Abstract
BACKGROUND
Behcet's disease (BD) is an inflammatory disorder known for various symptoms, including oral and genital ulcers and ocular inflammation. Panuveitis, a severe eye condition, is rare as the first sign of BD.
CASE SUMMARY
We present an unusual case of a 30-year-old man who developed panuveitis after receiving the mRNA-based coronavirus disease 2019 (COVID-19) vaccine (Moderna). Laboratory tests ruled out infections, but he had a positive HLA-B51 result and a history of genital ulcer and oral ulcers, leading to a BD diagnosis. Treatment with corticosteroids improved his condition. Interestingly, he had another episode of panuveitis after the second mRNA vaccine dose, which also responded to corticosteroids.
CONCLUSION
This case highlights the rare onset of BD following mRNA COVID-19 vaccination, suggesting a potential link between these vaccines and BD's eye symptoms, emphasizing the importance of quick treatment in similar cases.
Keywords: Behcet’s disease, mRNA COVID-19 vaccine, Ocular inflammation, Panuveitis, Case report
Core Tip: This case report highlights a rare instance of panuveitis as the first manifestation of Behcet's disease in a 30-year-old man following mRNA-based coronavirus disease 2019 vaccination (Moderna). The patient developed recurrent uveitis after both doses of the vaccine. This case suggests a potential link between mRNA vaccines and ocular inflammation in genetically predisposed individuals, particularly those with HLA-B51. It underscores the importance of considering Behcet's disease in patients presenting with panuveitis post-vaccination and calls for further research to confirm this association and understand the underlying mechanisms.
INTRODUCTION
Uveitis, a term used to describe inflammation of the uvea, can lead to significant vision loss if not diagnosed promptly and managed appropriately[1]. It can be triggered by various causes, including infections, autoimmune diseases, malignancies, and possibly, vaccines[2]. This array of etiologies highlights the complexity and the importance of recognizing the presenting symptoms of uveitis to guide effective management.
Behcet's disease, an inflammatory disorder of unknown cause, is one of the systemic diseases that can lead to uveitis[3]. The disease is characterized by recurrent oral and genital ulcers, skin lesions, and uveitis. Genetic predisposition, particularly the presence of HLA-B51, has been associated with Behcet’s disease[4]. With the ongoing global coronavirus disease 2019 (COVID-19) pandemic, the focus of medical attention has recently been on vaccines, particularly mRNA vaccines, as a potential trigger for uveitis[5]. These vaccines, including the mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (Moderna), have proven to be highly effective in combating the spread of the SARS-CoV-2. However, a handful of cases have reported the onset or recurrence of uveitis following the administration of these vaccines. In this article, we present a unique case of a 30-year-old Taiwanese male patient who experienced recurrent uveitis following administration of the mRNA-1273 vaccine. The case provides an opportunity to explore and elucidate potential links between COVID-19 vaccination and the onset or exacerbation of uveitis.
CASE PRESENTATION
Chief complaints
A 30-year-old healthy Taiwanese man visited the clinic with a complaint of foggy vision for about 5 d in both eyes.
History of present illness
The patient complained of foggy vision in both eyes approximately 1 wk after receiving his first dose of the mRNA-1273 SARS-CoV-2 vaccine (Moderna).
History of past illness
The patient's medical history was devoid of any conditions, including the absence of other diseases such as hypertension, diabetes mellitus, heart disease, or tuberculosis. However, since his adulthood, he had recurrent episodes of oral ulceration occurring approximately every 2 months.
Personal and family history
The patient reported no family history of any disease or cancerous growth.
Physical examination
At the time of presentation, the patient’s best-corrected visual acuity (BCVA) was 20/25 in the right eye and 20/20 in the left eye. Intraocular pressures were within the normal range in both eyes. On examination, bilateral fine keratic precipitates were observed, along with 3+ cells in the anterior chamber (AC) and 2+ cells in the anterior vitreous body of the right eye. Additionally, trace cells were noted in the AC, along with 1+ cells in the anterior vitreous of the left eye. In addition to intraocular inflammation, oral ulcer and genital ulcer were found in this patient.
Laboratory examinations
Laboratory investigations for infectious and inflammatory causes were all unremarkable, except for a slightly elevated erythrocyte sedimentation rate (18 mm/h) and a positive result for HLA-B51.
