Abstract
Background
Advantages to combining childhood vaccines include reducing the number of visits, injections and patient discomfort, increasing compliance and optimising prevention. The World Health Organization (WHO) recommends that routine infant immunisation programmes include a vaccination against Haemophilus influenzae (H. influenzae) type B (HIB) in the combined diphtheria‐tetanus‐pertussis (DTP)‐hepatitis B virus (HBV) vaccination. The effectiveness and safety of the combined vaccine should be carefully and systematically assessed to ensure its acceptability by the community.
Objectives
To compare the effectiveness of combined DTP‐HBV‐HIB vaccines versus combined DTP‐HBV and separate HIB vaccinations.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 4), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to week 1, November 2011), EMBASE (January 1990 to November 2011) and www.clinicaltrials.gov (up to April 2011).
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs comparing vaccination with any combined DTP‐HBV‐HIB vaccine, with or without three types of inactivated polio virus (IPV) or concomitant oral polio vaccine (OPV) in any dose, preparation or time schedule, compared with separate vaccines or placebo, administered to infants up to two years old.
Data collection and analysis
Two review authors independently inspected references identified by the searches and evaluated them against the inclusion criteria, extracted data and assessed the methodological quality of included trials.
Main results
Data for the primary outcome (prevention of disease) were lacking. We performed a meta‐analysis to pool the results of 20 studies with 5874 participants in an immunogenicity analysis and 5232 participants in the reactogenicity analysis. There were no data on clinical outcomes for the primary outcome (prevention of disease) and all studies used immunogenicity and reactogenicity (adverse events). The number of vaccine doses differed significantly between the studies. Heterogeneous interventions, study location, healthcare environment and combining research across disparate geographical locations, may have lead to bias. The risk of bias was unclear across most of the included studies. Comparisons found little heterogeneity. In two immunological responses the combined vaccine achieved lower responses than the separate vaccines for HIB and tetanus. No significant differences in immunogenicity were found for pertussis, diphtheria, polio and hepatitis B. Serious adverse events were comparable with mainly hospitalisation and acute bronchiolitis cases. Minor adverse events such as pain and redness were more common in children given the combined vaccine. Overall, the direction shown by the results is in favour of the DTPw (diptheria‐tetanus‐whole cell pertussis)‐HBV‐HIB vaccine rather than the DTPa (diptheria‐tetanus‐acellular pertussis)‐HBV‐HIB vaccine when compared to the separate vaccines (size of effect: risk ratio (RR) 1.43; 95% confidence interval (CI) 0.98 to 2.10, for 5269 participants).
Authors' conclusions
We could not conclude that the immune responses elicited by the combined vaccine were different from or equivalent to the separate vaccines. There was significantly less immunological response for HIB and tetanus and more local reactions in the combined injections. However, these differences rely mostly on one study each. Studies did not use an intention‐to‐treat (ITT) analysis and we were uncertain about the risk of bias in many of the studies. These results are therefore inconclusive. Studies addressing clinical end points whenever possible, using correct methodology and a large enough sample size should be conducted.
Keywords: Child, Preschool; Female; Humans; Infant; Infant, Newborn; Diphtheria; Diphtheria/immunology; Diphtheria/prevention & control; Diphtheria‐Tetanus‐Pertussis Vaccine; Diphtheria‐Tetanus‐Pertussis Vaccine/administration & dosage; Diphtheria‐Tetanus‐Pertussis Vaccine/immunology; Haemophilus Infections; Haemophilus Infections/immunology; Haemophilus Infections/prevention & control; Haemophilus Vaccines; Haemophilus Vaccines/administration & dosage; Haemophilus Vaccines/immunology; Hepatitis B; Hepatitis B/immunology; Hepatitis B/prevention & control; Hepatitis B Vaccines; Hepatitis B Vaccines/administration & dosage; Hepatitis B Vaccines/immunology; Tetanus; Tetanus/immunology; Tetanus/prevention & control; Vaccines, Combined; Vaccines, Combined/administration & dosage; Vaccines, Combined/immunology; Whooping Cough; Whooping Cough/immunology; Whooping Cough/prevention & control
Plain language summary
Combined DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines in healthy infants up to two years old
Childhood vaccinations provide an effective method of protection against diseases. The World Health Organization (WHO) recommends that routine infant immunisation programmes include a vaccination against Haemophilus influenzae (H. influenza) type B (HIB) in the combined diphtheria‐tetanus‐pertussis (DTP)‐hepatitis B virus (HBV) vaccination. We compared the combined DTP‐HBV‐HIB vaccine with the separate DTP‐HBV and HIB vaccines. Studies only reported on immunogenicity and reactogenicity.
We included 20 studies with 5874 participants in the immunogenicity analysis and 5232 in the reactogenicity analysis. In two immunological responses, the combined vaccine achieved lower responses than the separate vaccines for HIB and tetanus. We did not find any significant differences in immunogenicity for pertussis‐diphtheria‐polio and hepatitis B. Serious adverse events were comparable. Minor adverse events were more common with the combined vaccine. Overall, the level of evidence provided by the studies was low and we could not conclude that the immune responses with the combined vaccine were equivalent to the separate injections.
Background
Description of the condition
Despite the availability of proven vaccination, hepatitis B virus (HBV) and Haemophilus influenzae (H. influenzae) type B (HIB) infections continue to be endemic in many parts of the world. The benefits of effective immunisation against HBV and HIB disease during the first year of life are known and in 1996, the World Health Organization (WHO) set an objective for the development of a vaccine combining HBV with the established diphtheria‐tetanus‐whole cell pertussis (DTPw) antigens (Ortega‐Barria 2007). In 1998, the WHO further recommended the inclusion of HIB conjugate vaccines in infant immunisation programmes (WHO 1998).
Description of the intervention
Childhood vaccinations provide clinically‐effective and cost‐effective methods of protecting against many diseases. Combination vaccines have been widespread since the 1940s. Diphtheria‐tetanus‐pertussis (DTP) is one such vaccine and it is estimated that the DTP infant vaccine coverage exceeds 80% worldwide (Faingezicht 2002). There are multiple advantages to combining vaccines, for example, reducing the number of visits and injections, increasing compliance, reducing patient discomfort, optimising prevention and reducing operational costs. This might not be the case in some countries such as the United States (US), where combination vaccines are often more expensive than the separate components.
Assessment of the immune responses to combination vaccines has generally been based on randomised controlled comparative trials. The US Food and Drug Administration (FDA) recommends that clinical trials compare the immune responses elicited by the combination vaccine versus separate injections or other appropriate controls. End points commonly used for evaluating combination vaccines include the percentage of people responding to an antigen with a predefined antibody level and the geometric mean concentration (GMC) or geometric mean titre (GMT) of antibodies elicited by the component (Ball 2001).
How the intervention might work
The WHO recommends that routine infant immunisation programmes include a vaccination against HIB in the combined DTP‐HBV injection (WHO 1998). HIB is an important pathogen in both high‐income and low‐income countries. The DTP‐HBV combination vaccine would make an ideal partner for combining with HIB vaccines, because the DTP vaccine is mandatory in most immunisation programmes and the HBV vaccination is already in widespread use (Santos 2002).
Why it is important to do this review
The strategy of combining the HBV vaccine with the DTP vaccine has already been adopted into immunisation programmes (Riedemann 2002). The effectiveness and safety of adding a conjugate HIB vaccination to the DTP‐HBV vaccine, compared with separate administrations, for preventing these diseases has yet to be systematically assessed. The immunogenicity and reactogenicity (adverse events) results of five published clinical trials involving Tritanrix‐HBV/HIB in a variety of immunisation schedules and countries were reviewed for its suitability for use in national immunisation programmes (Aristegui 2003). Despite its use in accordance with the WHO recommendation in several countries, no systematic review of the effectiveness and safety of the combined vaccine is available.
Objectives
The objective of the review is to assess the clinical protection, immunogenicity (defined as antibody concentration responses to infectious diseases) and reactogenicity (adverse events) of a combined DTP vaccine, (including both Pw (whole cell pertussis) and Pa (acellular pertussis) vaccines), HBV and conjugate HIB vaccine, (with or without three types of inactivated polio virus (IPV) or concomitant oral polio vaccine (OPV)), in comparison with separate vaccinations of DTP, HBV, conjugate HIB, IPV and OPV, in infants up to two years of age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Healthy male and female infants up to two years of age.
