Abstract
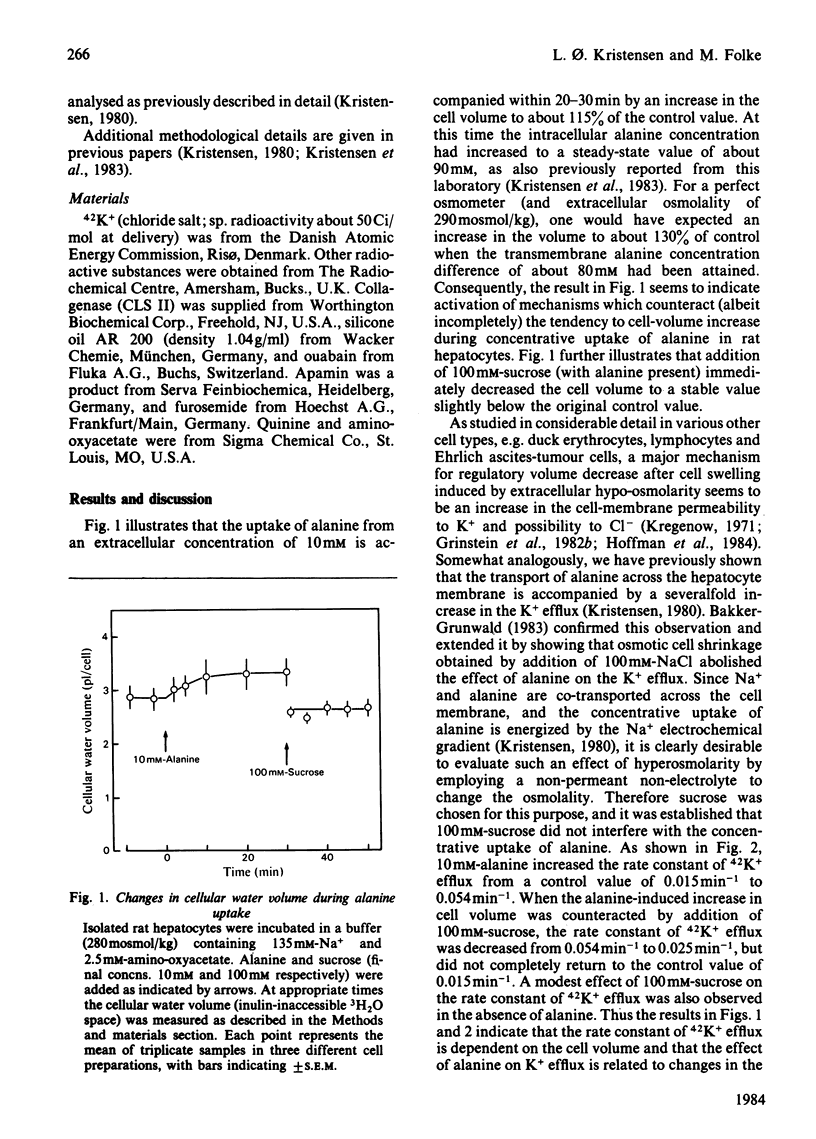
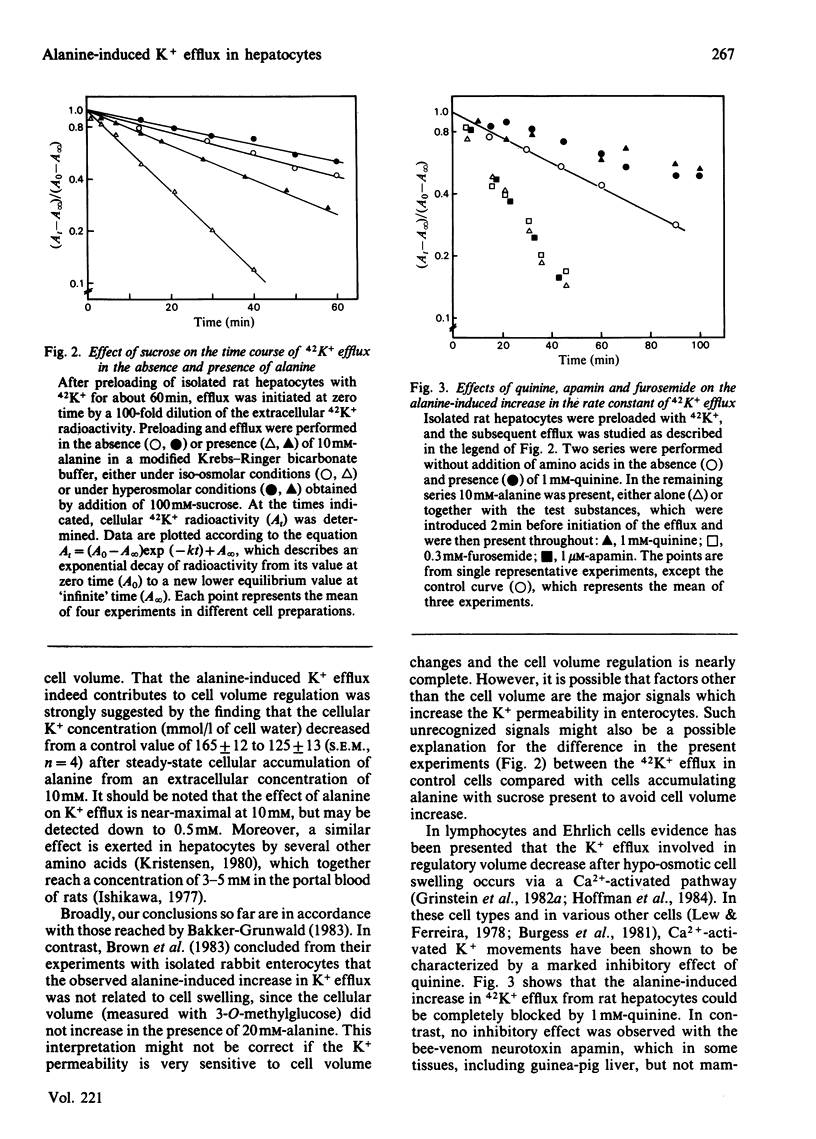
Changes in cell volume and 42K+ efflux associated with concentrative alanine uptake were studied in isolated rat hepatocytes suspended in Krebs-Ringer bicarbonate buffer. After addition of 10 mM-alanine, cellular water volume increased by 15% and the rate constant of 42K+ efflux by 250%. Alanine-induced 42K+ efflux was abolished by quinine and was strongly decreased when the cell-volume increase was counteracted by sucrose. The results suggest that K+ efflux during alanine uptake is implicated in a volume-regulatory response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker-Grunwald T. Potassium permeability and volume control in isolated rat hepatocytes. Biochim Biophys Acta. 1983 Jun 10;731(2):239–242. doi: 10.1016/0005-2736(83)90014-7. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Burton K. A., Sepúlveda F. V. Transport of sugars or amino acids increases potassium efflux from isolated enterocytes. FEBS Lett. 1983 Nov 14;163(2):203–206. doi: 10.1016/0014-5793(83)80819-9. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Dupre A., Rothstein A. Volume-induced increase of anion permeability in human lymphocytes. J Gen Physiol. 1982 Dec;80(6):801–823. doi: 10.1085/jgp.80.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Dupre A., Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982 May;79(5):849–868. doi: 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Grasset E., Schultz S. G. Sodium-coupled amino acid and sugar transport by Necturus small intestine. An equivalent electrical circuit analysis of a rheogenic co-transport system. J Membr Biol. 1982;66(1):25–39. doi: 10.1007/BF01868479. