Key Teaching Points.
-
•
Recurrent vasovagal syncope is a widespread condition. The norepinephrine reuptake seems to have a role in the mechanism involved in vasovagal syncope.
-
•
Atomoxetine 40 twice a day could be a possible medical treatment to reduce the frequency of asystole spells in patients suffering from recurrent vasovagal syncope.
-
•
Vasovagal sleep syncope is a rare condition and atomoxetine could have a role in the treatment of this condition.
Introduction
Recurrent vasovagal syncope (VVS) is a widespread condition that reduces quality of life, sleep syncope is an uncommon and underdiagnosed presentation of VVS. Medical therapies are often contraindicated in older patients and the effectiveness of cardiac pacing is undetermined. Promising results are from the use of norepinephrine transporter (NET) inhibition that increases synaptic norepinephrine. Atomoxetine, a highly selective NET inhibitor, is used to treat attention deficit disorder and has shown efficacy in reducing episodes of syncope.1,2 We describe a case of sleep syncope in which syncope and asystole resolved with the NET inhibitor atomoxetine.
Case report
History of presentation
A 70-year-old patient presented with a history of worsening recurrent VVS. The syncope began in 2019, and they fainted 11 times in the first year, with as many as 9 presyncopes per day. The events usually occurred while the patient was sitting quietly, with a prodrome of lightheadedness, nausea, warmth, and sweatiness, and lasting several minutes. When they stood up and tried to walk, the worsened orthostatic stress frequently culminated in unconsciousness for up to 60 seconds. When the patient regained consciousness, they were pale and diaphoretic.
In April 2020, the patient reported episodes differing from the previous episodes. During 3 spells, the patient fainted while sitting quietly, and in 1 spell the patient was supine in bed at night. There was a sudden onset of an episode of presyncope with a sensation of warmth, followed by syncope. This was witnessed by a highly observant partner. The partner was alarmed, insisting that other episodes of syncope had also occurred while the patient was asleep, characterized by disturbed breathing and brief unresponsiveness.
Past medical history
The comorbidities included dyslipidemia, prior cigarette smoking, and hypertension. The patient’s only medication was irbesartan 150 mg daily.
Physical examination and baseline investigations
Physical examination was unremarkable other than an elevated blood pressure of 150/95 mm Hg. The baseline electrocardiogram, transthoracic echocardiogram, ambulatory blood pressure monitor, and 24-hour Holter monitors were all normal. In the absence of an indication, the atropine test was not performed on this patient.
Differential diagnosis
The differential diagnosis included cardiac syncope owing to a cardiac arrhythmia and vasovagal sleep syncope.
Investigations
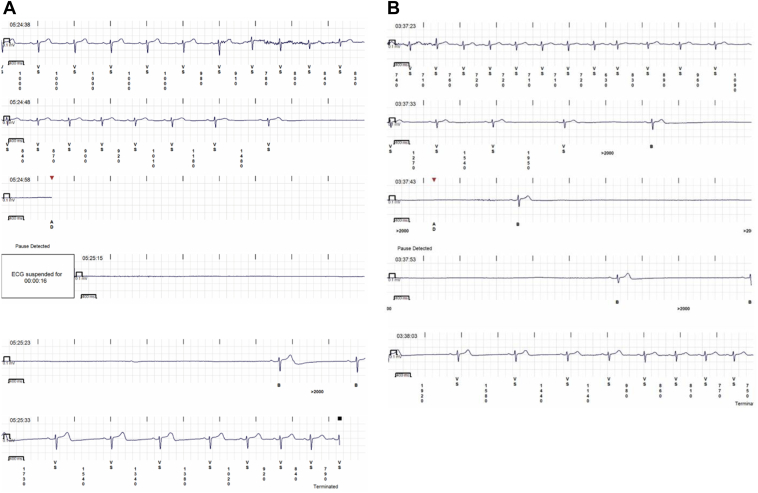
The history of nocturnal syncope raised concerns about a possible cardiac arrhythmia, and because of the high recurrence of spells no other tests were performed and a cardiac monitoring was proposed. The patient received an implantable loop recorder (ILR). One day after ILR insertion, the patient awoke at 5:24 AM with the characteristic prodrome and either lost consciousness or fell asleep. The ILR recorded a 35.6-second asystolic pause preceded by sinus slowing and followed by sinus bradycardia that progressively accelerated back to a normal rate (Figure 1A). This fits the European Heart Rhythm Association (EHRA) diagnostic criteria for vagally mediated asystole. A diagnosis of vasovagal sleep syncope was established.