Types of interventions
The interventions were vaccination with any combined DTP (applied to both DTPw and DTPa vaccines) ‐HBV‐conjugate HIB vaccine with or without three types of inactivated polio virus (IPV) or concomitant oral polio vaccine (OPV) given in any dose, preparation or time schedule, compared with separate vaccines or placebo, administered to infants aged up to two years. All identified trials tested the effectiveness of the combined DTP‐HBV‐conjugate HIB vaccine.
Types of outcome measures
Primary outcomes
The incidence of diphtheria, tetanus, pertussis, hepatitis B and HIB post‐vaccination.
Secondary outcomes
Immunogenicity, defined as antibody responses to tetanus, diphtheria, pertussis, hepatitis B and HIB.
Systemic and local adverse events, including fever, pain, redness, swelling, irritability, drowsiness, loss of appetite, vomiting and more generalised and severe signs, including potential adverse events which have been hypothesised in relation to the vaccination.
Search methods for identification of studies
Electronic searches
Previously we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 1); MEDLINE (January 1966 to March 2009); and EMBASE (January 1990 to March 2009).
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 4, www.thecochranelibrary.com (accessed 11 November 2011)); MEDLINE (March 2009 to November week 1, 2011); EMBASE (March 2009 to November 2011); and www.clinicaltrials.gov (March 2008 to April 2011).
We used the terms in Appendix 1 to search CENTRAL and MEDLINE. We combined the search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) Ovid format (Lefebvre 2011). We modified these search terms and used a filter developed by Wong (Wong 2006) to fit the EMBASE.com interface (see Appendix 2). We imposed no language or publication restrictions.
Searching other resources
In addition, we scrutinised clinical practice guideline reference lists to identify additional trials. We also checked relevant RCT references for additional studies. We looked for eligible titles and abstracts in electronic search results and obtained the full‐text articles that we identified as potentially eligible. We scanned the bibliographies of all included studies and pertinent reviews for additional references.
We searched the following conference proceedings for unpublished trials: Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 1995 to 2006 (available at www.icaac.org/icaacarch.asp); European Congress of Clinical Microbiology and Infectious Diseases 2001 to 2006 (available at www.akm.ch); and the Annual Meeting of the Infectious Diseases Society of America (IDSA) 2001 to 2006 (available at www.idsociety.org/).
Data collection and analysis
Selection of studies
Three review authors (ESB, EG, SH) independently inspected references identified by the searches and evaluated them against our inclusion criteria. We resolved disagreements in the selection of relevant studies by consensus. Three review authors (ESB, EG, SH) independently inspected the full‐text articles in cases of disagreement. We consulted a fourth review author (LL) in cases of continued disagreement. We have detailed the reasons for excluding studies.
Data extraction and management
Three review authors (EB, EG, SH) independently extracted data and assessed the methodological quality of each included trial. For each treatment group, we collected the following data.
Intervention characteristics: vaccination type, manufacturer, number of doses and schedule.
Characteristics of trial: publication year, start date, end date, study design, country where trial was preformed, data collection method, location of trial and date evaluated.
Quality assessment: blinding, unit of allocation, allocation generation and allocation concealment.
Case definitions ‐ characteristics of participants: exclusion, inclusion, age and number randomised.
Outcomes:
A. Immunogenicity ‐ antibody concentrations by serological analysis: number participated, exclusion (post‐random = evaluated for serology), number with antibody concentrations above the assay cut‐offs (PRP (polyribsylribitolphosphate), PRP‐T (vaccine conjugated to tetanus toxoid), FHA (filamentous haemagglutinin), PRN‐pertactin, BPT‐pertussis (PTox pertussis toxin), Bordetella pertussis (B. pertussis), HB‐hepatitis B, D‐diphtheria, T‐tetanus, polio type 1, polio type 2, polio type 3.
B. Reactogenicity ‐ adverse events: number of vaccines, number of participants and number of events:
serious adverse events
pain
redness
swelling
fever (elevated temperature)
fussiness or restlessness
poor appetite
vomiting
irritability or tenderness
diarrhoea
unusual crying
sleeping more than usual.
Assessment of risk of bias in included studies
For this update, we used the recommended new 'Risk of bias' tool to assess methodological quality according to: adequate sequence generation, allocation concealment, blinding, incomplete outcome data addressed, free of selective reporting and free of other bias (Higgins 2011). We used the categories 'high risk', 'low risk' and 'unclear risk' of bias to measure trial quality.
Measures of treatment effect
We analysed dichotomous (or binary) data, where each individual’s outcome is one of only two possible categorical responses, by calculating the risk ratio (RR) for each trial with the uncertainty in each result expressed using 95% confidence intervals (CIs).
Unit of analysis issues
No studies reported on the main outcome, i.e. incidence of diphtheria, tetanus, pertussis, hepatitis B and HIB post‐vaccination. All studies reported on immunogenicity, defined as antibody concentration responses to tetanus, diphtheria, pertussis, hepatitis B and HIB.
We performed a meta‐analysis to pool the results of 20 studies. We analysed vaccine immunogenicity in subcategories, according to two types of pertussis vaccination: acellular pertussis (Pa) and whole cell pertussis (Pw).
We defined infants with no seroprotective antibody titres (with titres below the assay cut‐off or without seroconversion) as events. Studies reported combined inactivated polio virus (IPV) in the DTP‐HBV‐HIB vaccine and oral polio vaccine (OPV) administered concurrently and therefore we included results of anti‐polio types 1, 2 and 3.
We analysed reactogenicity (adverse events) by events of total symptom scores (incidence of any solicited local and systemic adverse events). Serious adverse events were reported by investigators and data completed upon our request. Incidence of any solicited local and systemic adverse events included pain, redness, swelling, fever (elevated temperature), fussiness or restlessness, poor appetite, vomiting, irritability or tenderness, diarrhoea, unusual crying, or sleeping more than usual.
Dealing with missing data
Data of serious adverse events for some of the included trials are missing (although we did contact trial authors for additional information). We described missing participants due to drop‐outs and whether intention‐to‐treat (ITT) analysis was conducted in the studies in the 'Risk of bias' tables under 'Incomplete outcome data (attrition bias)'.
Assessment of heterogeneity
We initially assessed heterogeneity in the results of the trials by inspection of graphical presentations and by calculating an estimate of heterogeneity (Chi2 test and I2 statistic).
Assessment of reporting biases
We examined the funnel plot, using the method described in Egger 1997 to estimate the precision of trials (the inverse of the standard error plotted against the RR), in order to estimate potential selection bias (publication or other).
Data synthesis
We used a random‐effects model throughout the review because of heterogeneity. We pooled data, stratifying for number of doses received. We used a fixed‐effect model in the sensitivity analysis.
Subgroup analysis and investigation of heterogeneity
An approximate guide to interpretation of the I2 statistic is as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity.
Different subgroups contain different amounts of information and thus have different abilities to detect effects. Therefore, we did not use other methods for investigating heterogeneity of effects in the meta‐analysis.
Sensitivity analysis
We performed a post hoc sensitivity analysis for the anti‐PRP (polyribosylribitolphoshate) comparison by excluding this study from the analysis. It was influenced by one study with a large number of events (Pichichero 1997), which used pure (and not conjugated) PRP vaccines.
Results
Description of studies
Results of the search
We identified 246 studies (47 studies in this update), of which we considered 60 as potentially eligible, including eight studies in this update.
Included studies
We included 20 studies. Two different types of pertussis vaccination were used in the studies; 10 studies used acellular pertussis (DTPa) and 10 studies used whole cell pertussis (DTPw) (as part of the diptheria‐tetanus‐pertussis vaccine). In five studies inactivated polio virus (IPV) was combined with the DTP‐HBV‐HIB vaccine (Aristegui 2003; Avdicova 2002; Gabutti 2004; Mallet 2000; Schmitt 2000), while three studies reported oral polio vaccine (OPV) administered to all vaccinees in both groups concurrently (Nolan 2001; Omenaca 2001; Pichichero 1997).
Excluded studies
We excluded forty studies. Four studies were not true RCTs: one was an observational study (Kalies 2004); one trial was a single group design (Lopez 2002); one was a presentation of data from investigations on the nature and function of anti‐HIB antibodies (Poolman 2001); and one was a report of four primary and booster‐based paediatric clinical trials (Denoel 2007).