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O., Lambert I. H. Volume-induced increase of K+ and Cl- permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+. J Membr Biol. 1984;78(3):211–222. doi: 10.1007/BF01925969. [DOI] [PubMed] [Google Scholar]
- Ishikawa E. The regulation of uptake and output of amino acids by rat tissues. Adv Enzyme Regul. 1976;14:117–136. doi: 10.1016/0065-2571(76)90010-8. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):372–395. doi: 10.1085/jgp.58.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen L. O. Energization of alanine transport in isolated rat hepatocytes. Electrogenic Na+-alanine co-transport leading to increased K+ permeability. J Biol Chem. 1980 Jun 10;255(11):5236–5243. [PubMed] [Google Scholar]
- Kristensen L. O., Sestoft L., Folke M. Concentrative uptake of alanine in hepatocytes from fed and fasted rats. Am J Physiol. 1983 May;244(5):G491–G500. doi: 10.1152/ajpgi.1983.244.5.G491. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Schultz S. G. Studies on the electrical potential profile across rabbit ileum. Effects of sugars and amino acids on transmural and transmucosal electrical potential differences. J Gen Physiol. 1971 Jun;57(6):639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Ericson A. C. Epithelial cell volume modulation and regulation. J Membr Biol. 1982;69(3):167–176. doi: 10.1007/BF01870396. [DOI] [PubMed] [Google Scholar]