Imaging examinations
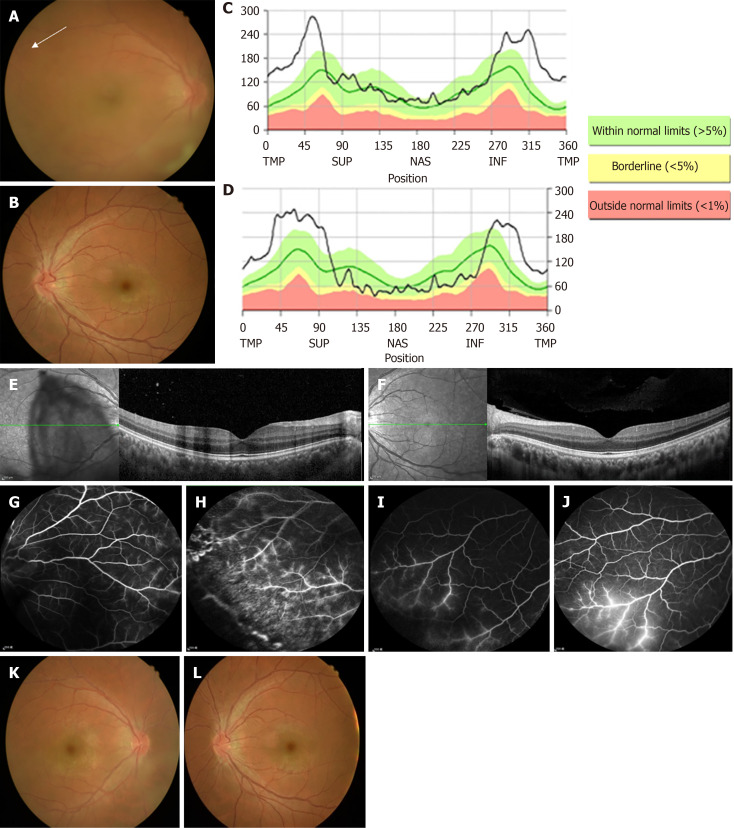
Fundus examination revealed prominent vitreous haze of the right eye and disc congestion and venous engorgement in both eyes (Figure 1A and B).
Figure 1.
Fundus examination. A and B: Fundus photographs revealing disc congestion, venous engorgement, and retinal hemorrhage at periphery (white arrow) in right eye (A) and left eye (B); C-F: Optical coherence tomography showed prominent bilateral disc edema (C and D) and increased inner retina thickness of macula in his right eye (E). The thickness of the left macula was unremarkable (F); G-J: Fluorescein angiography disclosed predominant phlebitis with perivascular staining at periphery in right eye (G and H) and left eye (I and J); K and L: Two weeks after treatment, the fundus of both eyes had cleared up.
Optical coherence tomography (OCT) demonstrated prominent bilateral disc edema (Figure 1C and D) and increased thickness of the inner retina in the macula of the right eye (Figure 1E and F). Fluorescein angiography (FA) revealed predominant phlebitis with perivascular staining at the periphery in both eyes (Figure 1G-J).
FINAL DIAGNOSIS
According to the international criteria for Behcet’s disease, all these features were consistent with a diagnosis of Behcet’s disease.
TREATMENT
The patient was initiated on topical 1% prednisolone acetate suspension, administered four times daily, and oral prednisolone at a dose of 40 mg per day.
OUTCOME AND FOLLOW-UP
Two weeks after the treatment, the BCVA improved to 20/20 in both eyes. The cells in the AC were reduced to trace in the right eye and were clear in the left eye. Fundus examination showed resolution of vitreous haze of the right eye and disc congestion in both eyes (Figure 1K and L). Four weeks after the initial treatment, the BCVA remained at 20/20 in both eyes. In the right eye, there were trace cells in the AC and anterior vitreous body, while both the AC and anterior vitreous body in the left eye were clear. Oral prednisolone was gradually tapered to a lower dose. However, as the patient and his wife were planning to undergo in vitro fertilization, he did not receive immunomodulatory therapy due to potential teratogenic side effects. Additionally, we delayed administering the patient's second dose of vaccine because the inflammation within his eye had not completely resolved. The patient was maintained on 7.5 mg of oral prednisolone, and his eye condition remained stable thereafter.
Unfortunately, 6 mo after the initial episode of uveitis, the patient experienced a recurrence of foggy vision in both eyes, approximately 1 wk after receiving his second dose of mRNA-1273 SARS-CoV-2 vaccine (Moderna). At the time of recurrence, his BCVA was 20/30 in the right eye and 20/25 in the left eye. Examination revealed 3+ cells in the AC, 2+ cells in the anterior vitreous body of the right eye, and 1+ cells in the AC and 1+ cells in the anterior vitreous body of the left eye. The patient was once again treated with topical 1% prednisolone acetate suspension administered four times daily, along with oral prednisolone at a dose of 40 mg per day. One week after treatment, the BCVA improved to 20/25 in the right eye and 20/20 in the left eye. The cells in the AC and anterior vitreous body decreased to 1+ in the right eye and trace in the left eye. Two weeks after treatment, the BCVA in both eyes was 20/20. The cells in the AC and anterior vitreous body were trace in the right eye, while the AC and anterior vitreous body were clear in the left eye. Oral prednisolone was tapered gradually to a lower dose.
