Abstract
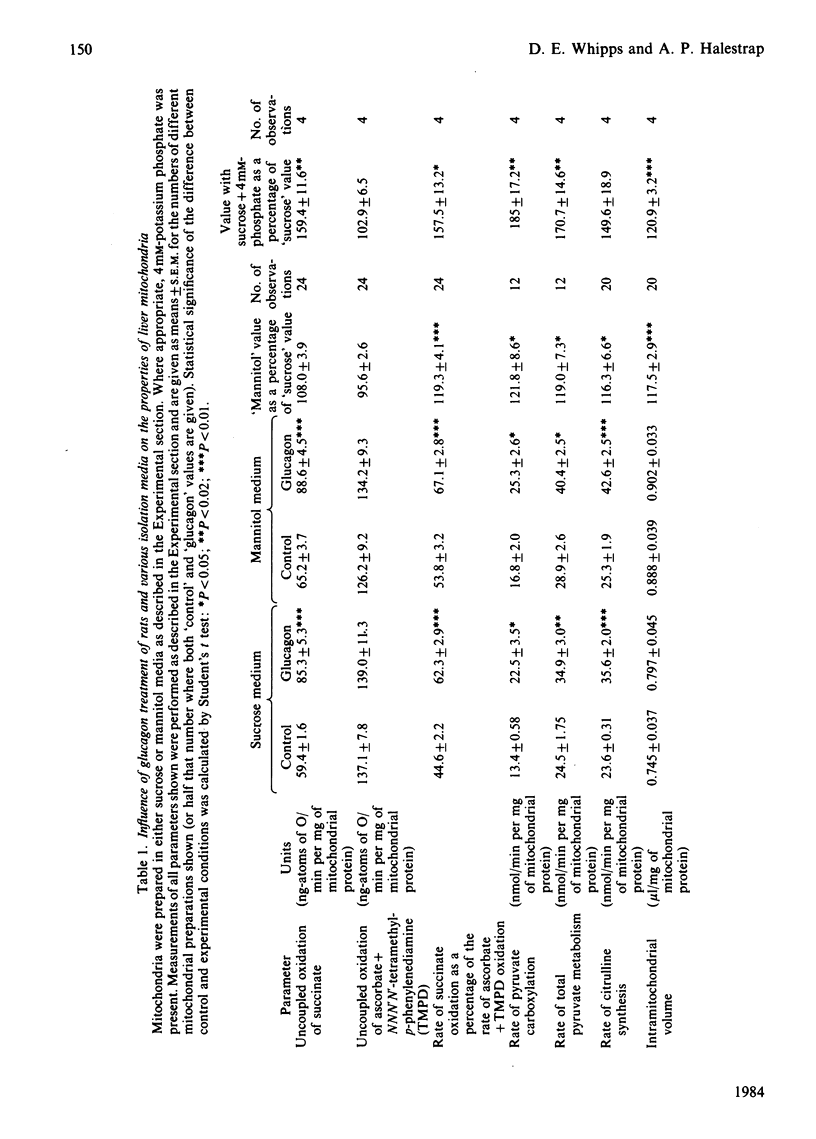
Liver mitochondria isolated from glucagon-treated rats by using both mannitol- and sucrose-based media showed enhanced uncoupled succinate oxidation, pyruvate metabolism and citrulline synthesis. Mitochondria prepared in mannitol medium showed some stimulation of these parameters compared with those prepared in sucrose medium. This was accompanied by an increase in matrix volume of about 20%. Some [14C]mannitol became permanently associated with mitochondria during preparation. It is suggested that mannitol may enter mitochondria during their preparation and cause swelling. The presence of 4mM-phosphate in the sucrose isolation medium stimulated the same parameters as did glucagon treatment, and also caused an increase in matrix volume of about 20%. These results confirm the conclusion that the mitochondrial volume may be important in the regulation of mitochondrial metabolism. They contradict the conclusion of others [Siess (1983) Hoppe-Seyler's Z. Physiol. Chem. 364, 279-290, 835-838] that mannitol rather than sucrose should be used when studying hormonal effects on mitochondrial metabolism. Reasons for the discrepancies in the results between groups studying the effects of hormones on mitochondrial metabolism are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E. H., Chisholm A. B., Titheradge M. A. Hormonal stimulation of mitochondrial pyruvate carboxylation in filipin-treated hepatocytes. Biochem J. 1983 May 15;212(2):417–426. doi: 10.1042/bj2120417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armston A. E., Halestrap A. P., Scott R. D. The nature of the changes in liver mitochondrial function induced by glucagon treatment of rats. The effects of intramitochondrial volume, aging and benzyl alcohol. Biochim Biophys Acta. 1982 Sep 15;681(3):429–439. doi: 10.1016/0005-2728(82)90185-2. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Gutfreund H. The scope of moderate pressure changes for kinetic and equilibrium studies of biochemical systems. FEBS Lett. 1976 Dec 31;72(2):199–207. doi: 10.1016/0014-5793(76)80971-4. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Quinlan P. T. The intramitochondrial volume measured using sucrose as an extramitochondrial marker overestimates the true matrix volume determined with mannitol. Biochem J. 1983 Aug 15;214(2):387–393. doi: 10.1042/bj2140387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Stimulation of pyruvate transport in metabolizing mitochondria through changes in the transmembrane pH gradient induced by glucagon treatment of rats. Biochem J. 1978 Jun 15;172(3):389–398. doi: 10.1042/bj1720389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The nature of the stimulation of the respiratory chain of rat liver mitochondria by glucagon pretreatment of animals. Biochem J. 1982 Apr 15;204(1):37–47. doi: 10.1042/bj2040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman H. C., Haynes R. C., Jr Elevated intramitochondrial adenine nucleotides and mitochondrial function. Arch Biochem Biophys. 1983 May;223(1):85–94. doi: 10.1016/0003-9861(83)90574-x. [DOI] [PubMed] [Google Scholar]
