Key Teaching Points.
-
•
We present the first case of an Aveir (Abbott, Abbott Park, IL) atrial leadless pacemaker (LP) to dislodge and pass through a patent foramen ovale (PFO) since the Aveir DR was approved by the US Food and Drug Administration. Our patient had postprocedural dislodgement into the left ventricular outflow tract the day after implantation. The only other reported case of atrial LP dislodgement through a PFO was discussed in the Aveir DR i2i IDE Study prior to US Food and Drug Administration approval.
-
•
Percutaneous snaring and retrieval was performed via a retrograde aortic approach from the femoral artery. A femoral cutdown was performed given the anticipated need for a 27F outer diameter (25F inner diameter) introducer sheath to accommodate the Aveir retrieval catheter; however, we discovered that it is technically feasible to insert this retrieval catheter through a smaller 25F outer diameter (22F inner diameter) sheath, albeit in an off-label approach. The Aveir retrieval catheter facilitates percutaneous retrieval by enabling counterclockwise rotation and unscrewing of the device.
-
•
The PFO was percutaneously closed, with subsequent successful reimplantation of an Aveir atrial LP. Further data are needed to determine whether to consider screening patients for the presence of a PFO and whether to consider empirically closing PFOs prior to atrial LP implantation.
Introduction
The Abbott Aveir DR (Abbott, Abbott Park, IL) is the first and only dual-chamber leadless pacemaker (LP) available for clinical use. The results of the first clinical trial using the Aveir DR were published on May 20, 2023.1 The device was approved by the US Food and Drug Administration (FDA) on July 5, 2023, and it became commercially available for implantation on November 6, 2023. We report a unique case of one of the first patients to receive a commercially implanted Aveir DR dual-chamber LP, whose atrial device dislodged through a patent foramen ovale (PFO) to the left ventricle, requiring percutaneous snaring and retrieval.
Case report
The patient is a 73-year-old man with a history of sinus pauses and intermittent complete heart block. Additional history included a PFO diagnosed 1 year prior after cryptogenic stroke, loop recorder implantation, hypertension, dyslipidemia, diabetes mellitus type II, obstructive sleep apnea, and aortic valve sclerosis. He underwent implantation of an Aveir VR single-chamber LP for complete heart block (Figure 1A and 1B). This device was chosen for this patient after FDA approval of the Aveir DR and shortly before commercial availability, with the intent of upgrading to a dual-chamber system. Postprocedure, he reported new intermittent symptoms of racing heart, lightheadedness, and flushing owing to ventricular pacing and atrioventricular dyssynchrony. Device interrogation 16 days postoperatively showed 21% ventricular pacing. He was admitted 17 days after his ventricular LP implantation for an upgrade to a dual-chamber LP with the addition of an Aveir AR atrial LP.
Figure 1.
Chest radiographs showing ventricular and atrial leadless pacemakers. Loop recorder is present. A, B: Posteroanterior (A) and lateral (B) views obtained after implantation of an Aveir VR (Abbott, Abbott Park, IL) single-chamber leadless pacemaker (arrows) in the right ventricle. C, D: Posteroanterior (C) and lateral (D) views obtained on postoperative day 1 after implantation of an Aveir AR (Abbott) atrial leadless pacemaker. The atrial leadless pacemaker is dislodged and located in the left ventricle (arrows).
