Key Teaching Points.
-
•
Purkinje-related polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF) storm is one of the life-threatening complications with myocardial infarction, and Purkinje de-networking has recently gained attention as a novel catheter ablation strategy.
-
•
This case report demonstrated the significance of the extensive ablation targeting the Purkinje-muscle junction for Purkinje de-networking. The partial modification of the Purkinje system sparing the distal region of the left septal fascicle resulted in the emergence of monomorphic VT involving the Purkinje network, and additional ablation for the spared region successfully suppressed the VT.
-
•
Purkinje de-networking targeting the Purkinje-muscle junction of all the left fascicles can be a novel endpoint of the ablation procedure for Purkinje-related ventricular arrhythmias.
Introduction
Incessant Purkinje-related ventricular tachycardia (VT) and ventricular fibrillation (VF), alternatively known as an electrical storm (ES), is a life-threatening complication of myocardial infarction (MI).1,2 The Purkinje potentials preceding the local ventricular myocardial potential during polymorphic VT and VF suggest the involvement of the Purkinje network.3,4 Conventionally, catheter ablation targeting the VT/VF-triggering premature ventricular contractions (PVCs) from the injured Purkinje network has been performed.5,6
Purkinje de-networking has recently gained attention as a novel approach for Purkinje-related ventricular arrhythmias (VAs) beyond trigger ablation.3,7, 8, 9 De-networking is believed to involve disruption of re-entry circuits within the Purkinje network, thereby preventing the maintenance of VAs. However, the treatment strategy and endpoints of Purkinje de-networking have not yet been fully established. Herein, we report a case of ablation of Purkinje-related VAs, signifying the importance of the complete Purkinje de-networking incorporating the left septal fascicle area.
Case report
A 64-year-old Japanese male patient was transferred to the National Cerebral and Cardiovascular Center in Suita, Japan, for catheter ablation of treatment-refractory VT/VF ES. He had been admitted to another hospital for heart failure secondary to a recent MI. From the ninth day of hospitalization, multiple episodes of polymorphic VTs developed, which were refractory to deep sedation, temporary pacing, and antiarrhythmic agents, including amiodarone, nifekalant, and lidocaine. The percutaneous coronary intervention was performed for total occlusion of the left anterior descending artery; however, unstable VT and VF episodes repeatedly occurred (Supplemental Figure 1A), and percutaneous cardiopulmonary support was initiated.
Under general anesthesia with cardiopulmonary support, left ventricular (LV) endocardial mapping was obtained during sinus rhythm using the 3-dimensional (3D) electroanatomical mapping system (CARTO; Biosense Webster Inc, Irvine, CA) and multipolar mapping catheter (DECANAV; Biosense Webster Inc). An extensive low-voltage area was observed in the anteroseptal region of the left ventricle (Figure 1A). Late potentials during sinus rhythm were mainly observed in the anteroapical region of the low-voltage area. The polymorphic VT was repeatedly initiated with PVCs, which were associated with the Purkinje activities (Supplemental Figure 1B and 1C). When the polymorphic VTs occurred, the multipolar mapping catheter placed along the left posterior fascicle indicated that the Purkinje potentials preceded and drove the local myocardial potentials (Figure 1B). The VT was easily induced by the ventricular burst pacing from the proximal region of the left fascicles. Hence, the patient was diagnosed with Purkinje-related VTs.10 Since the earliest activation site of triggered PVCs was distributed at the mid-left posterior fascicle, and the electrocardiogram revealed sinus rhythm with left anterior fascicular block, radiofrequency ablation targeting the Purkinje-muscle junction at the distal region of the left posterior fascicle was performed owing to concern regarding the risk of atrioventricular block. We carefully ablated the Purkinje-muscle junction using an irrigated tip bidirectional (D/F curve) ablation catheter (SmartTouch; Biosense Webster Inc) at 30 watts for 30 seconds. After the procedure, inferior axis monomorphic VT, probably originating from the left anterior fascicle area, was induced. We additionally performed radiofrequency ablation targeting the Purkinje-muscle junction at the distal region of the left anterior fascicle. Finally, Purkinje-related VTs were not induced by triple extrastimuli from both ventricles. The session was completed after an additional ablation to eliminate the abnormal potentials recorded in the apical and lateral left ventricle (Figure 1A).
Figure 1.