Six trials compared two different types of combined vaccines (Aristegui 2001; Gatchalian 2005; Gylca 2001; Scheifele 2006; Tichmann 2005; Tichmann‐Schumann 2005).
Three trials compared combined DTP/HIB and separate DTP + HIB vaccination without HBV vaccination (Botet‐Asensi 2003; Calbo 2002; Huang 1998).
One trial compared combined DTPa‐HBV‐IPV with separate DTPa‐HBV and IPV vaccines (Meriste 2006).
Two trials compared combined DTPw‐HBV‐HIB vaccine with separately administered DTPw‐HIB and HBV vaccines (Kanra 2006; Lim 2007)).
One trial compared primary and booster combined vaccines (Hla 2006).
One trial compared the fourth dose of combined DTPa‐IPV/PRP‐T with the third dose of combined vaccine (Scheifele 2005).
One trial compared combined DTPa‐HBV‐IPV‐HIB vaccine and pneumococcal conjugate vaccine (PCV7) with DTPa‐HBV‐IPV‐HIB vaccine (Knuf 2006).
One trial compared three lots of HIB conjugate vaccines (Aristegui 1998).
One study compared lot‐to‐lot consistency of combined vaccines and not with separate vaccines (Lagos 2005).
One trial compared a new combined DTPw‐HBV/HIB vaccine of HIB Lot 001A44 to HIB Lot 002A41 (Usonis 1999b).
One trial was a comparison between a five‐component pertussis combination vaccine CPDT‐IPV/PRP‐T to that of whole cell pertussis combination vaccine DPT‐IPV/PRP‐T (Mills 1998).
One trial compared a five‐component vaccine DTPa‐HBV‐IPV‐PCV7 and HIB with separate vaccines concurrently, or staggered (delayed) administration of PCV7 (Pichichero 2007).
We excluded another two trials that compared novel and local licensed DTPw/HIB vaccines (Clemens 2003) and the reactogenicity (adverse events) and immunogenicity of four commercial HIB vaccines (Usonis 1999a). We excluded another five trials that had no comparison between vaccines (Bavdekar 2007; Hogg 2003; Pichichero 1999; Trollfors 2005; Zepp 1997). We excluded three additional trials: in the first trial only data of safety and reactogenicity (adverse events) were provided (Zepp 2004); the second trial included the same trials reported elsewhere and only safety data was provided (Saenger 2005); and in the third excluded study, only data on antibody persistence (immunogenicity) of plain PRP and conjugate PRP‐T was provided (Nolan 2004).
In this 2011 update, we excluded six more studies: two studies compared different formulas of combined vaccines (Diaz‐Mitoma 2011; Madhi 2011) and three studies had no comparison between separate and combined vaccines (Gentile 2011; Halperin 2009; Kilpi 2009). One study compared DTPa‐IPV‐HBV‐PRP‐T vaccine with Pentaxim and Engerix B Pediatrico (HBV monovalent) vaccines in infants born to hepatitis B surface antigen seronegative mothers (Tregnaghi 2011).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
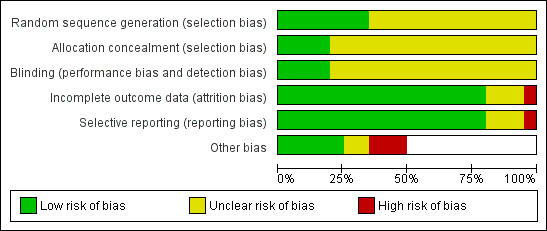
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
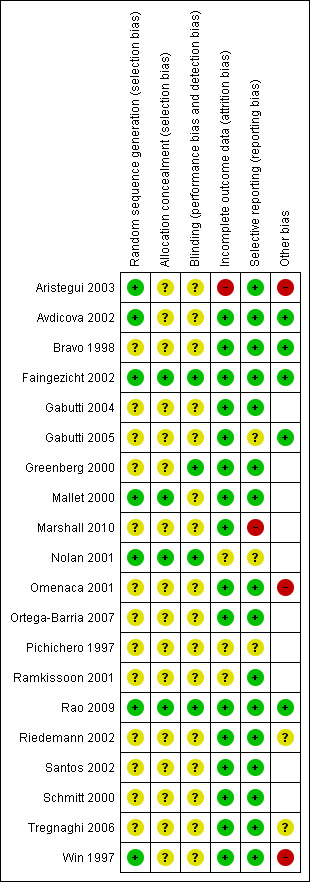
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation concealment
Four of the studies reported adequate allocation concealment (Faingezicht 2002; Mallet 2000; Nolan 2001; Rao 2009). One study reported inadequate allocation concealment (Bravo 1998).
Random sequence generation
Seven studies reported random sequence generation (Aristegui 2003; Avdicova 2002; Faingezicht 2002; Mallet 2000; Nolan 2001; Rao 2009; Win 1997).
Unit of allocation
All of the studies used infants or neonates as units of allocation.
Blinding
In one study where the term 'double‐blind' was used, it is not clear who was blinded (Nolan 2001). One study reported that three different production lots of the combined vaccine were used in a double‐blind manner but not for the control group (Tregnaghi 2006). In seven studies, blinding of assessors and/or laboratory personnel was reported (Faingezicht 2002; Greenberg 2000; Mallet 2000; Nolan 2001; Pichichero 1997; Rao 2009; Win 1997). Blinding of parents may not be relevant in the case of the infant's vaccination. Measurement of outcomes may not be influenced by the lack of blinding.
Incomplete outcome data
Incomplete outcome data were reported in most studies. Unclear risk was determined in three studies with no data (Nolan 2001; Pichichero 1997; Ramkissoon 2001). High risk of bias was determined in one study (Aristegui 2003).
Selective reporting
Reporting bias was determined by the method of collecting data for reactogenicity since immunogenicity data are not subject to reporting bias.
Most studies reported that parents documented the reactions (adverse events) for four days and therefore the reporting bias in the review is low. In one study there were no details how the adverse events were evaluated (Gabutti 2005). Two studies had no details on reporting method (Nolan 2001; Pichichero 1997) and in one study, serious adverse events were reported generally with no specification per study arm (Marshall 2010).
Other potential sources of bias
Intention‐to‐treat (ITT) analysis
No study clearly mentioned that the ITT principle was used in the analysis. Most studies excluded participants from analysis if they were leaving the study area, were lost to follow‐up, had an unsatisfactory compliance or protocol violation, parental request or consent was withdrawn, or experienced unrelated medical problems or death.
Effects of interventions
Immunogenicity: antibody concentrations by serological analysis
Data were not stratified for number of doses received. Last dose of the vaccines was extracted, excluding a booster dose.
Anti‐PRP (HIB) titres below the assay cutoff 0.15 µg/ml
Four studies of DTPa‐HBV‐HIB vaccines and three studies of DTPw‐HBV‐HIB vaccines were estimated. Four studies of DTPa‐HBV‐HIB vaccines and four studies of DTPw‐HBV‐HIB vaccines reported no events. No significant difference was found between combined and separate DTPa‐HBV‐HIB vaccines and DTPw‐HBV‐HIB vaccines (RR 1.82; 95% CI 0.98 to 3.38) (Analysis 1.1). No significant difference was found between combined and separate DTPw‐HBV‐HIB vaccines (RR 0.42; 95% CI 0.10 to 1.70). Significant difference was found between combined and separate DTPa‐HBV‐HIB vaccines (RR 2.60; 95% CI 1.33 to 5.08).
1.1. Analysis.
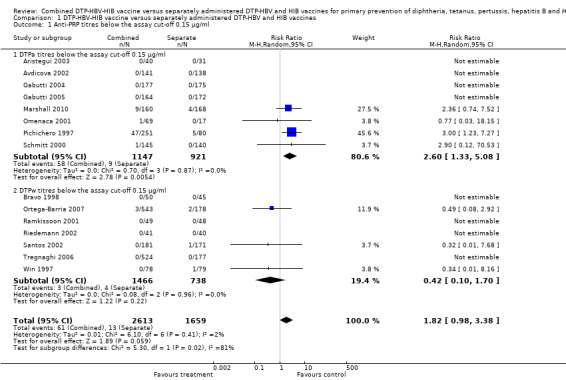
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 1 Anti‐PRP titres below the assay cut‐off 0.15 µg/ml.