Figure 1.
A: A 35.6-second asystole on day 1 of implantable loop recorder (ILR) monitoring. B: A 27-second asystole on day 9 of ILR monitoring.
The diagnosis was performed based on typical history and confirmed with our evidence-based criteria in the Calgary Syncope Symptom Score.
Management
Increased fluid and salt, fludrocortisone, and midodrine were contraindicated by the patient’s hypertension. Because of the high recurrence of spells, history of hypertension, and the documented asystolic events, we proposed to implant a pacemaker. The patient repeatedly declined the opportunity to receive a permanent cardiac pacemaker. Current evidence does not support neuroablation in patients older than 60; until now all studies of neuroablation have had a mean population younger than 60. Nonetheless, we did offer to arrange for this procedure to be done and the patient declined. Empiric treatment with atomoxetine was suggested and was chosen by the patient.
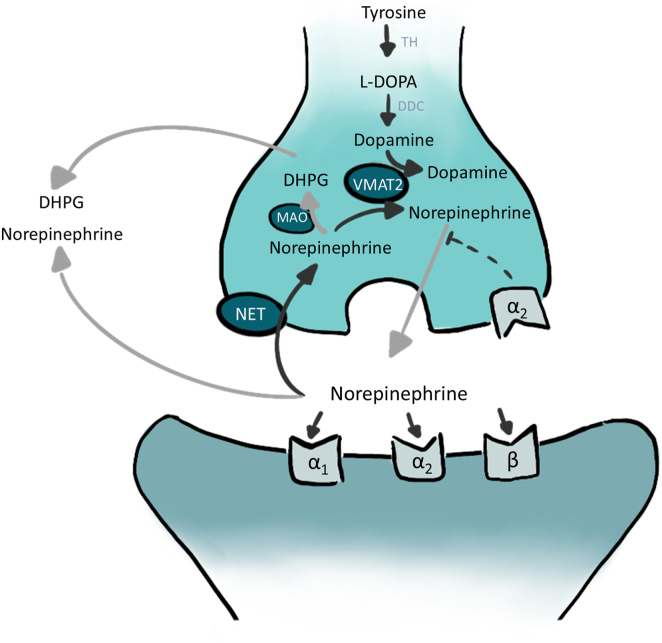
Atomoxetine is a norepinephrine transporter (NET) inhibitor (Figure 2) and, like other NET inhibitors, prevents VVS induced by head-up tilt table testing.3 Atomoxetine was associated with the suppression of VVS in case report series1 and suppressed VVS on tilt tests because it suppressed vagally mediated asystole.2 It has a pharmacokinetic half-life of 5–21 hours, depending on cytochrome CYP2D6 protein alleles, which determine the rate of atomoxetine metabolism.
Figure 2.
Norepinephrine transporter inhibitor mechanism of action. In the sinus node sympathetic synapse blocking norepinephrine reuptake increases intrasynaptic norepinephrine levels. Alpha and beta symbols denote subtypes of adrenergic receptors. DHPG = dihydroxyphenylglycol; L-DOPA = L-3,4 dihydroxyphenylalanine; MAO = monamine oxidase; NET = norepinephrine transporter; VMAT 2 = vesicular monoamine transporter 2.
