Abstract
Background
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is increasingly being used for temporary bleeding control in patients with trauma with non-compressible truncal hemorrhage (NCTH). In recent years, the technique is gaining popularity in postpartum hemorrhage and non-traumatic cardiac arrest, although still underutilized. In other surgical fields, however, there is not yet much awareness for the possible advantages of this technique. Consequently, for non-trauma indications, limited data are available.
Methods
Description of the use of REBOA in two patients with hemorrhagic shock due to exsanguinating non-traumatic NCTH.
Results
In the first case, REBOA was deployed at the emergency department in a patient in their 80s presenting with hemorrhagic shock due to a ruptured abdominal aortic aneurysm. Hemodynamic stability was obtained and a CT scan was subsequently performed for planning of endovascular aneurysm repair. After successful placement of the endograft, the REBOA catheter was deflated and removed. In the second case, REBOA was performed in a patient with shock due to iatrogenic epigastric artery bleeding after an umbilical hernia repair to prevent hemodynamic collapse and facilitate induction of anesthesia for definitive surgery. During laparotomy, blood pressure-guided intermittent aortic balloon occlusion was used to preserve perfusion of the abdominal organs. Patient made a full recovery.
Conclusion
REBOA deployment was successful in achieving temporary hemorrhage control and hemodynamic stability in patients with non-traumatic NCTH. REBOA facilitated diagnostic work-up, transportation to the operating room and prevented hemodynamic collapse during definitive surgical repair. In the right patient and skilled hands, this relatively simple endovascular procedure could buy precious time and prove lifesaving in a variety of non-compressible hemorrhage.
Keywords: aortic rupture, aorta, hemorrhage, gastrointestinal hemorrhage
Background
Catastrophic hemorrhage is a significant cause of potentially preventable death in both patients with trauma and non-trauma.1 Historically, resuscitative thoracotomy with aortic cross clamping has been used as hemorrhage control and resuscitative measure for patients with trauma in extremis from non-compressible truncal hemorrhage (NCTH). Nowadays, resuscitative endovascular balloon occlusion of the aorta (REBOA) is increasingly being used for control of traumatic NCTH.
With this technique, an aortic occlusion balloon catheter is inserted into the descending aorta via the femoral or brachial artery. By intraluminal occlusion of the aorta above the level of hemorrhage, REBOA achieves temporary distal hemorrhage control while maintaining proximal cerebral and myocardial perfusion. REBOA could be applied in the emergency department (ED) or in the prehospital arena to serve as bridge to definitive surgical hemostasis and prevent lethal exsanguination. REBOA is also increasingly being used in hybrid setting during definitive bleeding control treatment.2
Numerous studies describe the possible value of REBOA and its use in the trauma population. In recent years, the technique is gaining popularity in postpartum hemorrhage and non-traumatic cardiac arrest, although still underutilized.3 4 In other surgical fields, however, there is not yet much awareness for the possible advantages of this technique. Consequently, for non-trauma indications, limited data are available.
In the Netherlands, REBOA has solely been incorporated as standard care in the largest Dutch level 1 trauma center while potential benefit of more widespread REBOA use has been demonstrated.5 In other hospitals, REBOA may only be applied in rare cases when a surgical specialist acquainted with REBOA is on duty. In this report, we demonstrate two cases of non-traumatic REBOA use in urban Dutch hospitals, in a patient with a ruptured abdominal aortic aneurysm and a patient with iatrogenic surgical hemorrhage where REBOA was performed to facilitate diagnostic work-up and definitive surgery.