A: Findings from the first ablation procedure. The endocardial left ventricular voltage and activation maps during sinus rhythm are shown. An extensive low-voltage area is observed in the anteroseptal region from the base to the apex of left ventricle. The ablation points and Purkinje potentials are shown by red and yellow dots, respectively. Ablation areas for substrate modification and the Purkinje system modification areas are circled by blue and yellow lines, respectively. B: Intracardiac electrogram when polymorphic ventricular tachycardia (VT) occurred. The multipolar mapping catheter placed along the left posterior fascicle records the Purkinje potentials (red asterisks), which always precede the local myocardial potentials (black asterisks) in a 1:1 fashion during VT induction. Furthermore, the cycle length of the Purkinje activities is shorter than that of the ventricular potentials. A = atrial potential; D = distal; LV = left ventricle; P = proximal; RV, right ventricle; V = ventricular potential.
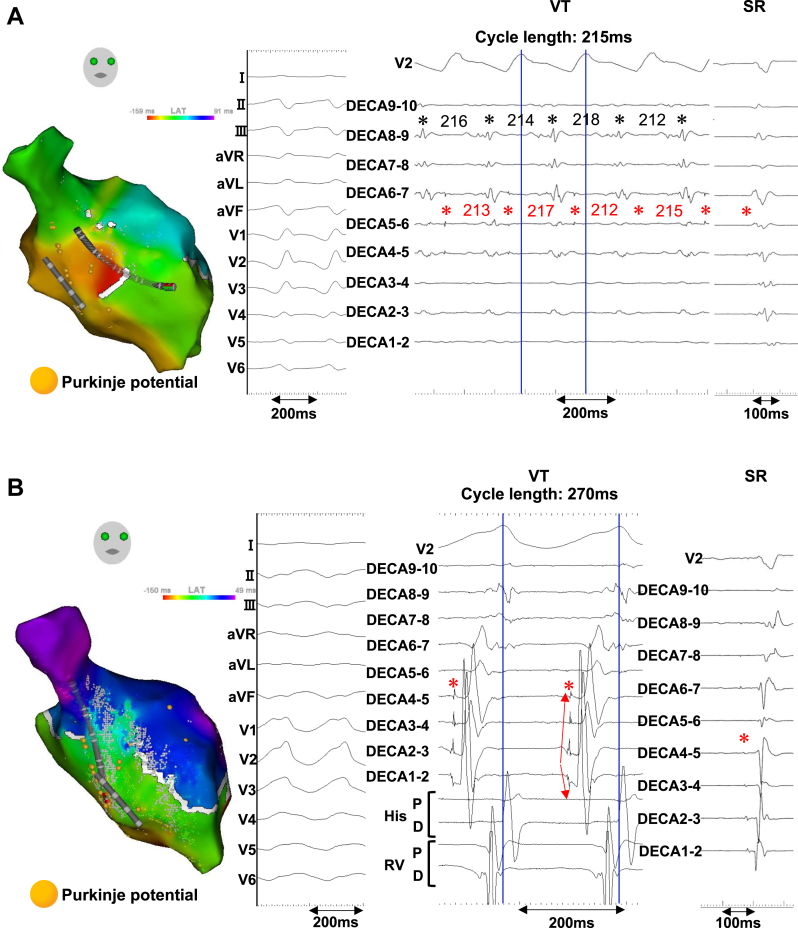
However, newly emerged monomorphic VTs frequently occurred the next day, and weaning from cardiopulmonary support failed. Therefore, we performed a second catheter ablation 2 days after the first session. The clinical VT was easily induced by the ventricular burst pacing from the proximal site of the left fascicles. LV endocardial mapping during the clinical VT showed a centrifugal activation pattern from the left septal fascicle area (Figure 2A). The Purkinje potentials that drove the local myocardial potentials suggested the involvement of the left septal fascicle (Figure 2A). This VT changed to another one during ablation of the distal left septal fascicle region. The Purkinje potentials of the left posterior fascicle preceded the local myocardial potentials during the second VT, and an activation map revealed a centrifugal activation pattern from the distal region of the left posterior fascicle (Figure 2B). Since the involvement of the left posterior fascicle was suspected, we spread the ablation area to the left posterior fascicle region, and could terminate the VT (Figure 3A). After the ablation at the Purkinje-muscle junction of the septal and posterior fascicles, VT became noninducible by triple extrastimuli from both ventricles. The PQ and QRS durations were slightly prolonged, from 164 ms and 116 ms at baseline to 180 ms and 142 ms at the end of the procedure (Figure 3A).
Figure 2.
