuclear extracts were prepared for analysis by the rapid method (33). The cell pellets containing 5 × 106 ACH-2/OX40 cells, ACH-2/mock cells, and ACH-2 cells were washed once with PBS, resuspended in 400 μl of buffer A (10 mM HEPES-KOH, pH 7.9; 1.5 mM MgCl2; 10 mM KCl; 0.5 mM dithiothreitol [DTT]) including 0.5% Nonidet P-40, incubated for 10 min on ice, vortexed briefly, and microcentrifuged at 13,000 × g for 10 s at 4°C. The supernatants were removed, and the nuclear pellets were suspended in 20 μl of buffer C (20 mM HEPES-KOH, pH 7.9; 2.5% glycerol; 420 mM NaCl; 1.5 mM MgCl2; 0.2 mM EDTA; 0.5 mM DTT; 0.5 mM PMSF), incubated for 20 min on ice, vortexed briefly, and microcentrifuged at 13,000 × g for 2 min at 4°C. The supernatants were collected, the protein concentrations were determined by the method of Bradford (5), and the supernatants were stored at −80°C before use. The sequences of the oligonucleotides used for the probes were as follows: wild-type NF-κB, 5′-AATTCAAGGGACTTTCCGCTGGGGACTTTCCAG and GTTCCCTGAAAGGCGACCCCTGAAAGGTCCTAG-5′; and mutant NF-κB, 5′-AATTCAACTCACTTTCCGCTGCTCACTTTCCAG and GTTGAGTGAAAGGCGACGAGTGAAAGGTCCTAG-5′. The NF-κB consensus binding sequence is shown in boldface type, and the mutated sites are italicized. Each oligonucleotide was annealed to its complementary strand and end labeled with [γ-32P]ATP (Amersham Pharmacia Biotech) using T4 polynucleotide kinase (TaKaRa, Tokyo, Japan). 0.2 pmol of γ-32P-labeled double-stranded probe (∼30,000 cpm) were incubated with 10 μg of nuclear extracts and 1 μg of poly[d(I-C)] (Roche Diagnostics) in 20 μl of buffer D (20 mM HEPES-KOH, pH 7.9; 2.5% glycerol; 100 mM KCl; 0.2 mM EDTA; 0.5 mM DTT; 0.5 mM PMSF) for 30 min at room temperature. Specific binding was controlled by competition with a 50-fold excess (10 pmol) of the wild-type or mutant nonradioactive oligonucleotide, incubated with the nuclear extract for 30 min at room temperature before the binding reaction was started. To identify the subunits constituting NF-κB complexes, specific Abs against p50, p52, p65, c-Rel, and Rel-B were used. Abs were added to the nuclear extract, and the mixture was allowed to stand for 30 min at room temperature before incubation with the radiolabeled probe. DNA-protein complexes were analyzed by electrophoresis in 5% (29:1) polyacrylamide gel in 0.5× TBE (44.5 mM Tris, 44.5 mM boric acid, 1 mM EDTA). After electrophoresis, the gels were dried and autoradiographed.
RESULTS
Triggering of OX40 by gp34 stimulates HIV-1 production from chronically infected T cells.
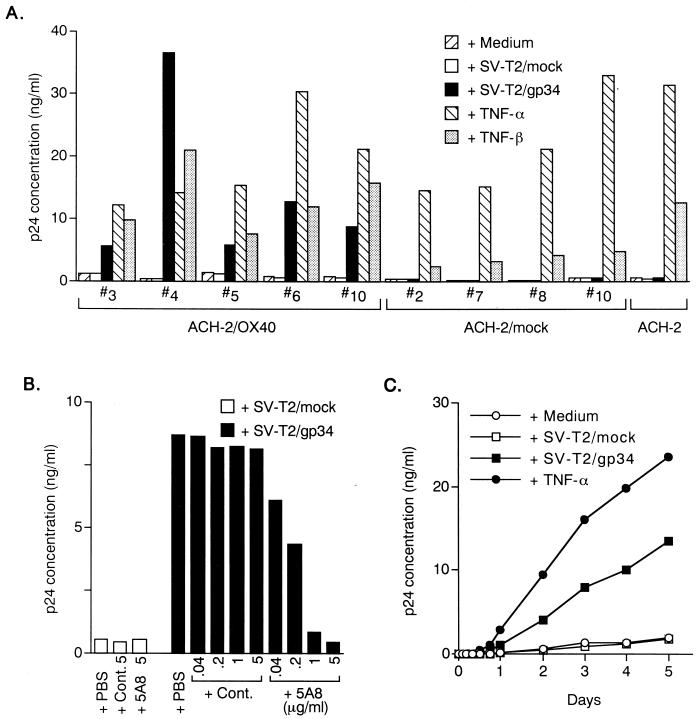
To investigate the potential role of interaction between gp34 and OX40 in HIV replication, we used chronically HIV-1-infected T-cell lines, ACH-2. The expression of OX40 and gp34 in this cell line was examined by FACS analysis. The ACH-2 cells did not express gp34 and OX40 on their surfaces (data not shown). Therefore, we established stable human OX40-expressing ACH-2, ACH-2/OX40 cells, and ACH-2/mock cells by electroporation of the pCAGIPuro/OX40 vector and the pCAGIPuro vector alone without an insert as a control, respectively. As a stimulator, we also established gp34-transfected mouse cell line SV-T2, SV-T2/gp34 cells and empty vector-transfected SV-T2, SV-T2/mock cells. As shown in Fig. 1A, all the ACH-2/OX40 cells (cell groups 3, 4, 5, 6, and 10) tested were apparently enhanced for HIV-1 expression through signaling from PFA-fixed SV-T2/gp34 cells. In the control coculture with OX40− ACH-2/mock cells (cell groups 2, 7, 8, and 10) or ACH-2 cell lines, the production of HIV-1 remained at the basal level. Importantly, enhanced expression of HIV-1 from ACH-2/OX40#10 cells by PFA-fixed SV-T2/gp34 cells was dose dependently inhibited by the anti-gp34 MAb 5A8 (Fig. 1B). The kinetic studies showed that HIV-1 expression by interaction of gp34 and OX40 could be detected as early as 18 h after cocultivation and that HIV-1 p24 increased over time (Fig. 1C). Similar results were also obtained in another cells, ACH-2/OX40#4 (data not shown). These results clearly showed that triggering of OX40 by its ligand, gp34/OX40L, stimulates HIV-1 production from T cells persistently infected with HIV-1.