Exclusion of Pichichero 1997, which donated most of the outcomes, resulted in a point estimate still in favour of the separate vaccines but no longer significant in a random‐effects model for the DTPa‐HBV‐HIB vaccines (RR 1.21; 95% CI 0.53 to 2.77). However, there is no significant heterogeneity for this comparison (Chi2 test 0.46, df 2, P = 0.8; and I2 statistic 0%) for the DTPa‐HBV‐HIB vaccines; and Chi2 test 3.93, df 5, P = 0.56; and I2 statistic 0% for all studies). Using a fixed‐effect model, there was no significant difference with the exclusion of Pichichero 1997 for the DTPa‐HBV‐HIB vaccines (RR 2.17; 95% CI 0.79 to 6.00) and RR 1.22; 95% CI 0.57 to 2.62 for all studies.
Anti‐PRP (HIB) titres below the assay cutoff 1.0 µg/ml
Nine studies of DTPa‐HBV‐HIB vaccines and six studies of DTPw‐HBV‐HIB vaccines reported on this outcome. No significant difference was found between combined and separate vaccines (RR 1.43; 95% CI 0.98 to 2.10) (Analysis 1.2). No significant difference was found between combined and separate DTPw‐HBV‐HIB vaccines (RR 0.83; 95% CI 0.44 to 1.58). A significant difference was found between combined and separate DTPa‐HBV‐HIB vaccines (RR 2.14; 95% CI 1.48 to 3.10). For the DTPa‐HBV‐HIB comparison we found little heterogeneity, I2 statistic ‐23%.
1.2. Analysis.
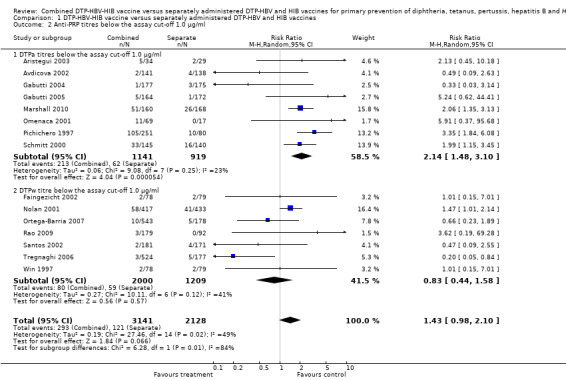
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 2 Anti‐PRP titres below the assay cut‐off 1.0 µg/ml.
Exclusion of Pichichero 1997, which donated most of the outcomes, resulted in a point estimate still in favour of the separate vaccines in a random‐effects model for the DTPa‐HBV‐HIB vaccines (RR 1.91; 95% CI 1.33 to 2.74). There was no significant heterogeneity for this comparison. Chi2 test 6.5, df 6, P = 0.4; and I2 statistic 8%) for the DTPa‐HBV‐HIB vaccines; and Chi2 test 21.5, df 13, P = 0.06; and I2 statistic 40% for all studies). Using a fixed‐effect model, the difference was significant even with the exclusion of Pichichero 1997 (RR 1.94; 95% CI 1.43 to 2.64) for the DTPa‐HBV‐HIB vaccines; and RR 1.51; 95% CI 1.21 to 1.88 for all studies.
Anti‐FHA (filamentous haemagglutinin) ‐ no seroprotective titres
No significant difference was found between combined and separate DTPa‐HBV‐HIB vaccines (RR 0.94; 95% CI 0.20 to 4.36) (Analysis 1.3). Four studies of DTPa‐HBV‐HIB were estimated with total of five events. Four studies had no events (Avdicova 2002; Gabutti 2005; Gabutti 2004; Omenaca 2001).
1.3. Analysis.
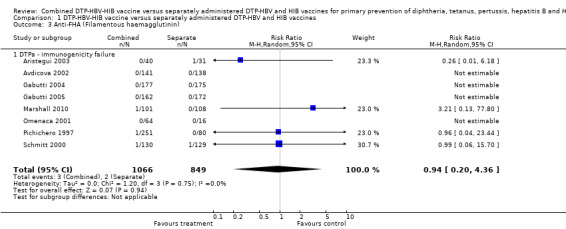
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 3 Anti‐FHA (Filamentous haemagglutinin).
Anti‐PRN ‐ no seroprotective titres
No significant difference was found between combined and separate DTPa‐HBV‐HIB vaccines (RR 0.71; 95% CI 0.34 to 1.50) (Analysis 1.4). Four studies of DTPa‐HBV‐HIB were estimated with total of 27 events. Four studies had no events (Gabutti 2004; Gabutti 2005; Omenaca 2001; Pichichero 1997).
1.4. Analysis.
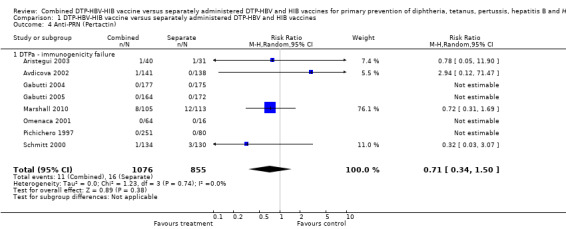
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 4 Anti‐PRN (Pertactin).
Anti‐BPT (pertussis) ‐ no seroprotective titres
No significant difference (RR 1.01; 95% CI 0.80 to 1.28) (Analysis 1.6) between combined and separate DTPa‐HBV‐HIB vaccines (RR 0.97; 95% CI 0.75 to 1.25) and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.33; 95% CI 0.69 to 2.57). Two studies of DTPa‐HBV‐HIB were included with a total of 117 events. Six studies of DTPw‐HBV‐HIB were estimated with a total of 10 events in the separate vaccines and 29 events in the combined vaccine. Three studies had no events (Ramkissoon 2001; Santos 2002; Win 1997).
1.6. Analysis.
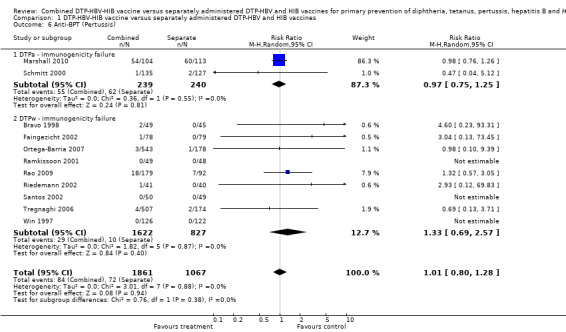
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 6 Anti‐BPT (Pertussis).
Anti‐D (diphtheria): titres below the assay cutoff
No significant difference (RR 0.91; 95% CI 0.59 to 1.38) (Analysis 1.7) between combined and separate DTPa‐HBV‐HIB vaccines (RR 0.91; 95% CI 0.73 to 1.14) and DTPw‐HBV‐HIB combined and separate vaccines (RR 0.87; 95% CI 0.39 to 1.91). Nine studies of DTPa‐HBV‐HIB and DTPw‐HBV‐HIB were estimated with a total of 193 events (Avdicova 2002; Aristegui 2003; Gabutti 2004; Omenaca 2001; Ortega‐Barria 2007; Pichichero 1997; Ramkissoon 2001; Rao 2009; Schmitt 2000). There were no events in eight studies (Aristegui 2003; Avdicova 2002; Gabutti 2004; Gabutti 2005; Omenaca 2001; Pichichero 1997; Ramkissoon 2001; Schmitt 2000).
1.7. Analysis.
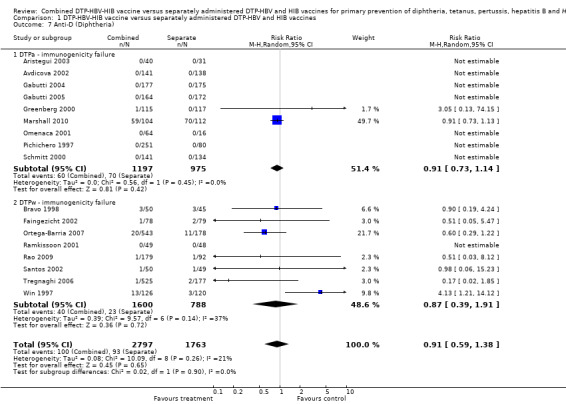
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 7 Anti‐D (Diphtheria).
