Key Teaching Points.
-
•
Pre–lead extraction computed tomography scan can help to identify areas of significant calcium.
-
•
Calcium modification with intravascular lithotripsy seems to be safe and can reduce lead extraction time for heavily calcified leads.
-
•
ntravascular ultrasound can be used to show calcium fracturing and may help guide intravascular lithotripsy use on heavily calcified leads.
Introduction
Transvenous lead extraction is indicated in a variety of scenarios, with one of the strongest indications being implantable cardioverter-defibrillator (ICD) infection. Long dwell times, severe lead adhesions, and dense calcifications increase the difficulty and risks associated with transvenous lead extraction. Intravascular lithotripsy for heavily calcified leads may be used as an adjunctive technique for extraction of these high-complexity cases.1 We present a case successfully using intravascular lithotripsy for a heavily calcified 30-year-old ICD lead with intravascular ultrasound (IVUS) showing fracture of intravascular calcium post lithotripsy therapy. To our knowledge this is the first reported use of IVUS to demonstrate effectiveness of lithotripsy for lead extraction.
Case report
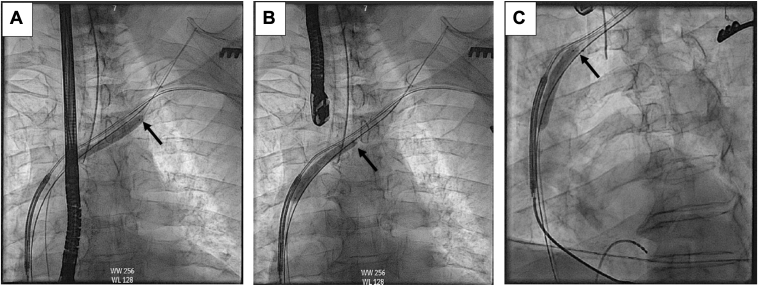
A 69-year-old male patient with history of nonischemic cardiomyopathy, left ventricular ejection fraction 35%, ventricular fibrillation arrest 30 years prior with an abdominal wall pocket, normally functioning dual-coil ICD lead (11.9F Endotak 0064; Guidant) was transferred for complex lead removal after development of ICD pocket infection following generator change despite an antibiotic pouch. Prior to transfer, once a plan for lead extraction had been made, the referring electrophysiologist debulked the pocket of foreign material by removing the current generator and cutting the header yoke. There was no expectation this would cure the infection, but it was done to better identify the causative bacteria and potentially limit systemic infection while awaiting transfer to our facility for lead extraction. The lead pin grew Enterococcus faecalis, and he was started on intravenous vancomycin. Chest radiography showed stable position of the lead (Figure 1A). Transesophageal echocardiogram showed a mobile echodensity attached to the lead in the middle of the right atrium, although this was suspected to be fibrin/thrombus given the clinical picture. Computed tomography (CT) scan revealed calcification surrounding the ICD lead from the first costosternal junction to the junction of the right brachiocephalic vein to the superior vena cava (Figure 1B and 1C). Owing to prolonged lead dwell time and heavy calcifications, intravascular lithotripsy-facilitated lead removal was pursued.
Figure 1.
A: Chest radiograph showing stable position of the implantable cardioverter-defibrillator (ICD) lead. B, C: Computed tomography scan showing areas of calcification (arrows) along the ICD lead.
