Abstract
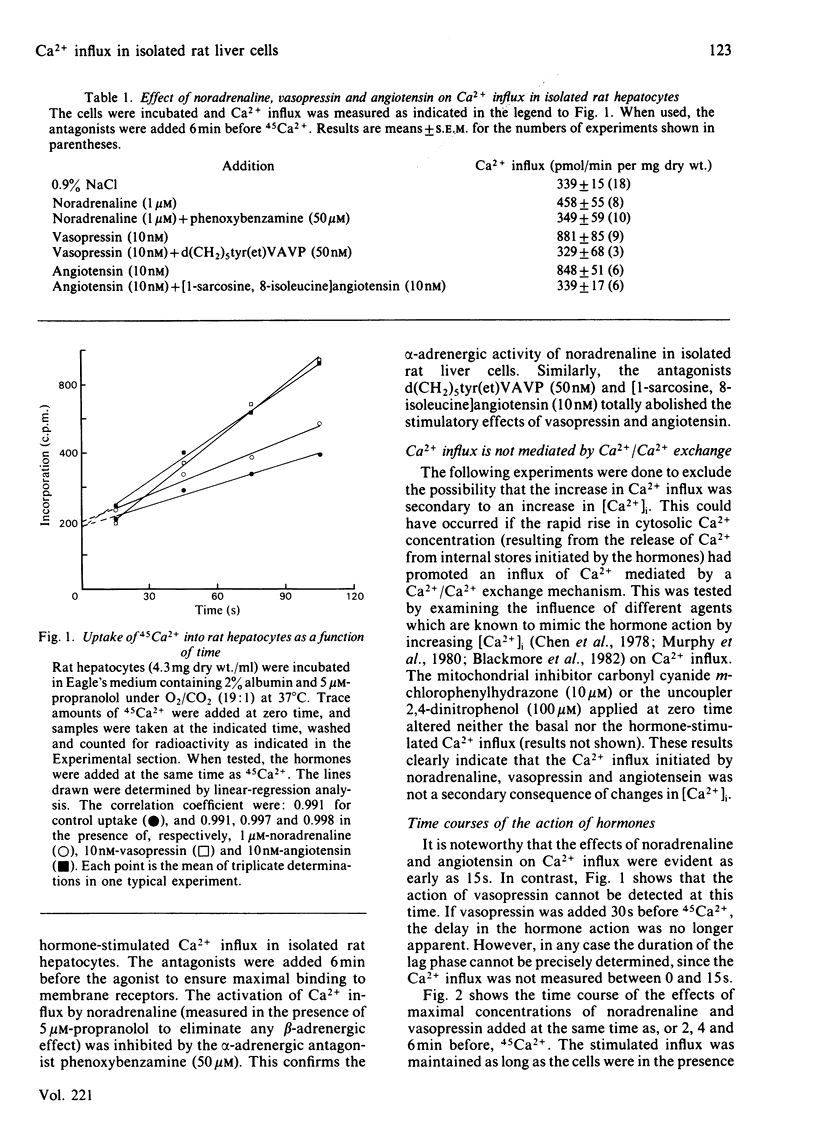
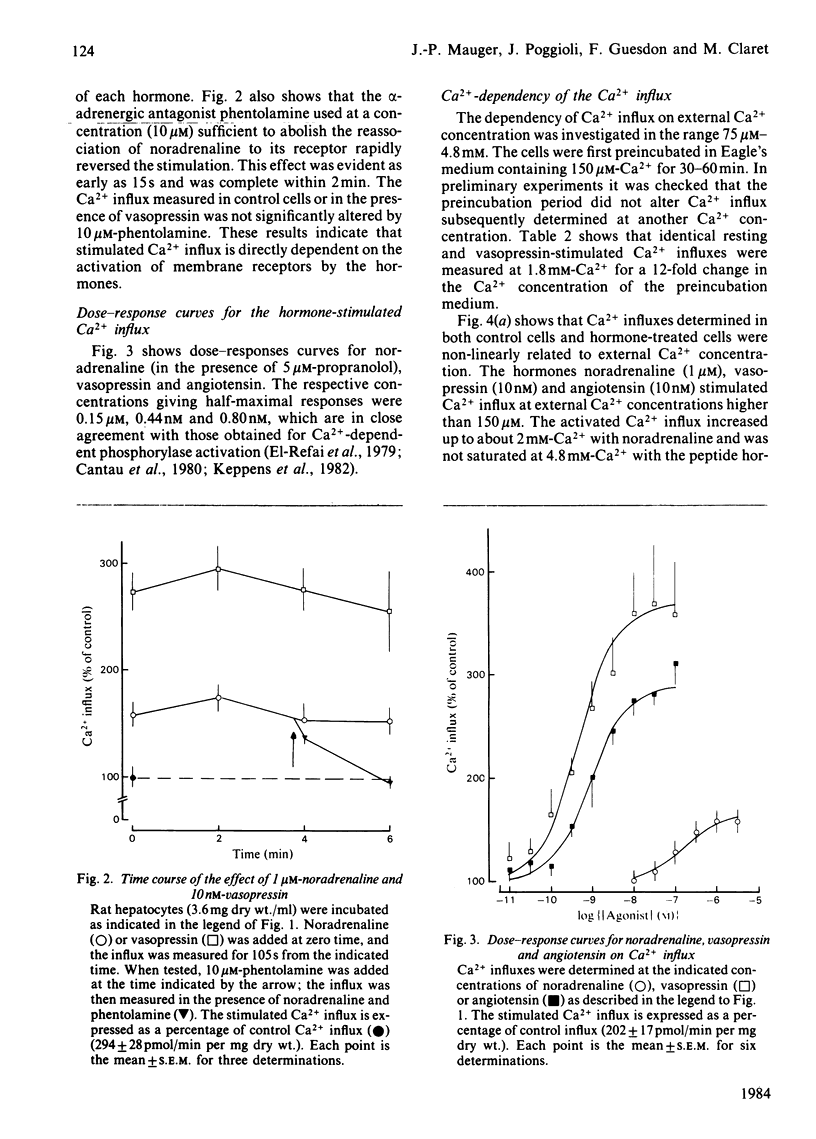
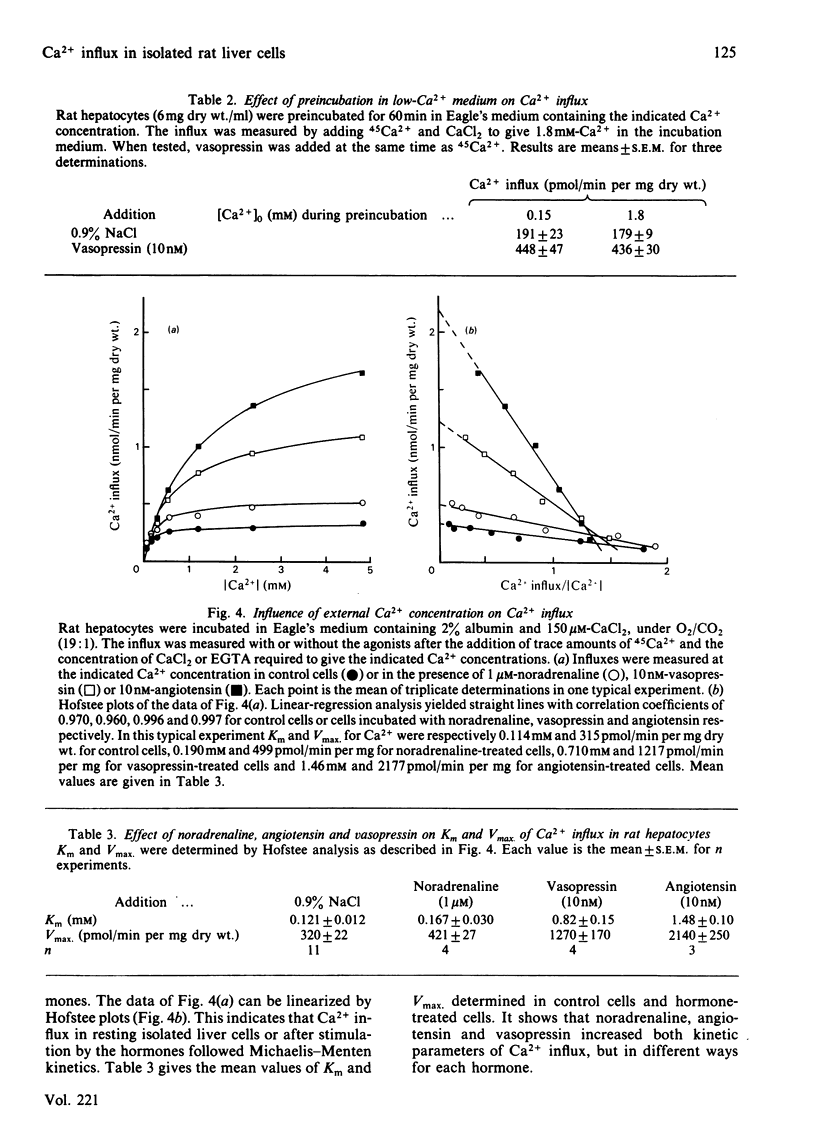
The effects of the Ca2+-mobilizing hormones noradrenaline, vasopressin and angiotensin on the unidirectional influx of Ca2+ were investigated in isolated rat liver cells by measuring the initial rate of 45Ca2+ uptake. The three hormones increased Ca2+ influx, with EC50 values (concentrations giving half-maximal effect) of 0.15 microM, 0.44 nM and 0.8 nM for noradrenaline, vasopressin and angiotensin respectively. The actions of noradrenaline and angiotensin were evident within seconds after their addition to the cells, whereas the increase in Ca2+ influx initiated by vasopressin was slightly delayed (by 5-15s). The activation of Ca2+ influx was maintained as long as the receptor was occupied by the hormone. The measurement of the resting and hormone-stimulated Ca2+ influxes at different external Ca2+ concentrations revealed Michaelis-Menten-type kinetics compatible with a saturable channel model. Noradrenaline, vasopressin and angiotensin increased both Km and Vmax. of Ca2+ influx. It is proposed that the hormones increase the rate of translocation of Ca2+ through a common pool of Ca2+ channels without changing the number of available channels or their affinity for Ca2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Barritt G. J., Parker J. C., Wadsworth J. C. A kinetic analysis of the effects of adrenaline on calcium distribution in isolated rat liver parenchymal cells. J Physiol. 