Anti‐T (tetanus) titres below the assay cutoff
No significant difference (RR 0.56; 95% CI 0.04 to 8.95) (Analysis 1.8) between DTPa‐HBV‐HIB and DTPw‐HBV‐HIB combined and separate vaccines. There were significant differences (RR 2.22; 95% CI 1.21 to 4.06) between combined and separate DTPa‐HBV‐HIb vaccines. There was no significant difference (RR 0.20; 95% CI 0.00 to 9.94) between combined and separate DTPw‐HBV‐HIB vaccines. Three studies were included with a total of 28 events in the combined vaccine and 18 in the separate vaccines. Most events were contributed by one study with 27 events in the combined vaccine and 13 events in the separate vaccines (Marshall 2010) and one study with five events in the separate vaccines (Ortega‐Barria 2007).
1.8. Analysis.
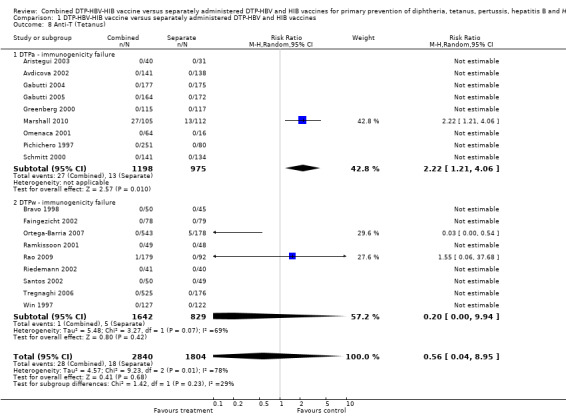
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 8 Anti‐T (Tetanus).
Anti‐HBV (hepatitis B) titres concentrations below the assay cutoff
No significant difference was found (RR 1.24; 95% CI 0.78 to 2.01) (Analysis 1.5) between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.51; 95% CI 0.68 to 3.34) and DTPw‐HBV‐HIB combined and separate vaccines (RR 0.96, 95% CI 0.43 to 2.16). Eight studies of DTPa‐HBV‐HIB were estimated with a total of 36 events. Eight studies of DTPw‐HBV‐HIB were estimated with a total of 174 events. Three studies had no events (Faingezicht 2002; Omenaca 2001; Ramkissoon 2001).
1.5. Analysis.
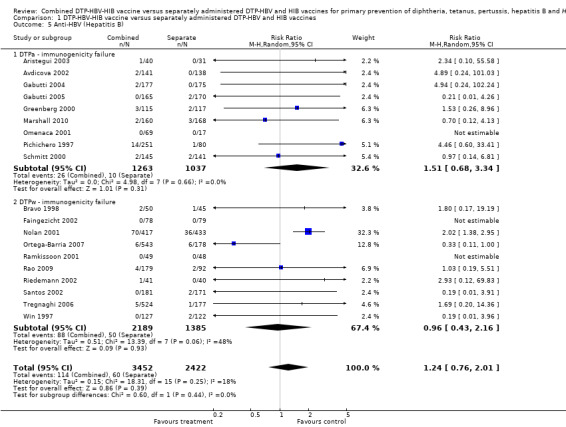
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 5 Anti‐HBV (Hepatitis B).
Anti‐polio type 1, 2 and 3 titres below the assay cutoff
No significant difference was found between combined and separate DTPa‐HBV‐HIB vaccines of anti‐polio type 1 (RR 1.22; 95% CI 0.20 to 7.56) (Analysis 1.9), of anti‐polio type 2 (RR 1.84; 95% CI 0.66 to 5.12) (Analysis 1.10) and of anti‐polio type 3 (RR 1.87; 95% CI 0.59 to 5.94) (Analysis 1.11). Four studies of DTPa‐HBV‐HIB were estimated. Three studies (Avdicova 2002; Gabutti 2004; Schmitt 2000) combined IPV vaccine with DTP‐HBV‐HIB vaccine and one study combined OPV with DTP‐HBV‐HIB vaccine (Pichichero 1997).
1.9. Analysis.
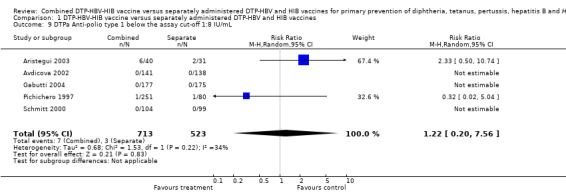
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 9 DTPa Anti‐polio type 1 below the assay cut‐off 1:8 IU/mL.
1.10. Analysis.
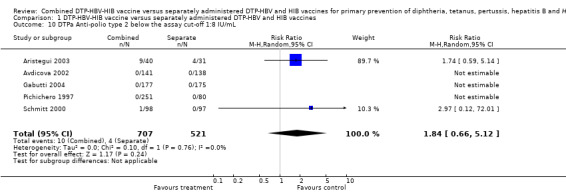
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 10 DTPa Anti‐polio type 2 below the assay cut‐off 1:8 IU/mL.
1.11. Analysis.
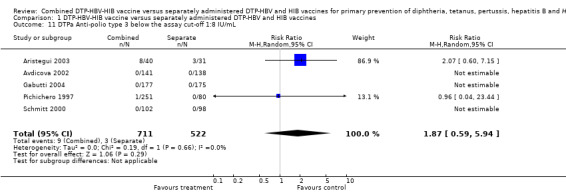
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 11 DTPa Anti‐polio type 3 below the assay cut‐off 1:8 IU/mL.
Reactogenicity (adverse events ‐ number of reported events by number of vaccines given)
Serious adverse events ‐ number of reported events by number of participants
Nine studies with a total of 5239 participants were estimated. No significant difference between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 0.94; 95% CI 0.58 to 1.53) (Analysis 1.12). Three studies of DTPa‐HBV‐HIB were estimated with 18 events in the combined group and 24 events in the separate group. No significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 0.75; 95% CI 0.41 to 1.37). Six studies of DTPw‐HBV‐HIB were estimated with 25 events in the combined group and eight events in the separate group. No significant difference was found between combined and separate DTPw‐HBV‐HIB vaccines (RR 1.41; 95% CI 0.64 to 3.13). See Table 1 and Table 2 for details.
1.12. Analysis.
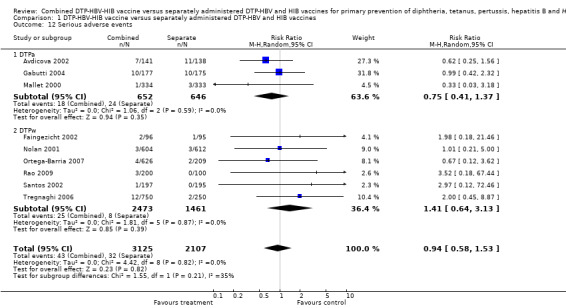
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 12 Serious adverse events.
1. Serious adverse events (DTPw): details.
| Combined group | Separate group | Not given |
| A few hours after the first vaccine dose, 1 child experienced seizures, which resolved spontaneously 2 weeks after the first vaccine dose, another child was diagnosed with acute bronchiolitis and subsequently died due to respiratory distress (Faingezicht 2002) |
1 acute bronchiolitis case, due to respiratory syncytials virus infection, occurred 3 days after the first vaccination. The child recovered after treatment and hospitalisation (Faingezicht 2002) | 5 participants were hospitalised or experienced a serious adverse event, including 1 participant who died as a secondary result of sudden infant death syndrome 52 days after the first dose vaccine (Greenberg 2000) |
| 3 events: one hypotonic‐hyporesponsiveness, 2 seizures (Nolan 2001) | 3 events of seizures (Nolan 2001) | |
| In 1 case 4 booster doses were followed by unsolicited grade '3' symptoms (pharyngitis and severe asthma) (Santos 2002) | ||
| 12 serious adverse events were reported by 10 participants (Tregnaghi 2006) | 2 serious adverse events after the primary vaccination course were reported by 2 participants (Tregnaghi 2006) | |
| 4 serious adverse events occurred in subjects receiving DTPw‐HBV/HIB 2.5 vaccine. 2 hypotonic‐hyporesponsive episodes (HHE) in HIB‐078 and 2 cases of convulsions in HIB‐079. All 4 participants recovered (Ortega‐Barria 2007) | 2 events occurred following the administration of Tritanrix™‐Hep B and Hiberix™ vaccines in HIB‐078. 1 case of HHE and one case of viral meningoencephalitis (Ortega‐Barria 2007). | |
| 2 siblings (twins) presented with symptoms of fever and decreased feeding 17 days after the second dose of vaccine (Shan 5) with one of them progressing to seizures. A diagnosis of septicaemia with meningitis was made. Another infant presented 10 days after the first dose of Shan 5 with symptoms of fever, irritability and breathlessness, a condition which upon investigation was diagnosed as bronchiolitis (Rao 2009) |
DTPw‐HBV/HIB: diphtheria, tetanus, whole cell pertussis/hepatitis B virus/H. influenzae type B HHE: hypotonic‐hyporesponsive episode