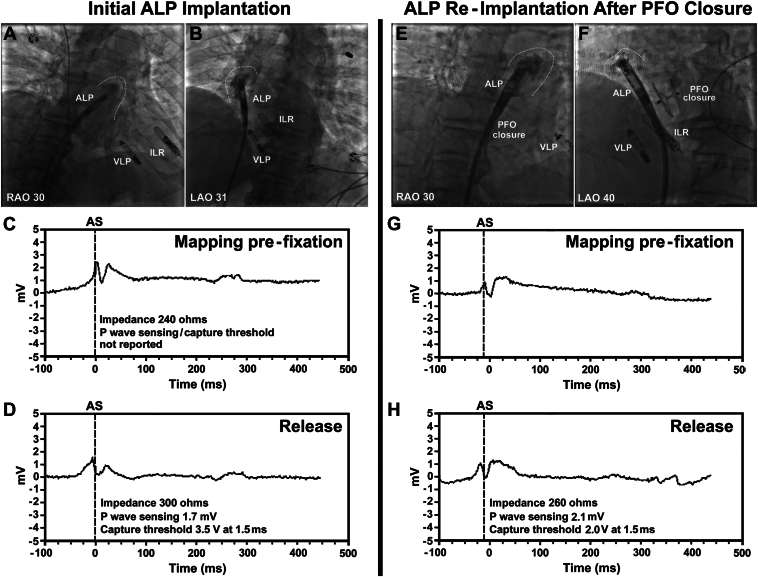
The delivery system was introduced through the right femoral vein. Iodinated contrast injection through a pigtail catheter in the right atrium verified the right atrial appendage location. The atrial LP was advanced into the right atrial appendage. Additional contrast injections through the protective sleeve and fluoroscopic anatomical landmarks confirmed appropriate atrial device placement (Figure 2A and 2B). The atrial LP was deployed at the anterior base of the right atrial appendage. A tension test confirmed the stability of the atrial device, and, after ensuring adequate current of injury, impedance, P-wave sensing, and capture threshold, the device was released (Figure 2C and 2D). The atrial and ventricular LPs were paired with implant-to-implant (i2i) communication. Intraprocedural device interrogation showed that the atrial device had a threshold of 3.5 V at 1.5 ms, sensing at 1.7 mV, and impedance of 300 ohms. The atrial capture threshold was anticipated to improve by the following day with resolution of tissue injury.
Figure 2.
Intraprocedural fluoroscopy images and measurements during initial implantation of an Aveir AR (Abbott, Abbott Park, IL) atrial leadless pacemaker (ALP), and subsequent ALP reimplantation after patent foramen ovale (PFO) closure. A, B: Initial ALP implantation. Contrast injection through the protective sleeve highlights the inner border of the right atrial appendage (dashed lines). The ALP was deployed at the anterior base of the right atrial appendage. The following day, this device was dislodged to the left ventricle. C, D: Contact mapping can assess current of injury via intracardiac electrograms (images), impedance, sensing amplitudes, and pacing capture thresholds during stages of index implantation. E, F: ALP reimplantation after PFO closure. The ALP was deployed at the anterior base of the right atrial appendage in a similar location to prior. G, H: Current of injury and measurements during reimplantation. ILR = implantable loop recorder; LAO = left anterior oblique; RAO = right anterior oblique; VLP = ventricular leadless pacemaker.
The following morning, a routine postprocedure chest radiograph showed that the atrial LP was dislodged and located in the ventricle (Figure 1C and 1D). Upon transfer to our tertiary care center, an echocardiogram confirmed that the atrial LP was in the left ventricular outflow tract, with the docking button located just below the aortic valve (Figure 3A). Given the known PFO, we suspected that the docking end of the atrial LP had gotten caught in the PFO with atrial contractility and the device had dislodged through the PFO into the left atrium, traveled to the left ventricle, and ultimately entangled in the mitral subvalvular apparatus, remaining in the left ventricular outflow tract.
Figure 3.
Radiologic images of dislodged atrial leadless pacemaker (ALP) and percutaneous retrieval from left ventricular outflow tract. A, B: Transthoracic (A) and transesophageal (B) echocardiograms showing the ALP (arrow) in the left ventricular outflow tract. C–F: Fluoroscopy images of the percutaneous snare retrieval of the ALP in left ventricular outflow tract. Traction on the ALP stretched the fixation helix (arrow). The Aveir retrieval catheter (Abbott, Abbott Park, IL) enabled counterclockwise rotation of the ALP to free the fixation helix. G: ALP postretrieval. AO = aorta; LA = left atrium; LV = left ventricle; other abbreviations as in Figure 2.