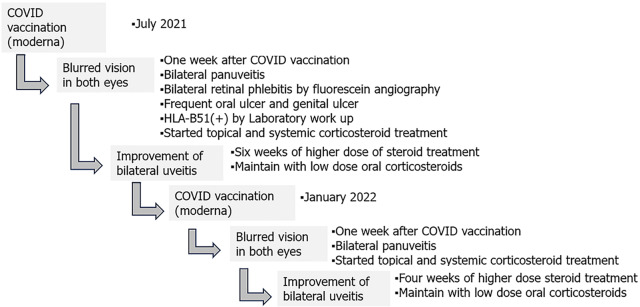
The patient is currently maintained on a daily dose of 7.5 mg of oral prednisolone, and the condition of both eyes has remained stable without any recurrence over the following 18 mo. Timeline of the disease course of this patient is illustrated in Figure 2.
Figure 2.
The patient’s timeline. COVID: Coronavirus disease.
DISCUSSION
This case raises the possibility of an association between mRNA-based COVID-19 vaccines and the development of panuveitis as the first manifestation of Behcet's disease in susceptible individuals. Behcet's disease is a rare systemic vasculitis with ocular involvement, typically uveitis, occurring in approximately 50%-70% of patients[6]. Panuveitis is a more severe form of uveitis, involving inflammation of the entire uveal tract, and is associated with poorer visual prognosis[7]. The exact etiology of Behcet's disease is unknown, but it is believed to involve genetic and environmental factors, as well as dysregulation of immune responses. The HLA-B51 allele is strongly associated with Behcet's disease[8], and it is hypothesized that exposure to certain environmental factors or infections may trigger an immune response in genetically susceptible individuals, leading to the development of the disease.
The role of vaccines in triggering autoimmune and inflammatory reactions has been previously reported[9]. In our case, the patient developed panuveitis following two mRNA-based COVID-19 vaccine administrations, suggesting a potential association. The mRNA vaccines have a novel mechanism of action, as they introduce synthetic mRNA encoding the SARS-CoV-2 spike protein, which then leads to the production of the protein and an immune response[10]. It is possible that in genetically predisposed individuals, the immune response to the vaccine could trigger an aberrant reaction, leading to the development of Behcet's disease and panuveitis. However, the exact mechanism underlying this association remains unknown. This case report raises several important questions. First, it highlights the need for further research to determine whether mRNA-based COVID-19 vaccines can trigger panuveitis as the first manifestation of Behcet's disease in susceptible individuals. Second, it emphasizes the importance of a comprehensive evaluation for underlying systemic diseases in patients presenting with panuveitis following vaccination. Finally, this case underscores the need for a tailored approach to the management of Behcet's disease-associated panuveitis, taking into consideration the potential risks and benefits of immunosuppressive therapy.
In addition to ocular adverse effects, the majority of adverse effects reported following Moderna vaccine administration are mild to moderate. Common adverse effects are injection site reactions (pain, swelling, and redness), fatigue, headache, muscle pain, fever, etc. These effects are generally considered signs of the body's immune response to the vaccine and are not a cause for significant concern[11]. Some less frequent but notable adverse effects have been reported, including lymphadenopathy and delayed local reactions[12]. While rare, some serious adverse effects have been associated with the Moderna vaccine such as anaphylaxis[13], myocarditis and pericarditis[14], and thrombosis with thrombocytopenia syndrome[15]. Most vaccine-associated adverse effects resolve within days to weeks after treatment. However, there are also rare side effects that can cause long-term effects. Long post-COVID vaccination syndrome (LPCVS) is a rare but severe adverse effect, causing patients to develop various neurocognitive symptoms, including headache, dizziness, and impaired thinking and concentration, and there is no effective treatment so far[16].
CONCLUSION
In summary, we present a unique case of panuveitis as the first manifestation of Behcet's disease following mRNA-based COVID-19 vaccination. Although the association between mRNA-based COVID-19 vaccines and panuveitis in Behcet's disease remains to be confirmed, this case highlights the importance of considering such a possibility in the evaluation and management of patients with similar presentations. Further studies are needed to elucidate the underlying mechanisms and establish a definitive causal relationship between mRNA-based COVID-19 vaccines and the development of panuveitis in Behcet's disease.
ACKNOWLEDGEMENTS
We would like to express our gratitude to our patient and his family for allowing us to publish this case report.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for the publication of this case report.
Conflict-of-interest statement: All authors declare that there are no conflicts of interest for this paper.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Ophthalmological Society of Taiwan, A1261; Taiwan Academy of Ophthalmology, M0121.