Case description
Case 1
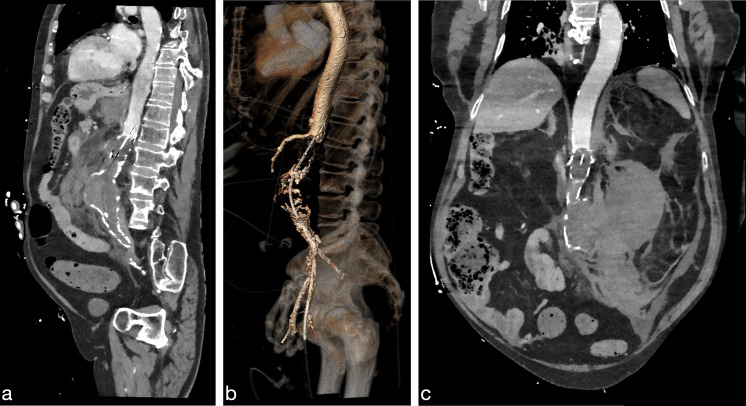
A patient in their 80s presented to the ED with suspected pneumonia not responding to antibiotic treatment. On admission, the patient was hemodynamically and neurologically stable with a respiratory rate of 18/min, oxygen saturation of 97%, heart rate of 92 bpm, blood pressure of 131/95 mm Hg (mean arterial pressure 107 mmHg) and body temperature of 36.1°C. Soon after admission, the patient became restless with tachypnea 33/min, desaturation below 80% requiring oxygen therapy, irregular tachycardia up to 200 bpm and a non-measurable blood pressure. The abdomen was enlarged and tender. There was suspicion of hemorrhagic shock. Ultrasound examination revealed free intra-abdominal fluid, suspect for a ruptured abdominal aorta aneurysm (rAAA). Laboratory examinations and arterial blood gas revealed: hemoglobin 9.4 g/dL, hematocrit 28%, thrombocytes 3.17×109/L, sodium 139 mmol/L, potassium 4.4 mmol/L, chloride 105 mmol/L, pH 7.17, bicarbonate 13.9 mmol/L, base excess −13.9 mmol/L and lactate 8.8 mmol/L. Massive transfusion protocol was initiated, the patient was sedated and intubated, and a vascular surgeon was consulted. In the ED, the vascular surgeon placed a REBOA-catheter (ER-REBOA PLUS, Prytime Medical Devices, Boerne, Texas) via ultrasound-guided percutaneous femoral artery access with an 8 Fr introducer sheath (Avanti+, Cordis, Santa Clara, California). The balloon was positioned using anatomical landmarks.6 After REBOA deployment, patient became hemodynamically stable and a CT angiography (CTA)-scan could be obtained. CTA-scan confirmed the presence of an rAAA and the patient was transferred into the operating room (OR) for endovascular aneurysm repair. Concurrently exact measurements were performed in a vascular planning tool (3Mensio Vascular, Pie Medical Imaging BV, Maastricht, The Netherlands). The REBOA balloon was repositioned to zone 1 as CTA revealed the balloon was initially placed just distally of the superior mesenteric artery in zone 2 (figure 1). After successful placement of the endograft, the REBOA-catheter was deflated and removed. Total occlusion time was approximately 30 min. Laparotomy was performed because of high suspicion of abdominal compartment syndrome. During laparotomy, the patient developed pulseless electrical activity. Resuscitation was unsuccessful. The patient was transferred to the intensive care unit (ICU) where he died.
Figure 1. CT images of the fully inflated aortic occlusion balloon above the ruptured abdominal aortic aneurysm with clear distinction between the proximal contrast enhanced blood-filled aorta and the absence of distal blood flow in sagittal (a) and coronal (c) plane and 3D reconstruction (b).
Case 2
A patient in their 50s received laparoscopic repair of an umbilical hernia. One day postoperatively, patient collapsed at home. He was transported to the ED by ambulance. Initial vital signs at the ED were respiratory rate 20/min, non-measurable oxygen saturation, heart rate 91 bpm, blood pressure 60/40 mm Hg and Glasgow Coma Score 15. He had a large peri-umbilical hematoma. There was no hemodynamic response to resuscitation with 3 units of red blood cells (RBC, 275 mL/unit). Arterial blood gas values were hemoglobin 11.9 g/dL, pH 7.31, bicarbonate 16 mmol/L, base excess −9.6 mmol/L and lactate 8.2 mmol/L. Point-of-care ultrasound showed a large amount of free intra-abdominal fluid. Subsequent abdominal CT-scan revealed a large intraperitoneal hematoma ventrally and around the liver, spleen and in the lower pelvis. The patient was transported into the OR for laparotomy. In the OR, prior to laparotomy and under local anesthesia, a vascular surgeon placed a balloon catheter (Reliant Stent Graft Balloon Catheter, Medtronic Plc, Minneapolis, Minnesota) via ultrasound-guided percutaneous femoral artery access with a 12 Fr sheath (Sentrant introducer sheath 64 cm, Medtronic Plc, Minneapolis, Minnesota) to avoid lethal hemodynamic collapse during induction of general anesthesia. The balloon was positioned at the height of the diaphragm in zone 1, which was confirmed with X-ray. Balloon occlusion resulted in 25 points increase of proximal mean arterial pressure to 75 mm Hg. During laparotomy, intermittent partial aortic occlusion was titrated in close collaboration with the anesthesiologist to maintain a target systolic blood pressure of 90 mm Hg, which was gradually reduced to 60 mm Hg to preserve renal perfusion. Five liters of blood were evacuated from the abdomen and the abdomen was packed with laparotomy pads. Surgical control of an epigastric artery bleeding was achieved. In total, the patient received 12 units RBC and 7 units Octaplas (200 mL/unit). Periprocedural lactate increased from 6.7 mmol/L to 8.1 mmol/L. After 1 hour, the balloon catheter and introducer sheath could be removed. An AbThera dressing (KCI USA, San Antonio, Texas) was applied for temporary abdominal closure. Lactate levels already decreased to 5.9 mmol/L 1 hour postoperatively. The next day, the abdomen was closed after second look surgery. Postoperatively, the patient went to the ICU. He developed acute kidney failure for which he received continuous venovenous hemodiafiltration (CVVHD) for 11 days. Eventually, the patient has made a full recovery.