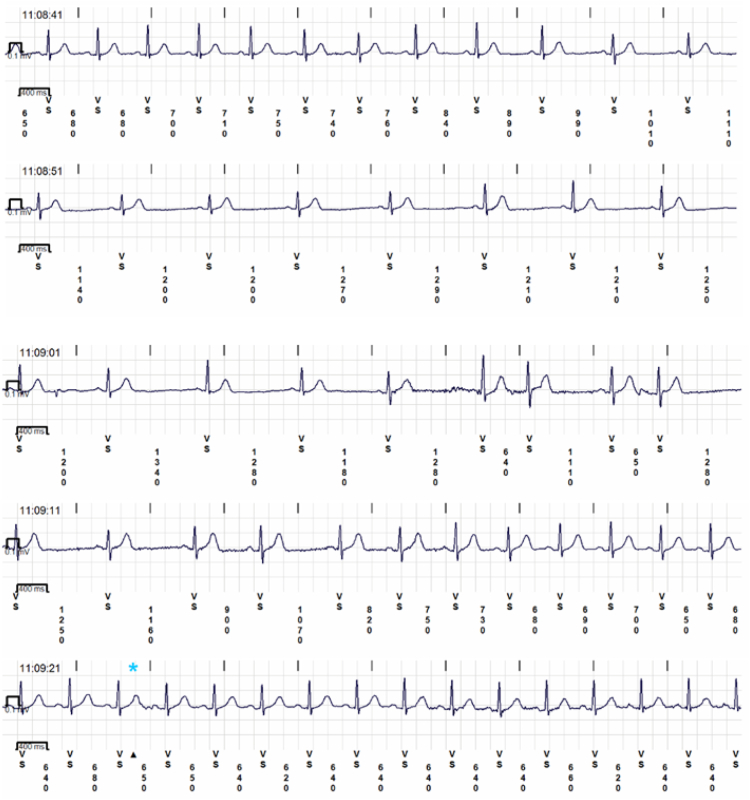
Two days later the patient started receiving atomoxetine 40 mg each morning. Six days thereafter an ILR recording was triggered by an asystolic event while the patient was asleep at 3:37 AM. It was a 27-second pause interrupted by 2 isolated beats (Figure 1B). No symptoms were reported by the patient, who was asleep at the time. The atomoxetine dose was then increased to 40 mg twice a day (bid). The patient was followed for another 124 days, during which time there was no sleep syncope and there were only 2 events in which the patient awoke with characteristic presyncopal prodromes, but did not faint. There were 2 nighttime transmissions with sinus node asystole lasting 5 and 6 seconds, and 2 nighttime transmissions of Mobitz I second-degree atrioventricular (AV) block and brief high-degree AV block. There were 14 awake presyncopal events, with no sinus node asystole and 1 awake presyncope associated with Mobitz I second-degree AV block and brief high-degree AV block. The awake presyncope had mean sinus rhythm rates of 52 ± 11 beats/min (range 40–75 beats/min). There were no auto-transmissions of rates below 40 beats/min (Table 1, Figure 3). The patient has had no clinical syncope and no nocturnal symptomatic or asystolic spells in the 125 days since beginning atomoxetine 40 mg bid.
Table 1.
Symptoms and implantable loop recorder recordings
| Day | State | Symptoms | Trough rhythm and rate | Therapy |
|---|---|---|---|---|
| 0 | ILR implanted | Sinus rhythm | None | |
| 1 | Sleep | Sleep syncope | 35.6 s pause | None |
| 9 | Sleep | Asleep | 27 s pause | Atomoxetine 40 mg daily |
| 10 | Awake | Presyncope | 40 bpm for 16 s | Atomoxetine 40 mg bid |
| 32 | Awake | Presyncope | 40 bpm for 30 s | Atomoxetine 40 mg bid |
| 33 | Awake | Presyncope ×3 | 50 bpm 50 s | Atomoxetine 40 mg bid |
| 34 | Awake | Presyncope | 42 bpm 42 s | Atomoxetine 40 mg bid |
| 70 | Awake | Presyncope | 75 bpm 30 s | Atomoxetine 40 mg bid |
| 72 | Awake | Presyncope | 60 bpm 30 s | Atomoxetine 40 mg bid |
| 73 | Awake | Presyncope | 54 bpm 20 s | Atomoxetine 40 mg bid |
| 112 | Awake | Presyncope | 33 bpm | Atomoxetine 40 mg bid |
| 114 | Asleep | Asleep | 5 s sinus pause | Atomoxetine 40 mg bid |
| 114 | Asleep | Asleep | 6 s sinus pause | Atomoxetine 40 mg bid |
| 129 | Awake | Presyncope ×3 | Mobitz 1 2nd degree AV block | Atomoxetine 40 mg bid |
| 131 | Asleep | Presyncope ×2 | 10 s 2nd degree AV block | Atomoxetine 40 mg bid |
| 133 | Awake, supine | Presyncope | 2nd degree AV block | Atomoxetine 40 mg bid |
AV = atrioventricular; bid = twice a day; bpm = beats per minute; ILR = implantable loop recorder.