2. Serious adverse events (DTPa): details.
| Combined group | Separate group | Not given |
| 7 SAEs were hospitalisations due to vaccination‐related common childhood infections (Avdicova 2002). 10 SAEs including one drop‐out following a serious adverse event and another following a non‐serious adverse event (Gabutti 2004). 1 case of large, local reactions after the second and third injections (Mallet 2000) |
11 SAEs were hospitalisations due to vaccination‐related common childhood infections (1 erythematous rash) (Avdicova 2002). 10 SAEs including one drop‐out following a serious adverse event (Gabutti 2004). 3 infants presented symptoms that were considered as a contradiction for further vaccination:inconsolable crying i.e. more than three hours after first dose (n = 1), second dose (n = 1) and third dose (n = 1) (Mallet 2000) |
2 episodes of "inconsolable crying" were reported within the context of multiple severe local reactions without further sequelae (Mallet 2000). 4 serious adverse events were reported (Aristegui 2003). 8 serious adverse events occurred (Schmitt 2000) |
| 1 SAE was assessed as probably related to vaccination, a hypotonic hyporesponsive episode (Marshall 2010) | 26 SAEs were reported during the study. All were considered unrelated to vaccination by the investigators (Marshall 2010) |
n: Number of participants SAEs: Serious Adverse Events
Pain
A total of 19,745 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. A significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.09; 95% CI 1.02 to 1.16) (Analysis 1.13). Eight studies of DTPa‐HBV‐HIB were estimated with 892 events in the combined group and 538 events in the separate group. A significant difference between combined and separate DTPa‐HBV‐HIB vaccines was found (RR 1.20; 95% CI 1.08 to 1.34). Ten studies of DTPw‐HBV‐HIB were estimated with 2889 events in the combined group and 1699 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 1.05; 95% CI 0.98 to 1.11).
1.13. Analysis.
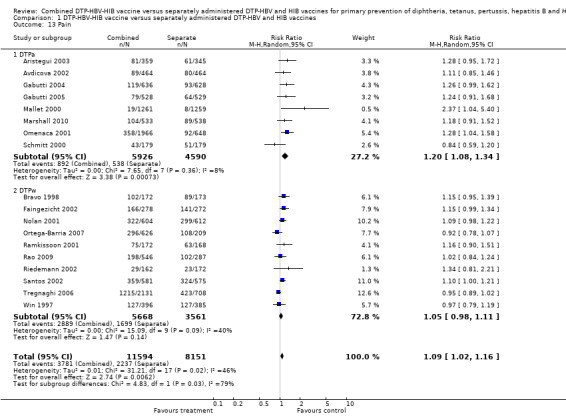
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 13 Pain.
Redness
A total of 19,745 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. A significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.09; 95% CI 1.01 to 1.18) (Analysis 1.14). Eight studies of DTPa‐HBV‐HIB were estimated with 1495 events in the combined group and 1013 events in the separate group. No significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.11; 95% CI 0.98 to 1.27). Ten studies of DTPw‐HBV‐HIB were estimated with 1751 events in the combined group and 1025 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 1.06; 95% CI 0.96 to 1.17).
1.14. Analysis.
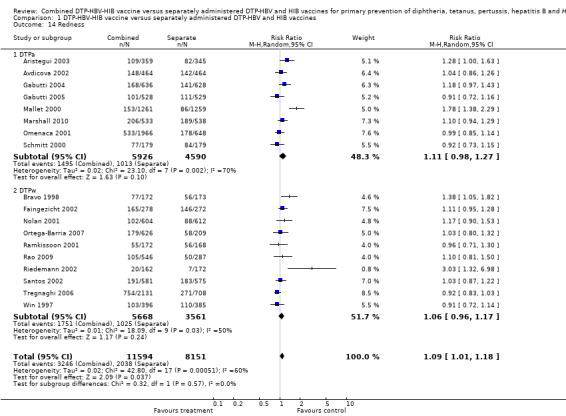
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 14 Redness.
Swelling
No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.04; 95% CI 0.98 to 1.11) (Analysis 1.15). Eight studies of DTPa‐HBV‐HIB were estimated with 1050 events in the combined group and 837 events in the separate group. There was no significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.06; 95% CI 0.95 to 1.18). Ten studies of DTPw‐HBV‐HIB were estimated with 1740 events in the combined group and 1047 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 1.03; 95% CI 0.95 to 1.12).
1.15. Analysis.
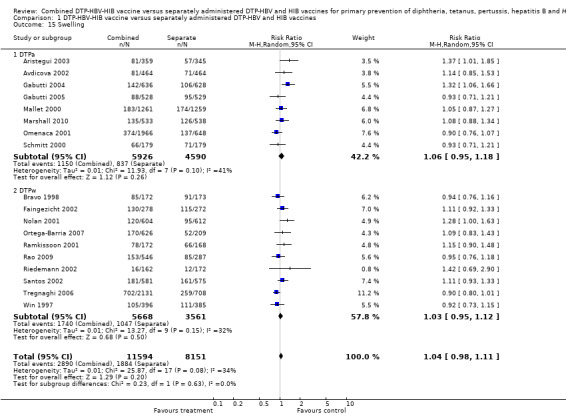
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 15 Swelling.
Fever
A total of 17,805 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.02; 95% CI 0.96 to 1.09) (Analysis 1.16). Seven studies of DTPa‐HBV‐HIB were estimated with 891 events in the combined group and 621 events in the separate group. There was no significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.11; 95% CI 1.00 to 1.24). Seven studies of DTPw‐HBV‐HIB were estimated with 1559 events in the combined group and 1028 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 0.98; 95% CI 0.92 to 1.04).
1.16. Analysis.
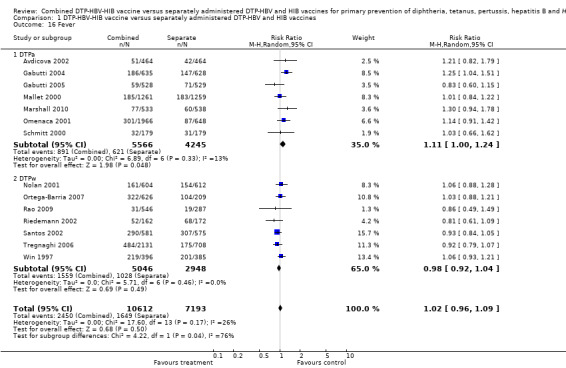
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 16 Fever.
Fussiness or restlessness
A total of 12,183 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.02; 95% CI 0.95 to 1.09) (Analysis 1.17). Seven studies of DTPa‐HBV‐HIB were estimated with 1532 events in the combined group and 1138 events in the separate group. No significant difference between combined and separate DTPa‐HBV‐HIB vaccines was found (RR 1.01; 95% CI 0.92 to 1.11). Two studies of DTPw‐HBV‐HIB was estimated with 498 events in the combined group and 477 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 1.05; 95% CI 0.90 to 1.23).
1.17. Analysis.
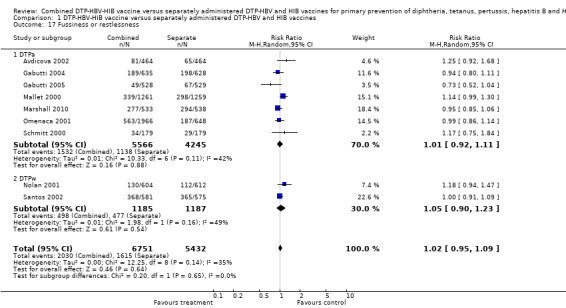
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 17 Fussiness or restlessness.