- Hamman H. C., Haynes R. C., Jr Hormonal regulation of mitochondrial function. Description of a system capable of mimicking several effects of glucagon. Biochim Biophys Acta. 1983 Aug 31;724(2):241–250. doi: 10.1016/0005-2728(83)90143-3. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979 Jul;195(2):460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979 Jul;195(2):453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Jensen C. B., Sistare F. D., Hamman H. C., Haynes R. C., Jr Stimulation of mitochondrial functions by glucagon treatment. Evidence that effects are not artifacts of mitochondrial isolation. Biochem J. 1983 Mar 15;210(3):819–827. doi: 10.1042/bj2100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., McGivan J. D., Meijer A. J. The stimulation of glutamine hydrolysis in isolated rat liver mitochondria by Mg2+ depletion and hypo-osmotic incubation conditions. Biochem J. 1981 Jan 15;194(1):35–41. doi: 10.1042/bj1940035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan P. T., Thomas A. P., Armston A. E., Halestrap A. P. Measurement of the intramitochondrial volume in hepatocytes without cell disruption and its elevation by hormones and valinomycin. Biochem J. 1983 Aug 15;214(2):395–404. doi: 10.1042/bj2140395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivarao D., Sitaramam V. Studies on the non-linear osmotic pressure-volume relationship in mitochondria and entry of sucrose into the matrix space during centrifugation. Biochim Biophys Acta. 1983 Feb 17;722(2):256–270. doi: 10.1016/0005-2728(83)90072-5. [DOI] [PubMed] [Google Scholar]
- Siess E. A. Different actions of mono- and disaccharides on rat liver mitochondria. Hoppe Seylers Z Physiol Chem. 1983 Jul;364(7):835–838. doi: 10.1515/bchm2.1983.364.2.835. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Fahimi F. M., Wieland O. H. Evidence that glucagon stabilizes rather than activates mitochondrial functions in rat liver. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1643–1651. doi: 10.1515/bchm2.1981.362.2.1643. [DOI] [PubMed] [Google Scholar]
- Siess E. A. Influence of isolation media on the preservation of mitochondrial functions. Hoppe Seylers Z Physiol Chem. 1983 Mar;364(3):279–289. doi: 10.1515/bchm2.1983.364.1.279. [DOI] [PubMed] [Google Scholar]
- Sitaramam V., Sarma M. K. Gravitational field enhances permeability of biological membranes to sucrose: an experimental refutation of sucrose-space hypothesis. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3441–3445. doi: 10.1073/pnas.78.6.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven A. J., Hensgens H. E., Meijer A. J., Tager J. M. On the nature of the stimulation by glucagon of citrulline synthesis in rat-liver mitochondria. FEBS Lett. 1982 Apr 19;140(2):270–272. doi: 10.1016/0014-5793(82)80911-3. [DOI] [PubMed] [Google Scholar]