Discussion
We report two successful REBOA deployments in hemodynamically unstable patients due to NCTH. In both patients, REBOA improved hemodynamics with elevated mean arterial pressures and hereby maintained cerebral and cardiac perfusion and oxygenation. In the first case, REBOA enabled performing diagnostic imaging and transportation to definitive surgery, while in the second case, it prevented cardiovascular collapse during induction of anesthesia and laparotomy until the bleeding was controlled.
The first case emphasizes the importance of proper training and direct availability of a complete REBOA kit at the ED. The surgeons could act straight away and no precious time was lost. In this case, mid-sternum was used as external landmark to position the balloon. Although literature has shown that this is a safe, reliable method for zone 1 positioning with low error rates, in this patient, the balloon was unintentionally placed in zone 2 (CT scan).6,8 Aortic lengths and tortuosity increase with age.6 9 When using REBOA in elderly patients with rAAA, this associated tortuosity can be challenging, especially when using guide-wire free deployment. There is a risk of placing the balloon ‘too low’ if aortic and iliofemoral elongation is not properly taken into consideration. One should also take into account the risk of creating a free rupture while blindly advancing the catheter into the aorta in rAAA patients. Hence, although imaging-free REBOA has successfully been performed,310,13 mostly in the younger trauma population, balloon position guidance and confirmation with an imaging modality currently remain recommended when readily available. No adverse effects of zone 2 occlusion have been described,14 but robust data substantiating the widespread recommendation to avoid this zone, where the visceral arteries originate, is currently lacking.
Intermittent aortic balloon occlusion was performed in the second case to prevent abdominal organ ischemia. During this process, anticipation and clear communication with the anesthesiologist are indispensable to avoid large pressure fluctuations or sudden collapse. A transient peri-procedural lactate elevation was observed, which already diminished 1 hour postoperatively. CVVHD was necessary for 11 days, but kidney function was normalized at follow-up 3 weeks later. Providers should be aware of the risk of (iatrogenic) ischemia and reperfusion injury and pro-actively mitigate this risk when possible, either through partial and intermittent inflation or through the use of balloons with flow channels.15,20 Deflating the balloon as much and as early as clinically possible is crucial.
In both cases, REBOA-trained and skilled surgeons were involved, highlighting the importance of REBOA awareness and proficiency. Knowledge of possible indications, as well as the practical know-how of the procedure, technical aspects and pitfalls in combination with a motivated multidisciplinary team, are essential aspects for successful and durable implementation of REBOA in clinical practices. Frequent training in obtaining femoral artery access and the REBOA procedure is a prerequisite to keep skills up to date.21,24
REBOA indications stretch beyond those of traumatic origin and include non-compressible obstetric and gastrointestinal hemorrhage.325,28 We advocate that all medical specialties treating bleeding patients should be involved in developing hospital-wide NCTH control protocols to ensure broad support in the future.
Aim of this dual case report was to raise awareness for the potential of REBOA in non-traumatic bleeding control at the ED and in the run-up to definitive hemorrhage control. Inherent to its working mechanism, REBOA is always a temporary solution serving as a bridge to definitive therapy. In the right patient and skilled hands, this relatively simple and elegant endovascular procedure could buy precious time and prove lifesaving in a variety of non-compressible hemorrhage.
Several authors are employees of the Dutch government. Opinions or assertions in this manuscript reflect personal views of the authors and are not to be construed as official or reflecting the views of the Dutch Department of Defense or Dutch government.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Consent obtained directly from patients.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Contributor Information
Jan C van de Voort, Email: j.vandevoort@erasmusmc.nl.
Suzanne M Vrancken, Email: smvrancken@alrijne.nl.
Eric R Manusama, Email: ermanusama@mcl.nl.
Boudewijn L S Borger van der Burg, Email: blsborgervanderburg@alrijne.nl.
Pieter Klinkert, Email: pklinkert@heelkundefriesland.nl.
Rigo Hoencamp, Email: rhoencamp@alrijne.nl.