A multidisciplinary team including electrophysiology, cardiac imaging, interventional cardiology, and cardiothoracic surgery specialists performed snare retrieval of the dislodged atrial LP. A preprocedural transesophageal echocardiogram confirmed the presence of a PFO with moderate left-to-right shunting by color Doppler. Fluoroscopy and intraoperative transesophageal echocardiography confirmed that the fixation helix was entangled in the mitral subvalvular apparatus and the docking button was oriented toward the aortic valve in the left ventricular outflow tract (Figure 3B–3F). The team determined that a retrograde aortic approach for snare retrieval was preferable to a transseptal approach. Given the anticipated need for a large introducer sheath, a femoral cutdown was performed by the cardiovascular surgeon. A 25F outer diameter (22F inner diameter) GORE DrySeal Flex Introducer Sheath (Gore, Newark, DE) was placed in the right femoral artery. The Aveir retrieval catheter was not used initially, owing to the inability to cross the aortic valve with this device. An 8F guide catheter was advanced over a guidewire across the aortic valve. An EN Snare (Merit Medical, South Jordan, UT) triple-loop endovascular snare was advanced through the guide catheter to snare the docking button of the atrial LP (Figure 3C). Despite attempted counterclockwise rotation and subsequent traction that deformed the fixation helix, the device could not be withdrawn because the helix was caught in the mitral subvalvular apparatus. Transesophageal echocardiography demonstrated that traction on the device was pulling on the chordae tendineae of the posterior mitral valve leaflet, tethering the leaflet. A second EN Snare and guide catheter were advanced through the same femoral arterial sheath and used to snare the fixation helix, but the device still could not be removed. With traction on the device, the fixation helix stretched sufficiently to allow the docking button to cross the aortic valve, making it accessible to the Aveir retrieval system. Note that the “sprung helix” is a safety feature; the helix was designed to straighten with traction to facilitate removal without damage to tissue. After confirming the LP would not embolize, the EN Snares were detached and removed. The Aveir retrieval catheter with triple-loop snare was advanced through the same femoral arterial introducer sheath to the aortic root and used to snare the docking button (Figure 3D). The protective sleeve was advanced over the atrial LP (Figure 3E), and the snare was retracted to dock the device in the docking cap (Figure 3F). The device was then rotated counterclockwise using the snare control handle and freed from the subvalvular apparatus. The device and the retrieval catheter were safely removed. The device was examined to confirm that no missing part was left behind (Figure 3G). Intraoperative transesophageal echocardiography confirmed that there was no damage to the mitral valve or subvalvular apparatus, no retained parts of the device, and no postprocedural pericardial effusion. A transthoracic echocardiogram the following day confirmed normal mitral valve function with intact subvalvular apparatus and no pericardial effusion.
The day after atrial LP retrieval, intermittent palpitations related to atrioventricular dyssynchrony returned. Five days postretrieval, a right femoral surgical site infection was diagnosed and successfully treated with antibiotics and drainage. Thirty days postretrieval, the patient underwent outpatient percutaneous transcatheter closure of his large PFO with a 30 mm Amplatzer septal occluder (Abbott). Fifty-seven days after PFO closure, he received a successful Aveir AR atrial LP reimplantation at the anterior base of the right atrial appendage (Figure 2E–2H). The intraprocedural atrial capture threshold was 3.5 V at 0.4 ms, sensing was 2.3 mV, and impedance was 270 ohms. The following day, the atrial device showed improvement in threshold (2.5 V at 0.4 ms), sensing (4.6 mV), and impedance (320 ohms). He required 70% atrial pacing. Fourteen days later, atrial threshold, sensing, and impedance further improved to 0.5 V at 0.4 ms, 5.3 mV, and 300 ohms, respectively. His symptoms of pacemaker syndrome owing to atrioventricular dyssynchrony resolved.
