Abstract
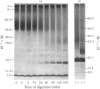
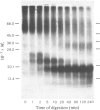
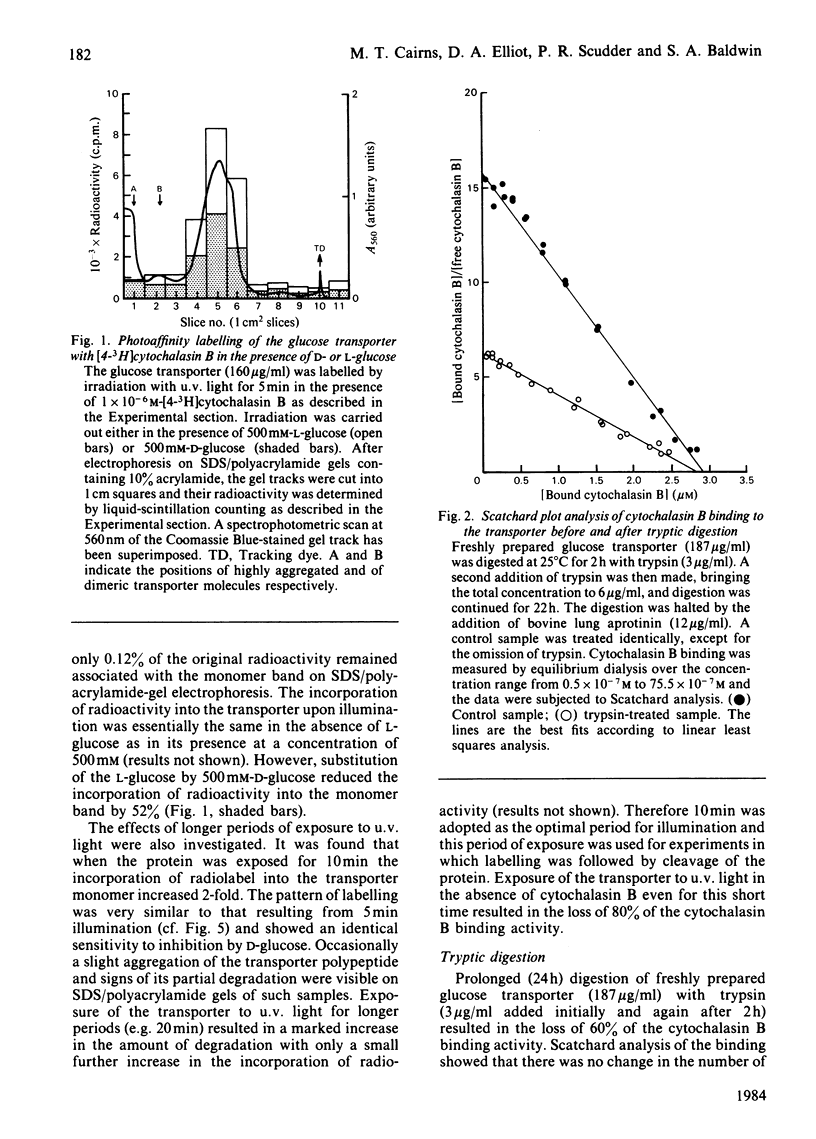
Treatment of the purified, reconstituted, human erythrocyte glucose transporter with trypsin lowered its affinity for cytochalasin B more than 2-fold, and produced two large, membrane-bound fragments. The smaller fragment (apparent Mr 18000) ran as a sharp band on sodium dodecyl sulphate (SDS)/polyacrylamide-gel electrophoresis. When the transporter was photoaffinity labelled with [4-3H]cytochalasin B before tryptic digestion, this fragment became radiolabelled and so probably comprises a part of the cytochalasin B binding site, which is known to lie on the cytoplasmic face of the erythrocyte membrane. In contrast, the larger fragment was not radiolabelled, and ran as a diffuse band on electrophoresis (apparent Mr 23000-42000). It could be converted to a sharper band (apparent Mr 23000) by treatment with endo-beta-galactosidase from Bacteroides fragilis and so probably contains one or more sites at which an oligosaccharide of the poly(N-acetyl-lactosamine) type is attached. Since the transporter bears oligosaccharides only on its extracellular domain, whereas trypsin is known to cleave the protein only at the cytoplasmic surface, this fragment must span the membrane. Cleavage of the intact, endo-beta-galactosidase-treated, photoaffinity-labelled protein at its cysteine residues with 2-nitro-5-thiocyanobenzoic acid yielded a prominent, unlabelled fragment of apparent Mr 38000 and several smaller fragments which stained less intensely on SDS/polyacrylamide gels. Radioactivity was found predominantly in a fragment of apparent Mr 15500. Therefore it appears that the site(s) labelled by [4-3H]cytochalasin B lies within the N-terminal or C-terminal third of the intact polypeptide chain.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M., Gorga J. C., Lienhard G. E. The monosaccharide transporter of the human erythrocyte. Transport activity upon reconstitution. J Biol Chem. 1981 Apr 25;256(8):3685–3689. [PubMed] [Google Scholar]
- Baldwin J. M., Lienhard G. E., Baldwin S. A. The monosaccharide transport system of the human erythrocyte. Orientation upon reconstitution. Biochim Biophys Acta. 1980 Jul;599(2):699–714. doi: 10.1016/0005-2736(80)90211-4. [DOI] [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Gorga F. R., Lienhard G. E. Purification of the cytochalasin B binding component of the human erythrocyte monosaccharide transport system. Biochim Biophys Acta. 1979 Mar 23;552(1):183–188. doi: 10.1016/0005-2736(79)90257-8. [DOI] [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Lienhard G. E. Monosaccharide transporter of the human erythrocyte. Characterization of an improved preparation. Biochemistry. 1982 Aug 3;21(16):3836–3842. doi: 10.1021/bi00259a018. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Matsumura G. Endo-beta-galactosidase of Escherichia freundii. Purification and endoglycosidic action on keratan sulfates, oligosaccharides, and blood group active glycoprotein. J Biol Chem. 1976 Oct 25;251(20):6218–6225. [PubMed] [Google Scholar]