FIG. 1.
Enhanced expression of HIV-1 by OX40 stimulation from HIV-1 chronically infected ACH-2/OX40 cells. (A) Five groups of ACH-2/OX40 cells (cell groups 3, 4, 5, 6, and 10), four groups ACH-2/mock cells (cell groups 2, 7, 8, and 10), and the parental ACH-2 cell line were stimulated by PFA-fixed SV-T2/gp34 cells or PFA-fixed SV-T2/mock cells at a cell ratio of 2:1 with TNF-α (2 ng/ml) or TNF-β (10 ng/ml). After 3 days, HIV-1 production was determined quantitatively by p24 assay using the culture supernatants. (B) PFA-fixed SV-T2/gp34 cells and PFA-fixed SV-T2/mock cells were preincubated with or without anti-gp34 neutralizing MAb (5A8) or control MAb (Cont.) at 5, 1, 0.2, or 0.04 μg/ml for 1 h at 37°C and then were added to ACH-2/OX40 cells. Culture supernatants were harvested 3 days after coculture. (C) Kinetics of HIV-1 expression after stimulation with gp34 in ACH-2/OX40 cells. Supernatants were harvested at each time point, i.e., at 0, 2, 4, 8, 12, 18, 24, 48, 72, 96, and 120 h, and then tested for p24 titers. All results are the means of values obtained in triplicate. A representative result from two independent experiments is shown.
Triggering of OX40 by gp34 induces apoptosis in chronically infected T cells.
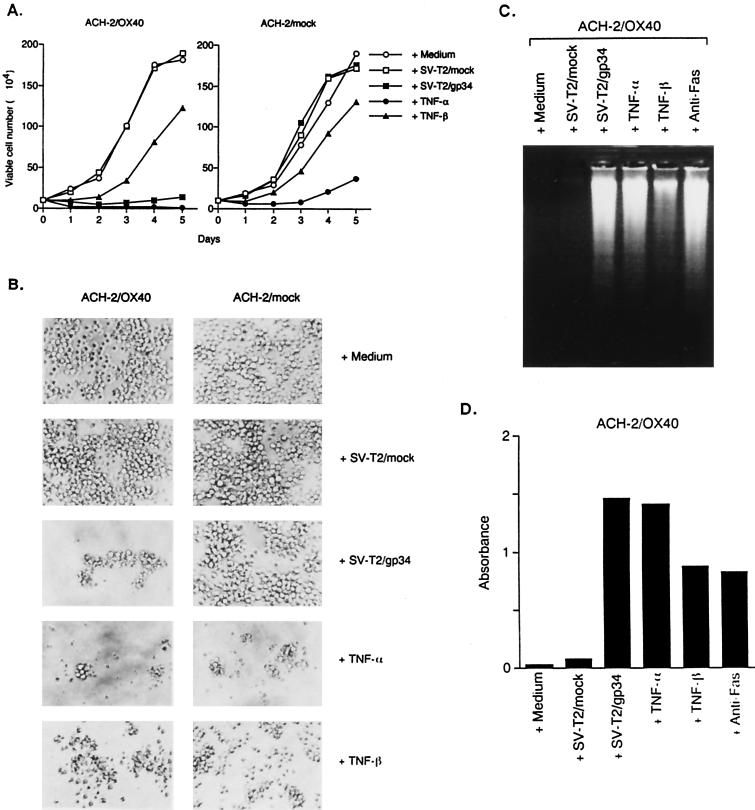
Since the gp34/OX40 system has been implicated in the modulation of proliferation in different cellular systems, we investigated whether HIV-1 induction by coculture was associated with an effect on the proliferation of T lymphocytes. Interestingly, when cells of the ACH-2/OX40#10 cells were induced for HIV-1 production by gp34, they apparently underwent cell growth retardation (Fig. 2A) and morphological change (Fig. 2B). The cell viability was only about 40% 2 days after coculture and, after that, surviving cells recovered and increased gradually in numbers. This pattern of cell growth and morphology was similar to the effect of TNF-α. TNF-α treatment of ACH-2/OX40 cells resulted in much stronger cell killing. On the other hand, TNF-β induced a little cell death in each cell. Furthermore, we demonstrated by electrophoresis on an agarose gel of the DNA (Fig. 2C) and with the use of an ELISA kit that measures the presence of histone-associated DNA fragments (Fig. 2D) that this cell death was due to apoptosis. Very similar results were obtained in another ACH-2/OX40#4 cells (data not shown). In ACH-2/mock#7 cells and the parental ACH-2 cell line, apoptosis was induced by TNF-α but was not induced by gp34 (data not shown).