Findings from the second ablation procedure. A: The endocardial left ventricular activation mapping and intracardiac electrograms during clinical monomorphic ventricular tachycardia (VT) and sinus rhythm (SR) are shown. The VT cycle length is 215 ms. The diastolic Purkinje potentials (red asterisks), which precede the local myocardial potentials (black asterisks), are repeatedly observed in the left septal fascicle area. Furthermore, a shortening in the Purkinje cycle length immediately precedes the shortening in the ventricular cycle length, suggesting the involvement of the Purkinje system. B: Activation mapping and intracardiac electrograms obtained during another VT (cycle length: 270 ms), which occurred after the left septal fascicle area ablation, are shown. A centrifugal activation pattern from the distal region of the left posterior fascicle is exhibited, and the diastolic Purkinje potentials at the left posterior fascicle (red asterisks) show polarity change at the distal electrodes of the multipolar mapping catheter (red arrows) and precede the local myocardial potentials in a 1:1 fashion.
Figure 3.
Ablation points of the 2 sessions. A: The ablation points (red dots) and pre- and postablation electrograms of the second session are shown. The PQ and QRS durations are slightly prolonged from 164 to 180 ms and 116 to 142 ms, respectively. B: The combined ablation points of the 2 procedures are shown. The virtual triangle was created with its apex formed by the proximal left bundle branch and its base by the most distal Purkinje potentials. The left anterior (A) and posterior (P) fascicles were marked as the margins of the triangle. Additional Purkinje de-networking incorporating the distal region of the left septal (S) fascicle in the second procedure (black dots: ablation points in the first procedure; red dots: ablation points in the second procedure) resulted in successful suppression of the ventricular arrhythmias.
Through the 2 procedures, the extensive ablation targeting the Purkinje-muscle junction of the left anterior, septal, and posterior fascicles for Purkinje de-networking was performed (Figure 3B). After the second procedure, there was no recurrence of VAs during the hospital stay, and this patient was discharged from the hospital 39 days after the second procedure. There have been 2 antitachycardia pacing deliveries for VT in 8 months after the discharge.
Discussion
In this study, we report a case of Purkinje-related VAs, which was successfully treated with Purkinje de-networking. During the first ablation session, we only ablated the distal region of the left posterior and anterior fascicles owing to concern regarding the risk of atrioventricular block. However, monomorphic VTs involving the septal and posterior fascicles newly emerged, which necessitated additional Purkinje de-networking. This case highlights the importance of extensive Purkinje de-networking incorporating the left septal fascicle region.
There were several limitations in this electrophysiological study. First, although the slight prolongation in PQ and QRS durations was considered the result of extensive ablation targeting the Purkinje-muscle junction, there is a possibility that the left fascicles were slightly damaged. However, the earliest activation sites of triggered PVCs were in more proximal areas. Therefore, we considered the extensive Purkinje de-networking effective. Second, we acknowledge that the presence of the Purkinje potentials preceding local ventricular activation does not necessarily prove the re-entry within the Purkinje network. However, the Purkinje system involvement was indicated by the following supportive findings in this case: (1) the VTs were repeatedly initiated with PVCs, which were associated with the preceding Purkinje activities; (2) the VTs were also induced by the ventricular burst pacing from the proximal site of the left fascicle; (3) the multipolar mapping catheter indicated that the Purkinje activities drove each of the local ventricular potentials in a 1:1 fashion during VT induction10; and (4) the Purkinje de-networking was effective for the VTs.
Catheter ablation targeting the Purkinje network, namely Purkinje de-networking, has proven to be efficacious in suppressing Purkinje-related polymorphic VT and VF.9 However, an optimal ablation strategy has not been established enough. Imnadze and colleagues7 have proposed an effective method for Purkinje de-networking from their experience of 10 patients with Purkinje-related VAs. After annotating the left bundle branch and LV Purkinje potentials using the 3D electroanatomical mapping, a virtual triangle was created with its apex formed by the proximal left bundle branch and its base by the most distal Purkinje potentials. A T-shaped, linear ablation is performed at the triangle’s base, followed by ablation within the left septal fascicle. With this method, 80% of the patients were free from VAs at a 1-year follow-up.