Discussion
To our knowledge, this is the first reported case of an Aveir atrial LP to dislodge and pass through a PFO since the device became commercially available. Our patient had early postprocedural dislodgement into the left ventricular outflow tract, diagnosed on chest radiograph and echocardiogram, which was successfully percutaneously snared and retrieved via a retrograde aortic approach owing to the orientation of the docking button. During the Aveir Dual-Chamber Leadless implant-to-implant (i2i) Investigational Device Exemption Study, 1 patient had intraprocedural dislodgement of an atrial LP through a suspected PFO into the left ventricle, which was percutaneously retrieved via transseptal snaring; an atrial LP was subsequently successfully implanted during the index procedure1 (personal communication, Abbott Medical, Abbott Park, IL). These are the only 2 known cases of atrial LP dislodgement through a PFO.
On initial atrial LP implantation, the intraprocedural capture threshold was 3.5 V at 1.5 ms. However, adequate increase in impedance during fixation (>250 ohms; recommendation by Abbott Medical) and current of injury via intracardiac electrograms (Figure 2A–2D) both indicated appropriate fixation into atrial tissue. With tissue injury and local tissue inflammation, capture threshold may be higher at implantation, with anticipated improvement once tissue injury resolves, as seen in a majority of LP implantations. This trend was noted on atrial LP reimplantation with an intraprocedural capture threshold of 3.5 V at 0.4 ms (Figure 2E–2H), which improved to 2.5 V at 0.4 ms the following day and 0.5 V at 0.4 ms 14 days later.
Abbott recommends using a 27F outer diameter (25F inner diameter) hydrophilic Aveir Introducer sheath (50 cm or 30 cm) for their Aveir delivery and Aveir retrieval catheters.2 In anticipation of requiring this large sheath, we performed a surgical arterial cutdown initially. During the procedure a 25F outer diameter (22F inner diameter) GORE DrySeal Flex Introducer Sheath was initially used, and we discovered it is technically feasible to insert the Aveir retrieval catheter through this smaller sheath, with no difficulty in manipulating the Aveir retrieval catheter. The docked LP (6.5 mm diameter) covered by the protective sleeve cannot fit through a 22F sheath (7.3 mm inner diameter), however. During retrieval of the snared LP, the protective sleeve was retracted and withdrawn into the sheath first, exposing the LP in the femoral artery. The exposed LP was then withdrawn into the sheath. With this technique, a percutaneous nonsurgical arterial approach with a smaller introducer sheath may be feasible, albeit in an off-label approach. Avoiding a femoral cutdown may minimize the risk of access site infection, which our patient suffered, as well as other potential groin and/or vascular complications.
The primary concerns regarding LPs include the risk of device dislodgement, pericardial effusion, cardiac perforation and tamponade, complications related to vascular access, elevated thresholds, battery longevity, and the feasibility of long-term retrievability.3, 4, 5, 6, 7, 8 Several issues were addressed with the redesign of the Nanostim leadless cardiac pacemaker (Nanostim Inc, Sunnyvale CA; subsequently St. Jude Medical Inc, Saint Paul, MN; now Abbott, Abbott Park, IL) to the Aveir platform, which demonstrates improved battery longevity, shorter length, a modified docking button to better enable retrievability, a more ergonomic delivery system, commanded electrograms in addition to pacing and sensing mapping features to optimize device site selection prior to fixation, and the ability to support a dual-chamber pacing system.3,4,7,8 Clinical trials of the Nanostim showed up to 1.7% incidence of dislodgement (LEADLESS II-Phase 1).3,4 The LEADLESS II-Phase 2 Trial of the Aveir ventricular LP reported 1.5% intraprocedural premature deployment (2 patients with device migration to the left pulmonary artery and 1 whose device remained in the right ventricle) and 0% postprocedural device dislodgement.7,8 The Micra LP (Medtronic, Minneapolis, MN) showed a 0.13% incidence of postprocedural dislodgement (reported in 1 patient on postimplant day 2) through 30 days of follow-up.5,6 The Aveir DR i2i Study,1 the first in-human clinical trial of the Aveir dual-chamber LP, reported 6 intraprocedural dislodgements (5 atrial and 1 ventricular) in 5 patients (1.7%)—5 related to inadequate fixation and the sixth due to mechanical dislodgement by an intracardiac echocardiography catheter—and 5 postprocedural atrial LP dislodgements (1.7%) identified 0–40 days (mean 26 days) postimplantation. With the Aveir DR, intraprocedural and postprocedural atrial LP dislodgement appears to occur more frequently than ventricular LP dislodgement.1 The Aveir DR i2i Study1 noted that atrial LPs implanted in the middle or distal (deep) right atrial appendage were associated with a higher rate of postprocedural dislodgement compared with other locations, although not statistically significant; thus, it has been recommended to target the base (ostium) of the right atrial appendage for atrial LP implantation.