FIG. 2.
Detection of apoptosis of the ACH-2/OX40 cells stimulated with gp34. (A) ACH-2/OX40#10 cells and ACH-2/mock#7 cells were cultured for 5 days with medium alone, PFA-fixed SV-T2/mock cells, PFA-fixed SV-T2/gp34 cells, TNF-α, or TNF-β and then examined for viable cell number under a microscope. The results are expressed as mean numbers of living cells obtained in triplicate. (B) Morphological alteration of ACH-2OX40#10 cells and ACH-2/mock#7 cells 3 days after the introduction of various stimuli. Original magnification, ×200. (C) Nuclear DNA fragmentation of ACH-2/OX40#10 cells (106) by apoptosis was analyzed by 2% agarose gel electrophoresis 3 days after the stimuli. Anti-Fas MAb (CH-11) was used as positive control. (D) The apoptotic DNAs of ACH-2/OX40#10 cells (103/well) were measured by using an ELISA kit 3 days after various stimuli. The results are a mean number of absorbance values obtained in triplicate. Representative results are shown from two independent experiments.
HIV expression by triggered OX40 is not mediated by endogenous TNF secretion.
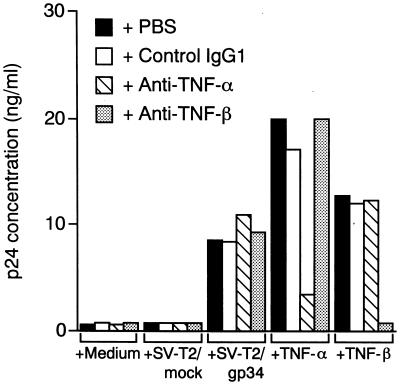
It is possible that autocrine secretion of TNF was responsible for HIV-1 expression caused by OX40-stimulation. Figure 3 shows that blocking Abs directed against TNF-α and TNF-β abrogated HIV production from ACH-2/OX40#10 cells induced by the respective cytokines, but these two Abs had no effect on the HIV-1 expression enhanced by PFA-fixed SV-T2/gp34 cells, indicating that interaction of gp34 and OX40 on ACH-2/OX40 cells leads to enhanced virus production independent of endogenous secretion of TNF-α or TNF-β. Indeed, the level of TNF-α and TNF-β in the supernatants from ACH-2/OX40#10 cells cocultivated with PFA-fixed SV-T2/gp34 cells was <10 pg/ml (data not shown). Similar results were also obtained in another cells, ACH-2/OX40#4 (data not shown).
FIG. 3.
Autocrine secretion of TNF-α and TNF-β does not account for enhanced HIV-1 expression in ACH-2/OX40 cells stimulated with gp34. ACH-2/OX40#10 cells were cocultured with PFA-fixed SV-T2/gp34 cells or PFA-fixed SV-T2/mock cells at a cell ratio of 2:1 or were cultured in the presence of TNF-α (2 ng/ml) or TNF-β (10 ng/ml) after preincubation with or without anti-human TNF-α neutralizing MAb (50 μg/ml), anti-human TNF-β neutralizing MAb (5 μg/ml), or control MAb (50 μg/ml) for 1 h at 37°C. Culture supernatants were harvested after 3 days, and then the HIV-1 p24 level was measured. The results are the means of values obtained in triplicate. Representative results are shown from three independent experiments.
κB sites in the LTR are required for HIV transcription induced by interaction of gp34 and OX40.
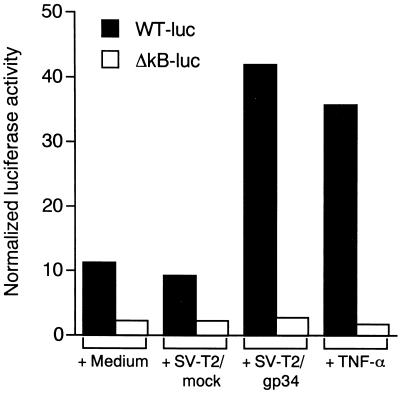
Because it has been described that NF-κB activation is necessary for the induction of HIV-1 transcription by TNF-α in ACH-2 cells, we investigated whether the same was true for HIV-1 expression by OX40-stimulation. ACH-2/OX40#4 cells were transiently transfected either with an HIV LTR construct linked to the reporter gene luciferase (pLTR-luc) or with its respective plasmid in which the κB sites were deleted (pLTR-lucΔNF-κB). Stimulation of OX40 by coculture with PFA-fixed SV-T2/gp34 cells resulted in the induction of luciferase activity when the cells were transfected with the pLTR-Luc wild-type construct (ca. fourfold higher than baseline; Fig. 4) but not with the deleted κB construct. Similar results were also obtained in another cells, ACH-2/OX40#10 (approximately twofold higher than baseline; data not shown). In the ACH-2/mock#7 cells and ACH-2 cell line, HIV-1 LTR transactivations were induced by TNF-α, but they remained at the basal level with gp34 (data not shown). These data clearly indicate that transactivation of the HIV-1 LTR by OX40 cross-linking requires the presence of NF-κB sequences in the LTR.