Drowsiness
A total of 12,011 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 0.99; 95% CI 0.89 to 1.09) (Analysis 1.18). Six studies of DTPa‐HBV‐HIB were estimated with 756 events in the combined group and 717 events in the separate group. No significant difference between combined and separate DTPa‐HBV‐HIB vaccines was shown (RR 1.02; 95% CI 0.88 to 1.19). Five studies of DTPw‐HBV‐HIB were estimated with 907 events in the combined group and 367 events in the separate group. No significant difference was found between combined and separate DTPw‐HBV‐HIB vaccines (RR 0.91; 95% CI 0.82 to 1.01).
1.18. Analysis.
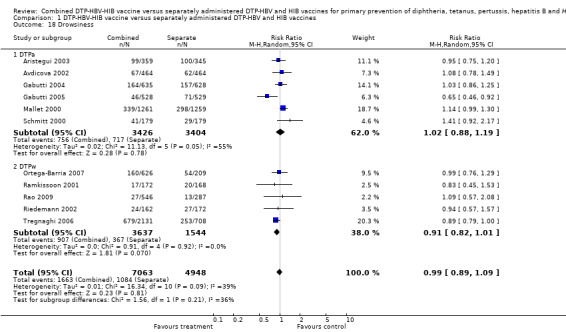
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 18 Drowsiness.
Poor appetite
A total of 13,922 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.01; 95% CI 0.94 to 1.08) (Analysis 1.20). Six studies of DTPa‐HBV‐HIB were estimated with 756 events in the combined group and 531 events in the separate group. There was no significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.05; 95% CI 0.94 to 1.18). Five studies of DTPw‐HBV‐HIB were estimated with 1118 events in the combined group and 425 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 0.97; 95% CI 0.89 to 1.06).
1.20. Analysis.
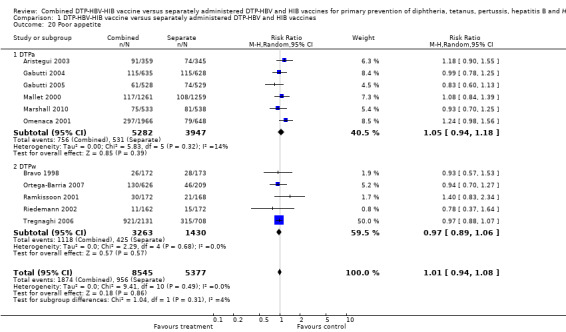
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 20 Poor appetite.
Vomiting
A total of 8281 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.05; 95% CI 0.90 to 1.23) (Analysis 1.21). Four studies of DTPa‐HBV‐HIB were estimated with 345 events in the combined group and 223 events in the separate group. There was no significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.05; 95% CI 0.89 to 1.23). Three studies of DTPw‐HBV‐HIB were estimated with 28 events in the combined group and 26 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 1.08; 95% CI 0.64 to 1.81).
1.21. Analysis.
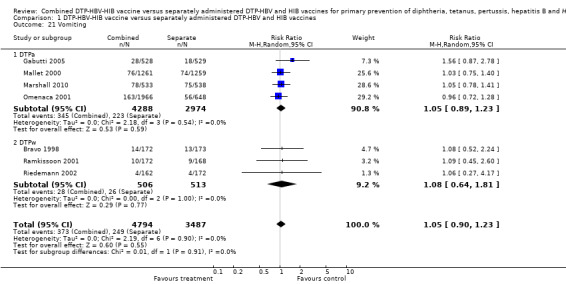
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 21 Vomiting.
Irritability or tenderness
A total of 8273 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 0.97; 95% CI 0.91 to 1.04) (Analysis 1.19). Two studies of DTPa‐HBV‐HIB were estimated with 255 events in the combined group and 242 events in the separate group. No significant difference between combined and separate DTPa‐HBV‐HIB vaccines was shown (RR 1.03; 95% CI 0.74 to 1.44). Seven studies of DTPw‐HBV‐HIB were estimated with 1987 events in the combined group and 982 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 0.95; 95% CI 0.90 to 1.01).
1.19. Analysis.
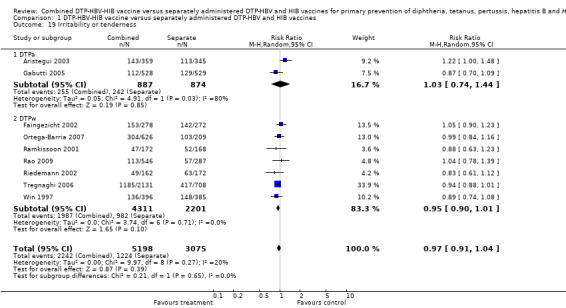
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 19 Irritability or tenderness.
Diarrhoea
A total of 5761 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.11; 95% CI 0.94 to 1.32) (Analysis 1.22). Three studies of DTPa‐HBV‐HIB were estimated with 308 events in the combined group and 166 events in the separate group. There was no significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.12; 95% CI 0.93 to 1.34). Three studies of DTPw‐HBV‐HIB were estimated with 34 events in the combined group and 30 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 1.11; 95% CI 0.69 to 1.77).
1.22. Analysis.
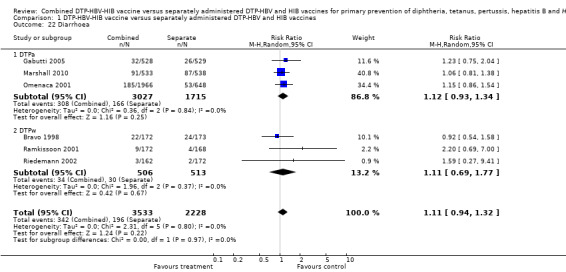
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 22 Diarrhoea.
Unusual crying
A total of 5890 DTPa‐HBV‐HIB and DTPw‐HBV‐HIB vaccines were estimated. No significant difference was found between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 0.72; 95% CI 0.49 to 1.05) (Analysis 1.23). Two studies of DTPa‐HBV‐HIB were estimated with 200 events in the combined group and 219 events in the separate group. There was no significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 1.45; 95% CI 0.27 to 7.84). Four studies of DTPw‐HBV‐HIB were estimated with 205 events in the combined group and 267 events in the separate group. There was no significant difference between combined and separate DTPw‐HBV‐HIB vaccines (RR 0.64; 95% CI 0.37 to 1.10).
1.23. Analysis.
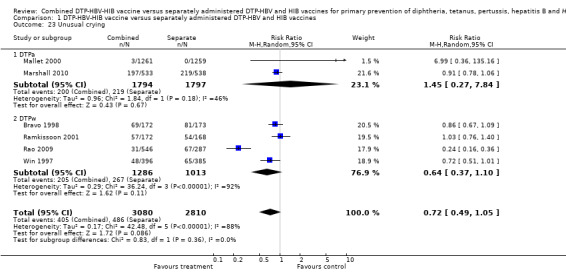
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 23 Unusual crying.
Sleeping more than usual
A total of 6563 DTPa‐HBV‐HIB vaccines were estimated. Four studies were estimated with 680 events in the combined group and 395 events in the separate group. There was no significant difference between combined and separate DTPa‐HBV‐HIB vaccines (RR 0.99; 95% CI 0.89 to 1.11) (Analysis 1.24).
1.24. Analysis.
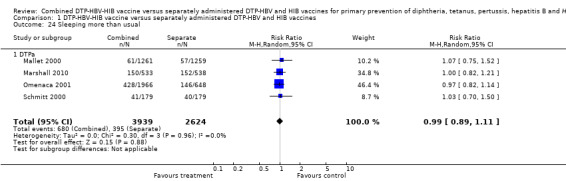
Comparison 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, Outcome 24 Sleeping more than usual.
Sensitivity analysis
We could not perform a sensitivity analysis to assess the impact of methods on the main results because only three studies had adequate allocation generation. We looked at the subgroups according to the antibody concentrations above the assay cut‐offs and found no difference between the subgroups.
Selection bias
We examined two funnel plot graphs of studies for anti‐PRP and they showed no significant selection bias (Figure 3; Figure 4).
3.