The suppression of vagal asystole was associated with atomoxetine 40 bid (P = .032, Fisher exact). There was no sleep syncope and a reduction in asystole while taking atomoxetine 40 mg bid.
Figure 3.
Sinus bradycardia while taking atomoxetine 40 mg twice daily.
Discussion
This is the first clinical case report that directly illustrates the novel uses of atomoxetine to suppress the terminal bradycardia of VVS. VVS is a common clinical condition that causes trauma and reduces the quality of life, yet it has few effective medical therapies. Both fludrocortisone4 and midodrine5 prevent recurrent syncope, but their use is limited by hypertension, heart failure, urinary retention, and glaucoma. Cardiac pacing may be effective in older patients with VVS and asystole but is invasive and can have complications.
The current concept of VVS is that it is a cascade of reflexes, generally beginning with a failure of venous return to adequately maintain cardiac preload and culminating in triggering of cardioinhibition. Within a few seconds cardiac output and blood pressure plummet, causing syncope.6 The role of arterial vasodilation remains contentious.
Atomoxetine is an NET inhibitor that is used to treat attention deficit hyperactivity disorder, with few side effects and contraindications. The contraindications include hypersensitivity to atomoxetine or any components, association with monoamine oxidase inhibitors, glaucoma, pheochromocytoma, and severe cardiovascular disease.7 Norepinephrine is released at neuronal synapses and is cleared by either diffusion or reuptake through active transport into terminals by the presynaptic NET, which recaptures as much as 90% of released norepinephrine in the sinus node synaptic clefts,8 likely owing to the narrow width of the cleft. This decreases intrasynaptic norepinephrine (Figure 2). The NET inhibition leads to a tachycardic response; a central sympatholytic action in the brain, which tends to lower pressure; and a stimulatory effect in the periphery, which tends to raise pressure.8,9 Under stress, NET inhibition predominantly causes sinus tachycardia rather than vascular effects. This may be owing to the narrower synaptic clefts in the sinus node than in the vasculature. The Prevention of Syncope Trial VI (POST 6) reported that atomoxetine prevented the terminal profound bradycardia and the conversion of vasovagal presyncope to VVS during drug-free head-up tilt table testing.2
This case report directly links the unusual pharmacodynamic effect of atomoxetine seen in an acute-phase tilt test study to clinical use. It is interesting especially because in this case we have the monitoring that reveals the absence of pause after the beginning of the full-dose therapy, compared to previous studies where just a reduction of symptoms or the hemodynamic changes during a tilt test were seen.1,2 This case was unique because the patient was monitored with an ILR.
This case report directly links the unusual pharmacodynamic effect of atomoxetine seen in an acute-phase tilt test study to clinical use. The effect was seen in a patient with sleep syncope, an uncommon variant of VVS occurring in about 5% of patients with VVS. Sleep syncope is a loss of consciousness in a nonintoxicated adult occurring during sleep. It is often associated with vagal symptoms such as nausea, vomiting, cramps, and diarrhea. Often the patient awakens, arises, and faints, but in some patients, syncope (defined by a lack of capacity for arousal) can occur while asleep.10,11
In older patients, traditional dietary and medical therapies for VVS are often contraindicated by hypertension, and pacemakers may be declined. The use of atomoxetine could be a possible therapy for patients suffering from sleep syncope, and from VVS more generally.