FIG. 4.
Transcriptional activity of HIV LTR induced by OX40 stimulation. ACH-2/OX40#4 cells were cotransfected by the DEAE-dextran transfection procedure with the constructs of wild-type pLTR-luc (WT-luc) or κB-deleted type pLTR-lucΔNF-kB (ΔkB-luc) (10μg/5 × 106 cells), and pRL-TK (1 μg/5 × 106 cells). The transfectants were cultured for 40 h at 37°C. Luciferase activities were determined 8 h after stimulation by PFA-fixed SV-T2/gp34 cells or PFA-fixed SV-T2/mock cells at a cell ratio of 2:1, by TNF-α (2 ng/ml), or with medium alone. Transcriptional activity of HIV LTR was expressed as normalized luciferase activity. The results are the means of values obtained in triplicate. Three independent experiments showed similar results.
Interaction of gp34 and OX40 leads to the activation of NF-κB in ACH-2/OX40 cells.
To determine whether OX40 stimulation by gp34 induces NF-κB binding activity in ACH-2/OX40 cells, we performed EMSA. ACH-2/OX40#10 cells were cultured with or without PFA-fixed SV-T2/gp34 cells, PFA-fixed SV-T2/mock cells, or TNF-α, and then the cells were harvested after 3 h (in the case of cocultivation with SV-T2/gp34 cells or SV-T2/mock cells) or 1 h (for TNF-α or medium alone); nuclear extracts were then prepared. The bands, indicated by an arrow in Fig. 5, of gp34-stimulated ACH-2/OX40 cells were NF-κB specific since they are apparently abrogated with a 50-fold excess of cold NF-κB oligonucleotide but not with a mutated NF-κB oligonucleotide (Fig. 5A, lanes 8 to 10). Since the NF-κB family is comprised of several molecules that can dimerize and thus result in the formation of different complexes, we further investigated which molecules were involved in the NF-κB specific bands of gp34-stimulated ACH-2/OX40 cells. For this purpose, supershift assays were performed with the nuclear extracts of gp34-stimulated ACH-2/OX40 cells in the absence or presence of Abs that specifically recognize the following members of the NF-κB family: p50, p52, p65, c-Rel, and Rel-B. The band was supershifted by p50 Ab and also by p65 Ab, demonstrating that the band contained transactivating p50 and p65 (Fig: 5B, lanes 3 and 5, open arrowheads). Similar results were obtained with TNF-α stimulation (Fig. 5A, lanes 11 to 13; Fig. 5B, lanes 10 and 12). We also obtained reminiscent results in other cells, i.e., ACH-2/OX40#4 (data not shown). The NF-κB bands were not detected in gp34-stimulated ACH-2/mock#7 cells or ACH-2 cell line (data not shown).
FIG. 5.
NF-κB binding activity induced by cross-linking of OX40. (A) Analysis of NF-κB binding activity by EMSA. ACH-2/OX40#10 cells were treated for 3 h with PFA-fixed SV-T2/gp34 cells or PFA-fixed SV-T2/mock cells at a cell ratio of 2:1 or were cultured for 1 h with TNF-α (2 ng/ml) or medium alone as a positive or a negative control, respectively. Nuclear extracts were preincubated with a 50-fold excess of cold wild- or mutant-type NF-κB competitor oligonucleotides and were treated with a 32P-labeled wild-type NF-κB oligonucleotide. The position of the NF-κB-specific band is indicated by the arrow (lanes 8, 10, 11, and 13). (B) Analysis of NF-κB components by supershift assay. ACH-2/OX40#10 cells were stimulated with PFA-fixed SV-T2/gp34 cells or TNF-α. Each nuclear extract was preincubated with the anti-p50, p52, p65, c-Rel, or Rel-B Ab for 30 min at room temperature and then was treated with a 32P-labeled wild-type NF-κB oligonucleotide. The arrow indicates the NF-κB-specific band. The lower and upper open arrowheads indicate the bands resulting from the supershift with the anti-p50 or p65 Ab (lanes 3, 5, 10, and 12). Representative results are shown from two independent experiments.
OX40 triggering by gp34 expressed on HTLV-1+ T cells enhances HIV-1 production from HIV-1-infected primary CD4+ T cells.