- Goodman D., Matzura H. An improved method of counting radioactive acrylamide gels. Anal Biochem. 1971 Aug;42(2):481–486. doi: 10.1016/0003-2697(71)90062-5. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Baldwin S. A., Lienhard G. E. The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochem Biophys Res Commun. 1979 Dec 14;91(3):955–961. doi: 10.1016/0006-291x(79)91972-7. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Changes in the intrinsic fluorescence of the human erythrocyte monosaccharide transporter upon ligand binding. Biochemistry. 1982 Apr 13;21(8):1905–1908. doi: 10.1021/bi00537a031. [DOI] [PubMed] [Google Scholar]
- Hashimoto F., Horigome T., Kanbayashi M., Yoshida K., Sugano H. An improved method for separation of low-molecular-weight polypeptides by electrophoresis in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1983 Feb 15;129(1):192–199. doi: 10.1016/0003-2697(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Schaffer M. H., Stark G. R., Vanaman T. C. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J Biol Chem. 1973 Oct 10;248(19):6583–6591. [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Scudder P., Uemura K., Dolby J., Fukuda M. N., Feizi T. Isolation and characterization of an endo-beta-galactosidase from Bacteroides fragilis. Biochem J. 1983 Aug 1;213(2):485–494. doi: 10.1042/bj2130485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Characterization of cytochalasin B photoincorporation into human erythrocyte D-glucose transporter and F-actin. Biochemistry. 1983 May 24;22(11):2750–2756. doi: 10.1021/bi00280a024. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Shanahan M. F., Olson S. A., Weber M. J., Lienhard G. E., Gorga J. C. Photolabeling of glucose-sensitive cytochalasin B binding proteins in erythrocyte, fibroblast and adipocyte membranes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):38–43. doi: 10.1016/0006-291x(82)91666-7. [DOI] [PubMed] [Google Scholar]
- Shelton R. L., Jr, Langdon R. G. Reconstitution of glucose transport using human erythrocyte band 3. Biochim Biophys Acta. 1983 Aug 24;733(1):25–33. doi: 10.1016/0005-2736(83)90087-1. [DOI] [PubMed] [Google Scholar]
- Sogin D. C., Hinkle P. C. Characterization of the glucose transporter from human erythrocytes. J Supramol Struct. 1978;8(4):447–453. doi: 10.1002/jss.400080407. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. Kinetic properties of the reconstituted glucose transporter from human erythrocytes. J Biol Chem. 1981 Sep 10;256(17):8907–8914. [PubMed] [Google Scholar]
- Zoccoli M. A., Baldwin S. A., Lienhard G. E. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem. 1978 Oct 10;253(19):6923–6930. [PubMed] [Google Scholar]