In the present case, partial de-networking of the Purkinje network sparing the left septal fascicle region was associated with a newly emerged VT involving the Purkinje network. Finally, we successfully suppressed monomorphic VTs by the extensive ablation targeting the Purkinje-muscle junction within the virtual triangle proposed by Imnadze and colleagues.7 Consistent with the findings of the present case, Masuda and colleagues11 reported that 4 of the 21 patients with Purkinje-related VF had newly emerged sustained monomorphic VTs after VF-triggering PVC ablation because of ischemic heart disease, which mostly arose from the Purkinje network and was successfully suppressed by the additional ablation targeting the Purkinje network. The conversion mechanism to monomorphic VT is postulated to be a re-entry circuit reorganization owing to the creation of a unidirectional block and conduction delay by partial modification within the Purkinje network. These data suggest that partial Purkinje de-networking can be arrhythmogenic, and the wide-range modification of the distal Purkinje network might be a preferable endpoint of the ablation for polymorphic VT and VF after MI.
Conclusion
We reported a case of Purkinje-related VAs after an MI. Partial Purkinje de-networking sparing the left septal fascicle region for polymorphic VT was associated with a newly emergent monomorphic VT. Additional ablation targeting the distal region of the spared left fascicle could suppress ES. Extensive modification targeting the Purkinje-muscle junction of all the left fascicles can be a new endpoint of the ablation procedure for the Purkinje-related VAs.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
The authors thank Editage for English-language editing.
Funding Sources
This work was supported by JSPS KAKENHI, Grant Number JP24K11205.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2024.06.002.
Appendix. Supplementary Data
The monitor electrocardiogram in lead II shows sinus rhythm (SR) and ventricular fibrillation (VF) initiated with premature ventricular conduction (PVC) with the superior axis (A). Polymorphic VTs are initiated with PVC1 or PVC2 (red dot-squares) associated with Purkinje activities (red asterisks) (B and C).
References
- 1.Haissaguerre M., Vigmond E., Stuyvers B., Hocini M., Bernus O. Ventricular arrhythmias and the His-Purkinje system. Nat Rev Cardiol. 2016;13:155–166. doi: 10.1038/nrcardio.2015.193. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e210–e271. doi: 10.1161/CIR.0000000000000548. [published correction appears in Circulation 2018;138:e415–e418] [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M., Cheniti G., Hocini M., et al. Purkinje network and myocardial substrate at the onset of human ventricular fibrillation: implications for catheter ablation. Eur Heart J. 2022;43:1234–1247. doi: 10.1093/eurheartj/ehab893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogami A., Komatsu Y., Talib A., et al. Purkinje-related ventricular tachycardia and ventricular fibrillation: solved and unsolved questions. JACC Clin Electrophysiol. 2023;9:2172–2196. doi: 10.1016/j.jacep.2023.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Komatsu Y., Hocini M., Nogami A., et al. Catheter ablation of refractory ventricular fibrillation storm after myocardial infarction. Circulation. 2019;139:2315–2325. doi: 10.1161/CIRCULATIONAHA.118.037997. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T., Schaeffer B., Tanigawa S., et al. Catheter ablation of polymorphic ventricular tachycardia/fibrillation in patients with and without structural heart disease. Heart Rhythm. 2019;16:1021–1027. doi: 10.1016/j.hrthm.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Imnadze G., Zerm T. Prevention of ventricular fibrillation through de-networking of the Purkinje system: proof-of-concept paper on the substrate modification of the Purkinje network. Pacing Clin Electrophysiol. 2019;42:1285–1290. doi: 10.1111/pace.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sciacca V., Fink T., Guckel D., et al. Catheter ablation in patients with ventricular fibrillation by purkinje de-networking. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.956627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamakura T., Kamoshida J., Toda K., Wayama K., Wada M., Kusano K. Ventricular fibrillation initiated by reentry involving the Purkinje network in a patient with after myocardial infarction. HeartRhythm Case Rep. 2023;9:618–623. doi: 10.1016/j.hrcr.2023.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haissaguerre M., Cheniti G., Escande W., Zhao A., Hocini M., Bernus O. Idiopathic ventricular fibrillation with repetitive activity inducible within the distal Purkinje system. Heart Rhythm. 2019;16:1268–1272. doi: 10.1016/j.hrthm.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda K., Nogami A., Kuroki K., et al. Conversion to Purkinje-related monomorphic ventricular tachycardia after ablation of ventricular fibrillation in ischemic heart disease. Circ Arrhythm Electrophysiol. 2016;9:e004224. doi: 10.1161/CIRCEP.116.004224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The monitor electrocardiogram in lead II shows sinus rhythm (SR) and ventricular fibrillation (VF) initiated with premature ventricular conduction (PVC) with the superior axis (A). Polymorphic VTs are initiated with PVC1 or PVC2 (red dot-squares) associated with Purkinje activities (red asterisks) (B and C).