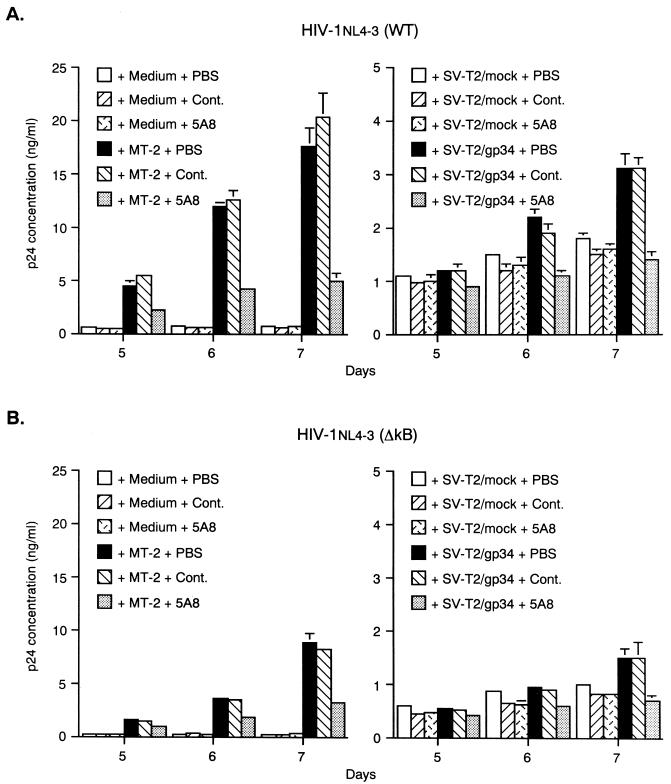
Finally, primary CD4+ T cells acutely infected with wild-type or NF-κB mutant HIV-1NL4-3 were cocultured with PFA-fixed gp34+ MT-2 cells or SV-T2/gp34 cells. On days 3, 4, 5, 6, and 7 after cocultivation, p24 levels in the culture supernatants were monitored. Cocultivation with PFA-fixed MT-2 cells resulted in the enhancement of wild-type HIV-1 production from CD4+ T cells on days 5, 6, and 7. Dramatic augmentation of HIV-1 production by gp34 was seen in CD4+ T cells infected with T-cell-line-tropic HIV-1. The enhanced HIV-1 production was inhibited by more than 50% by pretreatment of PFA-fixed MT-2 cells with anti-gp34 neutralizing MAb (5A8) (Fig. 6A, left panel). Similar results were also obtained in another stimulation system with PFA-fixed SV-T2/gp34 cells (Fig. 6A, right panel). These results showed that stimulation of OX40+ primary T cells acutely infected with HIV-1 with gp34 enhanced HIV-1 production. Interestingly, NF-κB mutant HIV-1 production was also enhanced by cocultivation with PFA-fixed MT-2 cells or SV-T2/gp34 cells on days 5, 6, and 7, although stimulation was less efficient. The enhanced HIV-1 production was significantly inhibited by 5A8 (Fig. 6B). These results showed that sites other than those for NF-κB binding were also involved in primary CD4+ T cells.
FIG. 6.
Enhanced HIV-1 expression in primary CD4+ T cells by coculture with gp34-expressing cells. Anti-CD3 MAb-stimulated primary CD4+ T cells cultured for 8 days in the presence of rhIL-2 and rhIL-4 were infected with wild-type (WT) (A) or NF-κB mutant (ΔkB) (B) HIV-1NL4-3 at an MOI of 0.002. They were cultured either alone or with a half number of PFA-fixed gp34+ MT-2 cells, SV-T2/gp34 cells, or SV-T2/mock cells pretreated with or without MAb (10 μg of anti-gp34 neutralizing MAb [5A8] per ml or a subtype-matched control MAb [Cont.]). After HIV-1 infection, culture supernatants were harvested to measure the HIV-1 p24 level. The results are the means ± standard error of the mean for values obtained in triplicate. Representative results are shown from three independent experiments.
DISCUSSION
AIDS is a clinically multifaceted disease, and primary infection with HIV is usually followed by a variably prolonged period of clinical latency. Multiple factors are involved in the complex interplay between HIV and host anti-HIV immune responses. It is thus important to understand the underlying mechanisms of activation of the large pool of latently infected cells and the progressive destruction of lymphoid tissues.
It has been reported that HIV-1 expression is augmented through signaling from some members of the TNF/TNFR superfamily, including TNF/TNFR (8, 23, 24), CD30L/CD30 (4, 21), and 4-1BBL/4-1BB (46), and some of the cytokine-receptor systems such as IL-1/IL-IR (33) and IL-6/IL-6R (35).
In the present study, we showed that stimulation of OX40 with gp34 in HIV-1-infected primary CD4+ T cells and a chronically infected ACH-2/OX40 cells leads to enhanced HIV-1 expression, as measured by the production of HIV-1 in the culture supernatants. Furthermore, we have examined the molecular mechanisms by which gp34-OX40 interaction activated HIV-1 expression in ACH-2/OX40 cells and have shown that the κB sites present in the HIV-1 LTR are required for HIV-1 transcription in this system. The lack of activity obtained using HIV-1 LTR-luc plasmids lacking the NF-κB sites strongly suggests that OX40 stimulation by gp34 proceeds primarily through κB-binding sites. In addition, we have observed that two NF-κB components, p50 and p65 subunits, are induced to bind to the κB sites upon OX40 stimulation of ACH-2/OX40 cells in EMSAs and supershift assays. All of these data support the notion that NF-κB activation is responsible for enhanced HIV-1 replication in HIV-1-infected cells by OX40 and gp34 interaction in ACH-2/OX40 cells. Enhancement of virus production by gp34-OX40 interaction was also reproduced in primary CD4+ T cells acutely infected with HIV-1. Thus, it is strongly suggested that a similar event may occur in vivo. However, it is important to note that in this experimental system, not only wild-type but also NF-κB mutant HIV-1NL4-3 were enhanced to produce virus upon stimulation with OX40, although the mutant virus was less efficiently enhanced. Thus, it is clear that sites other than those for NF-κB binding were also involved in primary CD4+ T cells. It is interesting to address further whether this difference is due to either the difference between acute and chronic infection or the difference between primary T cells and cell line cells.