References
- 1.Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80:324–34. doi: 10.1097/TA.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 2.Khan MZ, Bruce J, Baer D, Hoencamp R. Hybrid use of REBOA in a South African tertiary trauma unit for penetrating torso trauma. BMJ Case Rep. 2019;12:e229538. doi: 10.1136/bcr-2019-229538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stensæth KH, Carlsen MIS, Løvvik TS, Uleberg O, Brede JR, Søvik E. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as adjunct treatment in life threatening postpartum hemorrhage: Fourteen years’ experience from a single Norwegian center. Acta Obstet Gynecol Scand. 2024;103:965–9. doi: 10.1111/aogs.14767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamberini L, Coniglio C, Lupi C, Tartaglione M, Mazzoli CA, Baldazzi M, Cecchi A, Ferri E, Chiarini V, Semeraro F, et al. Resuscitative endovascular occlusion of the aorta (REBOA) for refractory out of hospital cardiac arrest. An Utstein-based case series. Resuscitation. 2021;165:161–9. doi: 10.1016/j.resuscitation.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Vrancken SM, de Vroome M, van Vledder MG, Halm JA, Van Lieshout EMM, Borger van der Burg BLS, Hoencamp R, Verhofstad MHJ, van Waes OJF. Non-compressible truncal and junctional hemorrhage: A retrospective analysis quantifying potential indications for advanced bleeding control in Dutch trauma centers. Injury. 2024;55 doi: 10.1016/j.injury.2023.111183. [DOI] [PubMed] [Google Scholar]
- 6.van der Burg BLSB, Vrancken S, van Dongen TTCF, Wamsteker T, Rasmussen T, Hoencamp R. Comparison of aortic zones for endovascular bleeding control: age and sex differences. Eur J Trauma Emerg Surg. 2022;48:4963–9. doi: 10.1007/s00068-022-02033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada Y, Narumiya H, Ishi W, Iiduka R. Anatomical landmarks for safely implementing resuscitative balloon occlusion of the aorta (REBOA) in zone 1 without fluoroscopy. Scand J Trauma Resusc Emerg Med. 2017;25:63. doi: 10.1186/s13049-017-0411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linnebur M, Inaba K, Haltmeier T, Rasmussen TE, Smith J, Mendelsberg R, Grabo D, Demetriades D. Emergent non-image-guided resuscitative endovascular balloon occlusion of the aorta (REBOA) catheter placement: A cadaver-based study. J Trauma Acute Care Surg. 2016;81:453–7. doi: 10.1097/TA.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 9.Tsurukiri J, Akamine I, Sato T, Sakurai M, Okumura E, Moriya M, Yamanaka H, Ohta S. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med. 2016;24:13. doi: 10.1186/s13049-016-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lendrum R, Perkins Z, Chana M, Marsden M, Davenport R, Grier G, Sadek S, Davies G. Pre-hospital Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for exsanguinating pelvic haemorrhage. Resuscitation. 2019;135:6–13. doi: 10.1016/j.resuscitation.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 11.de Schoutheete JC, Fourneau I, Waroquier F, De Cupere L, O’Connor M, Van Cleynenbreugel K, Ceccaldi JC, Nijs S. Three cases of resuscitative endovascular balloon occlusion of the aorta (REBOA) in austere pre-hospital environment—technical and methodological aspects. World J Emerg Surg. 2018;13:54. doi: 10.1186/s13017-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser J, Teeter W, Gerlach T, Fernandez N. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an Adjunct to Damage Control Surgery for Combat Trauma: A Case Report of the First REBOA Placed in Afghanistan. JEVTM . 2017;1:58–62. doi: 10.26676/jevtm.v1i1.16. [DOI] [Google Scholar]
- 13.Stensaeth KH, Sovik E, Haig INY, Skomedal E, Jorgensen A. Fluoroscopy-free Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for controlling life threatening postpartum hemorrhage. PLoS One. 2017;12:e0174520. doi: 10.1371/journal.pone.0174520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto S, Funabiki T, Kazamaki T, Orita T, Sekine K, Yamazaki M, Moriya T. Placement accuracy of resuscitative endovascular occlusion balloon into the target zone with external measurement. Trauma Surg Acute Care Open. 2020;5:e000443. doi: 10.1136/tsaco-2020-000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson AJ, Russo RM, Reva VA, Brenner ML, Moore LJ, Ball C, Bulger E, Fox CJ, DuBose JJ, Moore EE, et al. The pitfalls of resuscitative endovascular balloon occlusion of the aorta: Risk factors and mitigation strategies. J Trauma Acute Care Surg. 2018;84:192–202. doi: 10.1097/TA.0000000000001711. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro Junior MAF, Feng CYD, Nguyen ATM, Rodrigues VC, Bechara GEK, de-Moura RR, Brenner M. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) World J Emerg Surg. 2018;13:20. doi: 10.1186/s13017-018-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo RM, White JM, Baer DG. Partial Resuscitative Endovascular Balloon Occlusion of the Aorta: A Systematic Review of the Preclinical and Clinical Literature. J Surg Res. 2021;262:101–14. doi: 10.1016/j.jss.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 18.White JM, Ronaldi AE, Polcz JE, et al. A New Pressure-Regulated, Partial REBOA Device Achieves Targeted Distal Perfusion. J Surg Res. 2020;256:171–9. doi: 10.1016/j.jss.2020.06.042. [DOI] [PubMed] [Google Scholar]
- 19.Treffalls RN, DuBose JJ, Brenner M, Piccinini A, Inaba K, Scalea TM, Moore LJ, Kauvar DS, Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) Investigators Outcomes Associated With Aortic Balloon Occlusion Time in Patients With Zone 1 Resuscitative Endovascular Balloon Occlusion of the Aorta. J Surg Res. 2024;296:256–64. doi: 10.1016/j.jss.2023.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Polcz JE, Ronaldi AE, Madurska M, Bedocs P, Leung LY, Burmeister DM, White PW, Rasmussen TE, White JM. Next-Generation REBOA (Resuscitative Endovascular Balloon Occlusion of the Aorta) Device Precisely Achieves Targeted Regional Optimization in a Porcine Model of Hemorrhagic Shock. J Surg Res. 2022;280:1–9. doi: 10.1016/j.jss.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Hatchimonji JS, Sikoutris J, Smith BP, Vella MA, Dumas RP, Qasim ZA, Gallagher JJ, Reilly PM, Raza SS, Cannon JW. The REBOA Dissipation Curve: Training Starts to Wane at 6 Months in the Absence of Clinical REBOA Cases. J Surg Educ. 2020;77:1598–604. doi: 10.1016/j.jsurg.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Borger van der Burg BLS, Vrancken SM, van Dongen T, DuBose JJ, Bowyer MW, Hoencamp R. Feasibility Study of Vascular Access and REBOA Placement in Quick Response Team Firefighters. J Spec Oper Med. 2020;20:81–6. doi: 10.55460/T8SL-61MD. [DOI] [PubMed] [Google Scholar]
- 23.Borger van der Burg BLS, Hörer TM, Eefting D, van Dongen TTCF, Hamming JF, DuBose JJ, Bowyer M, Hoencamp R. Vascular access training for REBOA placement: a feasibility study in a live tissue-simulator hybrid porcine model. J R Army Med Corps . 2019;165:147–51. doi: 10.1136/jramc-2018-000972. [DOI] [PubMed] [Google Scholar]
- 24.Borger van der Burg BLS, Maayen R, van Dongen T, Gerben C, Eric C, DuBose JJ, Horer TM, Bowyer MW, Hoencamp R. Feasibility Study Vascular Access and REBOA Placement: From Zero to Hero. J Spec Oper Med. 2018;18:70–4. doi: 10.55460/G53H-UM93. [DOI] [PubMed] [Google Scholar]
- 25.Ando H, Kaszynski RH, Goto H. On-site placement of resuscitative endovascular balloon occlusion of the aorta (REBOA) in a hemorrhagic shock patient: A successful endeavor involving long-distance air transport. Am J Emerg Med. 2022;55:227. doi: 10.1016/j.ajem.2021.12.055. [DOI] [PubMed] [Google Scholar]
- 26.Sano H, Tsurukiri J, Hoshiai A, Oomura T, Tanaka Y, Ohta S. Resuscitative endovascular balloon occlusion of the aorta for uncontrollable nonvariceal upper gastrointestinal bleeding. World J Emerg Surg. 2016;11:20. doi: 10.1186/s13017-016-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashida T, Hata N, Higashi A, Oka Y, Otani S, Watanabe E. Case Report: Lifesaving Hemostasis With Resuscitative Endovascular Balloon Occlusion of the Aorta in a Patient With Cardiac Arrest Caused by Upper Gastrointestinal Hemorrhage. Front Med (Lausanne) 2021;8:777421. doi: 10.3389/fmed.2021.777421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Voort JC, Kessel B, Borger van der Burg BLS, DuBose JJ, Hörer TM, Hoencamp R. Consensus on resuscitative endovascular balloon occlusion of the aorta in civilian (prehospital) trauma care: A Delphi study. J Trauma Acute Care Surg. 2024;96:921–30. doi: 10.1097/TA.0000000000004238. [DOI] [PubMed] [Google Scholar]