During the follow-up, there was no further syncope, even if the patients suffered from some presyncope episodes. This is related to results from POST 6, which showed an almost complete suppression of asystole but not sinus bradycardia. That suggests that with this therapy a complete absence of symptoms is unlikely but more studies are needed to evaluate it. A placebo-controlled randomized clinical trial (POST 7) is testing the effectiveness of atomoxetine patients suffering from recurrence VVS in preventing VVS (NCT05159687).12
Conclusion
This report is the first to directly link the clinical effectiveness of atomoxetine with its unusual ability to prevent vagally mediated asystole. It appears to suppress both sleep syncope and vagally mediated sinus node asystole. POST 7, a randomized controlled trial that tests whether atomoxetine suppresses VVS, is funded by the Canadian Institutes of Health Research and is underway (NCT05159687).12
Disclosures
The authors have no conflicts to disclose. The Canadian Institutes of Health Research is funding the related Prevention of Syncope Trial 7 (NCT05159687).
Acknowledgments
Funding Sources
None.
References
- 1.Sheldon R., Parkash R., Sandhu R., Seifer C., Hamzeh R., Raj S. Atomoxetine for suppression of vasovagal syncope: a clinical case series. Can J Cardiol. 2022;38:S137. doi: 10.1007/s10286-022-00905-x. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon R.S., Lei L., Guzman J.C., et al. A proof of principle study of atomoxetine for the prevention of vasovagal syncope: the Prevention of Syncope Trial VI. Europace. 2019;21:1733–1741. doi: 10.1093/europace/euz250. [DOI] [PubMed] [Google Scholar]
- 3.Lei L.Y., Raj S.R., Sheldon R.S. Pharmacological norepinephrine transporter inhibition for the prevention of vasovagal syncope in young and adult subjects: a systematic review and meta-analysis. Heart Rhythm. 2020;17:1151–1158. doi: 10.1016/j.hrthm.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheldon R., Raj S.R., Rose M.S., et al. Fludrocortisone for the prevention of vasovagal syncope a randomized, placebo-controlled trial. J Am Coll Cardiol. 2016;68:1–9. doi: 10.1016/j.jacc.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Lei L.Y., Raj S.R., Sheldon R.S. Midodrine for the prevention of vasovagal syncope: a systematic review and meta-analysis. Europace. 2022;24:1171–1178. doi: 10.1093/europace/euab323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dijk J.G., van Rossum I.A., Thijs R.D. The pathophysiology of vasovagal syncope: novel insights. Auton Neurosci. 2021;236 doi: 10.1016/j.autneu.2021.102899. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration (FDA) approved product information. Revised January. US National Library of Medicine; 2022. Pharmacology of drugs used to treat attention deficit hyperactivity disorder in children and adolescents. Strattera (atomoxetine) capsules for oral use.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/021411s050lbl.pdf [Google Scholar]
- 8.Schroeder C., Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;303:H1273–H1282. doi: 10.1152/ajpheart.00492.2012. [DOI] [PubMed] [Google Scholar]
- 9.Strempel S., Schroeder C., Hemmersbach R., et al. Norepinephrine transporter inhibition alters the hemodynamic response to hypergravitation. J Appl Physiol. 2008;104:756–760. doi: 10.1152/japplphysiol.01128.2007. [DOI] [PubMed] [Google Scholar]
- 10.Jardine D.L., Davis J., Frampton C.M., Wieling W. Sleep syncope: a prospective cohort study. Clin Auton Res. 2022;32:19–27. doi: 10.1007/s10286-021-00842-1. [DOI] [PubMed] [Google Scholar]
- 11.Jardine D.L., Krediet C.T.P., Cortelli P., Wieling W. Fainting in your sleep? Clin Auton Res. 2006;16:76–78. doi: 10.1007/s10286-006-0314-y. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu R.K., Raj S.R., Hamzeh R., Sheldon R.S. The Seventh Prevention of Syncope Trial (POST VII)—a randomized clinical trial of atomoxetine for the prevention of vasovagal syncope: rationale and study design. Am Heart J. 2023;262:49–54. doi: 10.1016/j.ahj.2023.04.012. [DOI] [PubMed] [Google Scholar]