It was of note that apparent apoptosis was observed in ACH-2/OX40 cells when they were stimulated by gp34. This result is quite different from the case of CD30L-stimulated CD30+ ACH-2 cells, in which no cell death but rather a slight stimulation of cell proliferation was observed (4). Therefore, the effect of OX40 signaling on cells is similar to that of TNF-α. Thus, it is of particular importance to investigate a potential role of the OX40/gp34 system in vivo, not only from the aspect of enhancement of HIV-1 but also in light of the killing of HIV-1-infected cells as observed in the TNF/TNFR model (24). On the other hand, in the stimulation system of ACH-2/OX40 cells by gp34 or TNF-α, since FasL-neutralizing MAb could not inhibit apoptosis these apoptosis may be independent of the FasL/Fas system (data not shown).
It has been shown that extensive HIV-1 replication occurs in secondary lymphoid organs, where CD4+ T-helper cells meet antigens presented by antigen-presenting cells (APC) (9, 14) and are activated to express OX40 (31). DC are the most potent APC in vivo. Generally, it is considered that immature DC in peripheral tissues capture antigens efficiently and have the unique capacity to subsequently migrate to the T-cell areas of secondary lymphoid organs (2). The fact that gp34 is predominantly expressed on APC, such as DC and B cells, suggests the importance of OX40-gp34 in HIV-1 productive infection. Furthermore, gp34 on DC was shown to induce differentiation of naive CD4+ T cells to IL-4-secreting type 2 helper phenotype (31), in which virulent T- cell-line-tropic HIV-1 can proliferate preferentially (43). This may facilitate the production of Abs specific to HIV-1 antigens from B cells by means of IL-4 secreted from type 2 helper T cells. In addition, since gp34 has been shown on the cell surface of VEC (16), interaction of blood OX40+ T cells infected with HIV-1 may be activated by gp34+ VEC, which may enhance HIV-1 production in inflammatory sites.
The OX40-gp34 interaction has been shown to induce bidirectional signals. For example, gp34 stimulation by OX40 transduced a signal in DC, which resulted in enhanced TNF-α and IL-1β production (30). Since these cytokines are potent stimulators of HIV-1 production from infected T cells, HIV-1 infected OX40+ T cells contacting gp34+ DC may receive signals not only from OX40 stimulation but also from these cytokines. Furthermore, it would be interesting to examine whether the activation of DC with OX40 may enhance the expression of DC-SIGN, which is responsible for capturing HIV-1, in the periphery and facilitating its transport to secondary lymphoid organs rich in T cells and thus enhance infection in trans of these target cells (9).
Besides DC, B cells, and HUVEC (16, 28, 30), it has been demonstrated that HTLV-infected cells express gp34 (40). Therefore, it is of interest to study whether such gp34+ HTLV+ T cells enhance HIV-1 production in patients harboring both HTLV and HIV-1. Recent in vitro studies showed that (i) noninfectious HTLV-1 virions induced the production of large quantities of HIV-1 from T cells by mitogenic stimulation (47); (ii) primary CD4+ T cells treated with soluble Tax protein and cell-free supernatants from HTLV-1-infected MT-2 cell cultures, containing β-chemokines of RANTES (regulated upon activation, normal T-cell expressed and secreted), macrophage inflammatory protein 1α (MIP-1α), and MIP-1β became highly susceptible to T-cell-line-tropic HIV-1 (29); and (iii) coinfection of monocyte-derived macrophages with T-cell-line-tropic HIV-1 and HTLV-1 significantly enhanced HIV-1 replication (39). Since Tax induces both OX40 and gp34 (15, 26, 34), OX40-gp34 interaction induced by Tax may enhance HIV-1-production. Furthermore, since MT-2 culture supernatants contain soluble gp34 (Y. Tanaka et al., unpublished data), it will be worthwhile to test whether an anti-gp34 MAb, 5A8, can inhibit such enhancement.
We showed that OX40-gp34 interaction plays an important role in enhancing HIV-1 replication in vitro. Since in HIV-1-infected patients CD4+ T cells are activated to express OX40 in addition to CD40L and Fas (37), it is speculated that these OX40+ CD4+ T cells in peripheral blood and in lymphoid organs, where cell-to-cell contact can easily occur, can be costimulated by gp34 in vivo. This potential mechanism of induction of HIV-1 expression from both acutely and latently infected CD4+ T cells by direct cellular interaction may play a pivotal role in the pathogenesis of HIV-1 disease. Therefore, intervention of interactions between OX40 and gp34 will be considered as a potentially new therapeutic strategy for inhibiting enhanced HIV-1 replication in vivo.
ACKNOWLEDGMENTS
We thank Y. Tsunetsugu-Yokota of National Institute of Infectious Diseases for gifts of mutant viruses, H. Niwa of Osaka University for gifts of the pCAGIPuro vector, S. Yamaoka for helpful suggestions, M. Hoshi and N. Misawa for technical assistance, and S. R. Jennings of Louisiana State University for critical reading of the manuscript.
This work was supported in part by grants from Health Sciences of Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan, and CREST (Core Research for Evolutional Science and Technology) of The Japan Science and Technology Corporation.