Specialty type: Ophthalmology
Country of origin: Taiwan
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade A
Scientific Significance: Grade A
P-Reviewer: Said ZNA S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Yu HG
Contributor Information
Rou-Ting Lin, School of Medicine, College of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; Department of Clinical Education and Training, Kaohsiung Medical University Hospital, Kaohsiung 80756, Taiwan.
Pei-Kang Liu, Department of Ophthalmology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80737, Taiwan; Department of Ophthalmology, Kaohsiung Medical University Hospital, Kaohsiung 80756, Taiwan.
Chia-Wei Chang, Department of Clinical Education and Training, Kaohsiung Medical University Hospital, Kaohsiung 80756, Taiwan.
Kai-Chun Cheng, Department of Ophthalmology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80737, Taiwan; Department of Ophthalmology, Kaohsiung Medical University Hospital, Kaohsiung 80756, Taiwan.
Kuo-Jen Chen, Department of Ophthalmology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung 81201, Taiwan.
Yo-Chen Chang, Department of Ophthalmology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80737, Taiwan; Department of Ophthalmology, Kaohsiung Medical University Hospital, Kaohsiung 80756, Taiwan; Department of Ophthalmology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung 81201, Taiwan. ycchang@kmu.edu.tw.
References
- 1.Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, Huong DL, Piette JC, LeHoang P. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 2001;80:263–270. doi: 10.1097/00005792-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500; discussion 500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Davatchi F, Chams-Davatchi C, Shams H, Shahram F, Nadji A, Akhlaghi M, Faezi T, Ghodsi Z, Sadeghi Abdollahi B, Ashofteh F, Mohtasham N, Kavosi H, Masoumi M. Behcet's disease: epidemiology, clinical manifestations, and diagnosis. Expert Rev Clin Immunol. 2017;13:57–65. doi: 10.1080/1744666X.2016.1205486. [DOI] [PubMed] [Google Scholar]
- 4.de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behçet's disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009;61:1287–1296. doi: 10.1002/art.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacherini D, Biagini I, Lenzetti C, Virgili G, Rizzo S, Giansanti F. The COVID-19 Pandemic from an Ophthalmologist's Perspective. Trends Mol Med. 2020;26:529–531. doi: 10.1016/j.molmed.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evereklioglu C. Current concepts in the etiology and treatment of Behçet disease. Surv Ophthalmol. 2005;50:297–350. doi: 10.1016/j.survophthal.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Belkhadir K, Boutimzine N, Laghmari M, Amazouzi A, Tachfouti S, Cherkaoui O. [Prognostic factors of ocular involvement in Behçet's disease] J Fr Ophtalmol. 2019;42:612–617. doi: 10.1016/j.jfo.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Direskeneli H. Behçet's disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis. 2001;60:996–1002. doi: 10.1136/ard.60.11.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasaka Y, Hasegawa E, Keino H, Usui Y, Maruyama K, Yamamoto Y, Kaburaki T, Iwata D, Takeuchi M, Kusuhara S, Takase H, Nagata K, Yanai R, Kaneko Y, Iwahashi C, Fukushima A, Ohguro N, Sonoda KH JOIS Uveitis Survey Working Group. A multicenter study of ocular inflammation after COVID-19 vaccination. Jpn J Ophthalmol. 2023;67:14–21. doi: 10.1007/s10384-022-00962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, Dinnon KH, Elbashir SM, Shaw CA, Woods A, Fritch EJ, Martinez DR, Bock KW, Minai M, Nagata BM, Hutchinson GB, Wu K, Henry C, Bahl K, Garcia-Dominguez D, Ma L, Renzi I, Kong WP, Schmidt SD, Wang L, Zhang Y, Phung E, Chang LA, Loomis RJ, Altaras NE, Narayanan E, Metkar M, Presnyak V, Liu C, Louder MK, Shi W, Leung K, Yang ES, West A, Gully KL, Stevens LJ, Wang N, Wrapp D, Doria-Rose NA, Stewart-Jones G, Bennett H, Alvarado GS, Nason MC, Ruckwardt TJ, McLellan JS, Denison MR, Chappell JD, Moore IN, Morabito KM, Mascola JR, Baric RS, Carfi A, Graham BS. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, Hashimoto D, Banerji A, Li L, Anvari S, Shenoy ES. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimabukuro TT, Cole M, Su JR. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacNeil JR, Su JR, Broder KR, Guh AY, Gargano JW, Wallace M, Hadler SC, Scobie HM, Blain AE, Moulia D, Daley MF, McNally VV, Romero JR, Talbot HK, Lee GM, Bell BP, Oliver SE. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients - United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finsterer J. A Case Report: Long Post-COVID Vaccination Syndrome During the Eleven Months After the Third Moderna Dose. Cureus. 2022;14:e32433. doi: 10.7759/cureus.32433. [DOI] [PMC free article] [PubMed] [Google Scholar]