The abdominal pocket was first opened by cardiothoracic surgery. The right ventricular lead was then freed from the shoulder access site, cut within the small access pocket, and pulled from the presumably sterile shoulder pocket into the abdominal pocket to reduce the chance of pocket infection if the lead was unable to be removed. A #2 locking stylet was placed into the right ventricular lead to the passive lead tip. IVUS was performed of the superior vena cava and brachiocephalic vein (Figure 2A) via the femoral vein, which showed an area of significant calcium encasing the lead. Intravascular lithotripsy (Shockwave Medical, Santa Clara, CA) was performed via the femoral vein with 7 mm balloon inflation between 2 and 6 atmospheres with a series of 60–90 pulses delivered at each of 3 areas of expected calcification based on CT scan (Figure 3). Repeat IVUS along areas of delivery of intravascular lithotripsy revealed diffuse fracture of the calcium, identified in Figure 2B at the same location by the change in artifact from the proximal coil and this being the last area of significant calcification visualized moving inferiorly. A 13F rotating dilator sheath (SubC; Philips, Amsterdam, Netherlands), then a 13F TightRail (Philips) with outer sheath use successfully freed the lead, with extraction time of 20 minutes following lithotripsy to free the lead. There were no complications following the procedure. A wound vac was placed and the patient was fitted with a LifeVest (Zoll, Chelmsford, MA) and is considering subcutaneous ICD or a right-sided cardiac resynchronization therapy defibrillator.
Figure 2.
A: Intravascular ultrasound for pre–intravascular lithotripsy showing dense calcification (white) with shadowing dropout from the lead (blue arrow). B: Post–intravascular lithotripsy showing fractures of the calcium (red arrows).
Figure 3.
Shockwave lithotripsy balloon placement. A: Distal balloon marker tip (arrow) at the clavicular head. The darker, shaded area where the balloon is inflated denotes the approximate area being treated with shockwave lithotripsy. The balloon is then deflated and drawn toward the superior vena cava for the second (B) and third (C) sites of shockwave intravascular lithotripsy delivery.
Discussion
Dense calcifications of ICD leads during lead extraction are most commonly addressed with mechanical cutting tools over laser sheaths, which may result in complications including vascular laceration and pericardial effusion. Intravascular lithotripsy is a promising adjunct for heavily calcified leads and works by creating acoustic pressure waves to fracture calcified lesions Although intravascular lithotripsy is used primarily in arterial beds, the safety and effectiveness likely translates to venous beds, as there has been shown to be safe acoustic propagation through soft tissue.2 This pretreatment calcium modification technique could significantly reduce the time required for lead extraction, particularly during the highest-risk phase of the procedure, which may take several hours using conventional methods. A retrospective study of 14 cases using intravascular lithotripsy as an adjunct for lead extraction for calcified lesions described an average of 26 fewer minutes spent actively extracting leads following intravascular lithotripsy pretreatment.1 This study also found no difference in procedure-related complications in conventional and intravascular lithotripsy–facilitated extraction groups. However, notably, there were only 51 patients included in the conventional arm and 14 patients included in the intravascular lithotripsy arm.1 If larger sample sizes were compared, it is possible that there may be a signal for improved safety in the intravascular lithotripsy arm, especially if lithotripsy may allow for less utilization of mechanical cutting tools, resulting in less trauma to the venous endothelium.
The use of IVUS-facilitated lithotripsy in this case resulted in no known trauma to the venous endothelium. Our general practice is to perform prophylactic snaring of dual-coil ICD leads >10 years old from the femoral vein before extraction, for inferior traction to reduce the risk of superior vena cava injury. Because this lead was very large (11.9F), we expected a more robust rail than most cases, allowing for greater traction. In addition, the binding sites were proximal based on CT/IVUS, and lithotripsy resulted in steady progress with extraction tools; thus we opted not to snare in this case. Snaring and lithotripsy may be complementary, and in subsequent cases we have found the deflectable sheath used for our snaring technique can first be used to direct the lithotripsy balloon closer to the targeted lead.
CT and IVUS may be beneficial imaging modalities in patients requiring complex lead removal techniques. CT scan was used in this case to identify areas of calcification that would benefit from lithotripsy. An association with severe lead adhesions seen on preprocedure CT has been associated with more complex procedures.3 Preprocedure CT scan can help the operator recognize higher-risk patients and plan accordingly. This case also demonstrated the use of IVUS to identify successful calcium fracture following intravascular lithotripsy. One prior study has used IVUS to identify intravascular lead adherence and develop a grading system that correlated with extraction difficulty.4 It may be advantageous to incorporate IVUS guidance to identify areas of lead adherence and calcification prior to lead extraction, which may surpass the utilization of CT scan to identify calcification prior to lead extraction and reduce radiation to the patient.