REFERENCES
- 1.Baker S J, Reddy E P. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Baum P R, Gayle III R B, Ramsdell F, Srinivasan S, Sorensen R A, Watson M L, Seldin M F, Baker E, Sutherland G R, Clifford K N, Alderson M R, Goodwin R G, Fanslow W C. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas P, Smith C A, Goletti D, Hardy E C, Jackson R W, Fauci A S. Cross-linking of CD30 induces HIV expression in chronically infected T cells. Immunity. 1995;2:587–596. doi: 10.1016/1074-7613(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Bressler P, Pantaleo G, Demaria A, Fauci A S. Anti-CD2 receptor antibodies activate the HIV long terminal repeat in T lymphocytes. J Immunol. 1991;147:2290–2294. [PubMed] [Google Scholar]
- 7.Clouse K A, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci A S, Folks T M. Monokine regulaton of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 8.Duh E J, Maury W J, Folks T M, Fauci A S, Rabson A B. Tumor necrosis factor α activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-κB sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 10.Geijtenbeek T B H, Kwon D S, Torensma R, van Vliet S J, van Duijnhoven G C F, Middel J, Cornelissen I L M H A, Nottet H S L M, KewalRamani V N, Littman D R, Figdor C G, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey W R, Fagnoni F F, Harara M A, Buck D, Engleman E G. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–762. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gras G, Legendre C, Krzysiek R, Dormont D, Galanaud P, Richard Y. CD40/CD40L interactions and cytokines regulate HIV replication in B cells in vitro. Virology. 1996;220:309–319. doi: 10.1006/viro.1996.0319. [DOI] [PubMed] [Google Scholar]
- 13.Gruss H-J, Dower S K. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- 14.Haase A T. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 15.Higashimura N, Takasawa N, Tanaka Y, Nakamura M, Sugamura K. Induction of OX40, a receptor of gp34, on T cells by trans-acting transcriptional activator, Tax, of human T-cell leukemia virus type I. Jpn J Cancer Res. 1996;87:227–231. doi: 10.1111/j.1349-7006.1996.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T. Tumor necrosis factor receptor-associated factor (TRAF) family: adaptor proteins that mediate cytokine signaling. Exp Cell Res. 2000;254:14–24. doi: 10.1006/excr.1999.4733. [DOI] [PubMed] [Google Scholar]
- 18.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-κB activation. J Biol Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 20.Latza U, Dürkop H, Schnittger S, Ringeling J, Eitelbach F, Hummel M, Fonatsch C, Stein H. The human OX40 homolog: cDNA structure, expression and chromosomal assignment of the ACT35 antigen. Eur J Immunol. 1994;24:677–683. doi: 10.1002/eji.1830240329. [DOI] [PubMed] [Google Scholar]
- 21.Maggi E, Annunziato F, Manetti R, Biagiotti R, Giudizi M G, Ravina A, Almerigogna F, Boiani N, Alderson M, Romagnani S. Activation of HIV expression by CD30 triggering in CD4+ T cells from HIV-infected individuals. Immunity. 1995;3:251–255. doi: 10.1016/1074-7613(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura Y, Hori T, Kawamata S, Imura A, Uchiyama T. Intracellular signaling of gp34, the OX40 ligand: induction of c-jun and c-fos mRNA expression through gp34 upon binding of its receptor, OX40. J Immunol. 1999;163:3007–3011. [PubMed] [Google Scholar]
- 23.Matsuyama T, Hamamoto Y, Okamoto T, Shimotohno K, Kobayashi N, Yamamoto N. Tumor necrosis factor and HIV: a note of caution. Lancet. 1988;ii:1364. doi: 10.1016/s0140-6736(88)90897-5. [DOI] [PubMed] [Google Scholar]
- 24.Matsuyama T, Hamamoto Y, Soma G, Mizuno D, Yamamoto N, Kobayashi N. Cytocidal effect of tumor necrosis factor on cells chronically infected with human immunodeficiency virus (HIV): enhancement of HIV replication. J Virol. 1989;63:2504–2509. doi: 10.1128/jvi.63.6.2504-2509.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minowada J, Ohnuma T, Moore G E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972;49:891. [PubMed] [Google Scholar]
- 26.Miura S, Ohtani K, Numata N, Niki M, Ohbo K, Ina Y, Gojobori T, Tanaka Y, Tozawa H, Nakamura M, Sugamura K. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type 1 transactivator p40tax. Mol Cell Biol. 1991;11:1313–1325. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyoshi I, Kubonishi I, Yoshimoto S, Shiraishi Y. A T-cell line derived from normal human cord leukocytes by coculturing with human leukemic T-cells. Jpn J Cancer Res. 1981;72:978–981. [PubMed] [Google Scholar]
- 28.Morimoto S, Kanno Y, Tanaka Y, Tokano Y, Hashimoto H, Jacquot S, Morimoto C, Schlossman S F, Yagita H, Okumura K, Kobata T. CD134L engagement enhances human B cell Ig production: CD154/CD40, CD70/CD27, and CD134/CD134L interactions coordinately regulate T cell-dependent B cell responses. J Immunol. 2000;164:4097–4104. doi: 10.4049/jimmunol.164.8.4097. [DOI] [PubMed] [Google Scholar]