1981 Mar;312:29–55. doi: 10.1113/jphysiol.1981.sp013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Shuman E. A., Exton J. H. alpha-Adrenergic activation of phosphorylase in liver cells involves mobilization of intracellular calcium without influx of extracellular calcium. J Biol Chem. 1982 Jan 10;257(1):190–197. [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in cell cultures. 3. Effects of calcium and parathyroid hormone in kidney cells. J Gen Physiol. 1970 Feb;55(2):163–186. doi: 10.1085/jgp.55.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret-Berthon B., Claret M., Mazet J. L. Fluxes and distribution of calcium in rat liver cells: kinetic analysis and identification of pools. J Physiol. 1977 Nov;272(3):529–552. doi: 10.1113/jphysiol.1977.sp012058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M., Mazet J. L. Ionic fluxes and permeabilities of cell membranes in rat liver. J Physiol. 1972 Jun;223(2):279–295. doi: 10.1113/jphysiol.1972.sp009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Refai M. F., Blackmore P. F., Exton J. H. Evidence for two alpha-adrenergic binding sites in liver plasma membranes. Studies with [3H]epinephrine and [3H]dihydroergocryptine. J Biol Chem. 1979 Jun 10;254(11):4375–4386. [PubMed] [Google Scholar]
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. Effects of noradrenaline on potassium reflux, membrane potential and electrolyte levels in tissue slices prepared from guinea-pig liver. J Physiol. 1972 Sep;225(3):721–750. doi: 10.1113/jphysiol.1972.sp009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y., Iwasa Y., Higashi K., Matsui K., Miyamoto E. Demonstration of a high affinity Ca2+ ATPase in rat liver plasma membranes. Biochem Biophys Res Commun. 1982 Mar 30;105(2):488–494. doi: 10.1016/0006-291x(82)91461-9. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- Keppens S., De Wulf H., Clauser P., Jard S., Morgat J. L. The liver angiotensin receptor involved in the activation of glycogen phosphorylase. Biochem J. 1982 Dec 15;208(3):809–817. doi: 10.1042/bj2080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Kondo S., Schulz I. Ca++ fluxes in isolated cells of rat pancreas. effect of secretagogues and different Ca++ concentrations. J Membr Biol. 1976 Oct 20;29(1-2):185–203. doi: 10.1007/BF01868959. [DOI] [PubMed] [Google Scholar]
- Lotersztajn S., Hanoune J., Pecker F. A high affinity calcium-stimulated magnesium-dependent ATPase in rat liver plasma membranes. Dependence of an endogenous protein activator distinct from calmodulin. J Biol Chem. 1981 Nov 10;256(21):11209–11215. [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Parker J. C., Barritt G. J., Wadsworth J. C. A kinetic investigation of the effects of adrenaline on 45Ca2+ exchange in isolated hepatocytes at different Ca2+ concentrations, at 20 degrees C and in the presence of inhibitors of mitochondrial Ca2+ transport. Biochem J. 1983 Oct 15;216(1):51–62. doi: 10.1042/bj2160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer R. K., Borle A. B. Sex difference in cellular calcium metabolism of rat hepatocytes and in alpha-adrenergic activation of glycogen phosphorylase. Biochim Biophys Acta. 1983 Apr 5;762(2):302–314. doi: 10.1016/0167-4889(83)90085-x. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Hoek J. B. Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta. 1981 Dec 30;639(3-4):243–295. doi: 10.1016/0304-4173(81)90012-4. [DOI] [PubMed] [Google Scholar]