Although not performed in this case, another benefit of IVUS use includes sizing of the intravascular lithotripsy balloon 1:1 to the vessel undergoing calcium modification, as undersizing of the intravascular lithotripsy balloon results in less calcium fracture.2 It is unknown if the larger lithotripsy balloons (8–12 mm) available would be more effective for lead extraction, or if they could increase the risk for venous laceration, as the target is the lead binding sites rather than the entire vein. In our case, if no calcium fracture or inadequate calcium fracture were observed on repeat IVUS, additional lithotripsy could have been performed to targeted areas guided by IVUS. On the other hand, artifact related to leads and the Bridge wire (Philips) positioned in the left subclavian may reduce IVUS image quality compared to coronary use.
Although this case mainly used IVUS to identify calcium fracture, we believe IVUS can be a useful adjunct for clinical practice. Future studies may help to further delineate optimal utilization of IVUS as an adjunct to lead extraction with focus on the following: (1) identification of calcified areas with lead adherence for targeted intravascular lithotripsy use; (2) sizing of vessel prior to lithotripsy for optimal delivery of intravascular lithotripsy; and (3) potential delineation of vascular risk factors for vascular tear or injury.
There are potential limitations to use of intravascular lithotripsy. This case described adjunctive use of intravascular lithotripsy on a single ICD lead extraction. It is currently unknown if intravascular lithotripsy compromises lead integrity or function, which will need to be further investigated if this technique is to be used where a functional lead will remain.1 Another issue that will need to be clarified is the utilization of intravascular lithotripsy in the setting of high-grade central venous stenosis. It may be possible that the combination of vascular stress of dilation, intravascular lithotripsy, traction, and cutting tools may predispose to venous lacerations.1 The skeletal adverse effects from lithotripsy are unknown, and there is only 1 case report of vertebral fracture associated with lithotripsy.5 It is possible that an adverse effect of lithotripsy may be remodeling of the clavicular bone following lithotripsy; however, there are no reports of rib fracture with use of this technology in the coronary arteries, making this a less likely concern.
Conclusion
To our knowledge, this is the first published case of the use of IVUS showing intravenous calcium fractures following intravascular lithotripsy. Intravascular lithotripsy, potentially in conjunction with IVUS, shows promise for continuing to make lead extraction faster and safer for patients by decreasing the amount of time spent during the highest-risk phase of lead extraction.
FundingSources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: The authors have no conflicts of interest relative to this work to disclose.
References
- 1.Latanich C.A., Anderson J.A. Shockwave intravascular lithotripsy facilitated transvenous lead extraction. JACC Clin Electrophysiol. 2023;9:1585–1592. doi: 10.1016/j.jacep.2023.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Kereiakes D.J., Virmani R., Hokama J.Y., et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC Cardiovasc Interv. 2021;14:1275–1292. doi: 10.1016/j.jcin.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 3.Svennberg E., Jacobs K., McVeigh E., Pretorius V., Birgersdotter-Green U. Computed tomography-guided risk assessment in percutaneous lead extraction. JACC Clin Electrophysiol. 2019;5:1439–1446. doi: 10.1016/j.jacep.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaser A.D., Aziz Z., Besser S.A., et al. Characterization of lead adherence using intravascular ultrasound to assess difficulty of transvenous lead extraction. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007726. [DOI] [PubMed] [Google Scholar]
- 5.Kazimoglu H., Mungan M.U., Kirkali Z. Vertebral fracture associated with shockwave lithotripsy in a patient with granulomatous spondylitis. J Endourol. 2001;15:687–689. doi: 10.1089/08927790152596244. [DOI] [PubMed] [Google Scholar]