- 29.Moriuchi H, Moriuchi M, Fauci A S. Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-1-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1. J Exp Med. 1998;187:1689–1697. doi: 10.1084/jem.187.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohshima Y, Tanaka, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 31.Ohshima Y, Yang L-P, Uchiyama T, Tanaka Y, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4+ T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 32.Okamoto T, Matsuyama T, Mori S, Hamamoto Y, Kobayashi N, Yamamoto N, Josephs S F, Wong-Staal F, Shimotohno K. Augmentation of human immunodeficiency virus type 1 gene expression by tumor necrosis factor α. AIDS Res Hum Retrovir. 1989;5:131–138. doi: 10.1089/aid.1989.5.131. [DOI] [PubMed] [Google Scholar]
- 33.Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pankow R, Durkop H, Latza U, Krause H, Kunzendorf U, Pohl T, Bulfone-Paus S. The HTLV-1 Tax protein transcriptionally modulates OX40 antigen expression. J Immunol. 2000;165:263–270. doi: 10.4049/jimmunol.165.1.263. [DOI] [PubMed] [Google Scholar]
- 35.Poli G, Bressler P, Kinter A, Duh E, Timmer W C, Rabson A, Justement J S, Stanley S, Fauci A S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor α by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross E K, Buckler-White A J, Rabson A B, Englund G, Martin M A. Contribution of NF-κB and Sp1 binding motifs to the replicative capacity of human immunodeficiency virus type 1: distinct patterns of viral growth are determined by T-cell types. J Virol. 1991;65:4350–4358. doi: 10.1128/jvi.65.8.4350-4358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sousa A E, Chaves A F, Doroana M, Antunes F, Victorino R M M. Early reduction of the over-expression of CD40L, OX40 and Fas on T cells in HIV-1 infection during triple anti-retroviral therapy: possible implications for lymphocyte traffic and functional recovery. Clin Exp Immunol. 1999;116:307–315. doi: 10.1046/j.1365-2249.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki Y, Koyanagi Y, Tanaka Y, Murakami T, Misawa N, Maeda N, Kimura T, Shida H, Hoxie J A, O'Brien W A, Yamamoto N. Determinant in human immunodeficiency virus type 1 for efficient replication under cytokine-induced CD4+ T-helper 1 (Th1)- and Th2-type conditions. J Virol. 1999;73:316–324. doi: 10.1128/jvi.73.1.316-324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo J, Beck Z, Csoman E, Liu X, Andriko I, Kiss J, Basci A, Ebbesen P, Toth F D. Differential patterns of interaction between HIV type 1 and HTLV type 1 in monocyte-derived macrophages cultured in vitro: implications for in vivo coinfection with HIV type 1 and HTLV type 1. AIDS Res Hum Retrovir. 1999;15:1653–1666. doi: 10.1089/088922299309694. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Inoi T, Tozawa H, Yamamoto N, Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-1 (HTLV-I) Int J Cancer. 1985;36:549–555. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka Y, Yasumoto M, Nyunoya H, Ogura T, Kikuchi M, Shimotohno K, Shiraki H, Kuroda N, Shida H, Tozawa H. Generation and characterization of monoclonal antibodies against multiple epitopes on the C-terminal half of envelope gp46 of human T-cell leukemia virus type-1 (HTLV-1) Int J Cancer. 1990;46:675–681. doi: 10.1002/ijc.2910460421. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, Yoshida A, Tozawa H, Shida H, Nyunoya H, Shimotohno K. Production of a recombinant human T-cell leukemia virus type-1 trans-activator (tax1) antigen and its utilization for generation of monoclonal antibodies against various epitopes on the tax1 antigen. Int J Cancer. 1991;48:623–630. doi: 10.1002/ijc.2910480423. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, Koyanagi Y, Tanaka R, Kumazawa Y, Nishimura T, Yamamoto N. Productive and lytic infection of human CD4+ type 1 helper T cells with macrophage-tropic human immunodeficiency virus type 1. J virol. 1997;71:465–470. doi: 10.1128/jvi.71.1.465-470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tozawa H, Andoh S, Takayama Y, Tanaka Y, Lee B, Nakamura H, Hayami M, Hinuma Y. Species-dependent antigenicity of the 34-kDa glycoprotein found on the membrane of various primate lymphocytes transformed by human T-cell leukemia virus type-1 (HTLV-1) and simian T-cell leukemia virus (STLV-I) Int J Cancer. 1988;41:231–238. doi: 10.1002/ijc.2910410213. [DOI] [PubMed] [Google Scholar]
- 45.Tsunetsugu-Yokota Y, Kato T, Yasuda S, Matsuda Z, Suzuki Y, Koyanagi Y, Yamamoto N, Akagawa K, Cho M W, Takemori T. Transcriptional regulation of HIV-1 LTR during antigen-dependent activation of primary T cells by dendritic cells. J Leukoc Biol. 2000;67:432–440. doi: 10.1002/jlb.67.3.432. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Kim Y-J, Bick C, Kim S H, Kwon B S. The potential roles of 4-1BB costimulation in HIV type 1 infection. AIDS Res Hum Retrovir. 1998;14:223–231. doi: 10.1089/aid.1998.14.223. [DOI] [PubMed] [Google Scholar]
- 47.Zack J A, Cann A J, Lugo J P, Chen I S. HIV-1 production from infected peripheral blood T cells after HTLV-1 induced mitogenic stimulation. Science. 1988;240:1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]