In conclusion, the presence of a PFO presents additional challenges in LP implantation by allowing for the possibility of LP dislodgement into the left heart, with serious clinical consequences. It is feasible and safe to percutaneously snare and retrieve the Aveir LP using the Aveir retrieval catheter. The procedural approach depends on the LP location and orientation. Further data are needed to determine whether to consider screening patients for PFO and whether to consider empirically closing PFOs prior to atrial LP implantation.
Disclosures
B.Y. is a consultant with Abbott. M.E.M. has been involved in educational and advisory activities with honorarium with Abbott.
Acknowledgments
The authors acknowledge the following representatives of Abbott Medical for providing information about atrial leadless pacemaker implantation and the Aveir Retrieval Catheter: Fabio Di Lorenzo, Field Clinical Procedure Specialist; Nathan Wilkinson, Field Clinical Procedure Specialist; Smriti Sharma, Territory Manager, Cardiac Rhythm Management (CRM); Matthew Fiebig, CRM Leadless Clinical Specialist; Leonard Ganz, MD, DVP, Medical Affairs & CMO, CRM. The authors further acknowledge the following from Aurora Cardiovascular and Thoracic Services: Sarah Kennedy and Jennifer Pfaff for editorial preparation of the manuscript and Brian Schurrer for assistance with figures.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Knops R.E., Reddy V.Y., Ip J.E., et al. A dual-chamber leadless pacemaker. N Engl J Med. 2023;388:2360–2370. doi: 10.1056/NEJMoa2300080. [DOI] [PubMed] [Google Scholar]
- 2.Abbott Manuals. Aveir Introducer Instructions for Use, US; Aveir Retrieval Catheter Instructions for Use, US; Aveir DR Leadless Pacemaker and Delivery Catheter Instructions for Use, US. https://manuals.eifu.abbott/en/index.html Available via Abbott eLabeling website:
- 3.Reddy V.Y., Exner D.V., Cantillon D.J., et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–1135. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 4.Reddy V.Y., Knops R.E., Sperzel J., et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129:1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds D., Duray G.Z., Omar R., et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 6.Roberts P.R., Clementy N., Al Samadi F., et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm. 2017;14:1375–1379. doi: 10.1016/j.hrthm.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Reddy V.Y., Exner D.V., Doshi R., et al. Primary results on safety and efficacy from the LEADLESS II-Phase 2 Worldwide Clinical Trial. JACC Clin Electrophysiol. 2022;8:115–117. doi: 10.1016/j.jacep.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Reddy V.Y., Exner D.V., Doshi R., et al. 1-year outcomes of a leadless ventricular pacemaker: The LEADLESS II (Phase 2) Trial. JACC Clin Electrophysiol. 2023;9:1187–1189. doi: 10.1016/j.jacep.2023.01.031. [DOI] [PubMed] [Google Scholar]