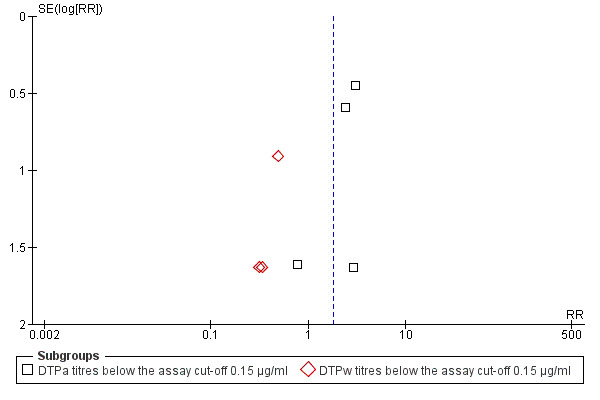
Funnel plot of comparison: 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, outcome: 1.1 Anti‐PRP titres below the assay cut‐off 0.15 µg/ml.
4.
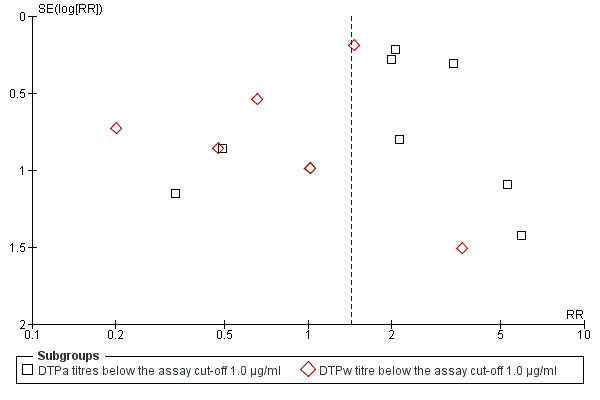
Funnel plot of comparison: 1 DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines, outcome: 1.2 Anti‐PRP titres below the assay cut‐off 1.0 µg/ml.
Discussion
We found no studies that addressed clinical outcomes, i.e. incidence of diphtheria, tetanus, pertussis, hepatitis B virus (HBV) and H. influenzae type B (HIB). For some of these diseases, past eradication programmes were effective in almost total eradication of the disease and thus trials addressing clinical outcomes demand an unrealistic sample size and follow‐up. However, for some of them, for example, HBV, tetanus and HIB, clinical outcomes could be expected. The lack of such outcomes weakens conclusions that can be drawn from published studies.
Immunogenicity
The number of vaccine doses differed significantly between the studies. We decided to extract data following the last dose of the vaccines, excluding a booster dose, because the sample size of booster groups differed significantly from the original groups.
In two immunological responses the combined vaccine achieved lower responses than the separate vaccines: anti‐PRP (HIB) and anti‐T (tetanus). These results changed slightly by the update when we added two studies to the meta‐analysis. The direction shown by the results is in favour of the DTPw‐HBV‐HIB vaccine rather than the DTPa‐HBV‐HIB vaccine when compared to the separate vaccines (size of effect: RR 1.43; 95% CI 0.98 to 2.10 for 5269 participants). For the other responses, no significant differences could be shown but the number of events (response below the threshold) was so low that the CIs are very large.
We should take note that the anti‐PRP comparison was influenced by one study with a large number of events (Pichichero 1997), which used pure (and not conjugated) PRP vaccines (polyribosylribitolphoshate). The anti‐T comparison is influenced by one study added in the update with a high number of serological failures (Marshall 2010).
Reactogenicity (adverse events)
We were unable to find data of serious adverse events for some of the included trials, although we did contact trial authors for additional information. We did not find any difference between combined and separate vaccines. However, nine studies with a total of 5232 participants is a relatively small number upon which to base conclusions. A significant increase in pain and redness was observed in the patients given the combination vaccine.
Limitations of the review
The quality of many of the studies included in the analysis is uncertain. The interventions are heterogeneous. While most of the studies were supported by the manufacturers GlaxoSmith\Kline Biologicals, Rixensart (Belgium) and by Aventis Pasteur (Lyon, France), combined vaccines were prepared as investigational formulations and reconstituted with different dilutents. Therefore, the findings may not generalise to all DTP‐HBV‐HIB vaccines. Though studies included in the meta‐analysis had similar vaccination schedules, immunogenicity was measured at different points of vaccination: after the first, second, or third vaccination and in some studies, after the booster vaccination. The meta‐analysis included immunogenicity data after the third vaccinations, while the immunogenicity profile might differ after the booster vaccination. The study location, the healthcare environment and combining research across disparate geographical locations, may lead to bias. The studies did not use an ITT analysis (excluding one study included at the update) (Rao 2009).
Summary of main results
Immunogenicity, defined as antibody concentration responses by serological analysis of diphtheria, tetanus, pertussis, HBV and HIB reported no significant difference between combined and separate vaccines. However, for anti‐PRP (HIB) below the assay cut‐off of 1.0 µg/ml, in nine studies of DTPa‐HBV‐HIB vaccines, we found a significant difference between combined and separate vaccines (RR 2.14; 95% CI 1.48 to 3.10). This cut‐off refers to the long‐term protection and that, according to many authors, a significant difference on this specific point is not clinically relevant. In six studies of DTPw‐HBV‐HIB vaccines we did not find a significant difference between combined and separate vaccines (RR 0.83; 95% CI 0.44 to 1.58).
We did not find a significant difference (RR 0.56; 95% CI 0.04 to 8.95) between DTPa‐HBV‐HIB and DTPw‐HBV‐HIB combined and separate vaccines in anti‐T (tetanus) immunological response. However, we did find a significant difference (RR 2.22; 95% CI 1.21 to 4.06) between combined and separate DTPa‐HBV‐HIB vaccines. Most events were due to one study added to the meta‐analysis.
Reactogenicity (adverse events) defined as incidence of any solicited local and systemic adverse event showed no difference between combined and separate vaccines. However, for pain, we found a significant difference between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.09; 95% CI 1.02 to 1.16). We found a significant difference between DTPa‐HBV‐HIB combined and separate vaccines and DTPw‐HBV‐HIB combined and separate vaccines (RR 1.09; 95% CI 1.01 to 1.18) for redness.
Overall completeness and applicability of evidence
The objective of the review was to assess the clinical protection, immunogenicity and reactogenicity (adverse events) of a combined DTP, applied to both DTPw (diptheria‐tetanus‐whole cell pertussis) and DTPa (diptheria‐tetanus‐acellular pertussis) vaccines, HBV and conjugate HIB vaccines, with or without three types of inactivated polio virus (IPV) or concomitant oral polio vaccine (OPV), in comparison with separate vaccinations of DTP, HBV, conjugate HIB, IPV and OPV, in infants up to two years of age.
We found no studies that addressed clinical outcomes, i.e. incidence of diphtheria, tetanus, pertussis, HBV and HIB. For some of these diseases, past eradication programmes were effective in almost total eradication of the disease and thus, trials addressing clinical outcomes demand an unrealistic sample size and follow‐up. However, for some of them, for example, HBV, tetanus and HIB, clinical outcomes could be expected. The lack of such outcomes weakens conclusions that can be drawn from published studies.
The number of vaccine doses differed significantly between the studies. We decided to extract data following the last dose of the vaccines, excluding a booster dose, because the sample size of booster groups differed significantly from the original groups.
Updating the review we included two more studies and the new data showed significantly less immunological response for HIB and tetanus and more local reactions to the injections with DTPa‐HBV‐HIB vaccines rather than DTPw‐HBV‐HIB vaccines.
The quality of many of the studies included in the analysis is uncertain and we could not conclude that the immune responses elicited by the combined vaccine are equivalent to the separate injections. However, the differences rely mostly on one study each; it is not clear whether the results can be generalised to all vaccines. The results of this review should be viewed with caution, mostly as an indication that high quality data are lacking.
Quality of the evidence
We included 20 studies in the meta‐analysis with 5874 participants in the immunogenicity analysis and 5232 participants in the reactogenicity analysis. Overall, the level of evidence provided by the studies was low and we could not conclude that the immune responses elicited by the combined vaccine are equivalent to the separate injections. The data showed significantly less immunological response for HIB and tetanus and more local reactions to the injections. However, the differences rely mostly on one study each; it is not clear whether the results can be generalised to all vaccines. The results of this review should be viewed with caution, mostly as an indication that high quality data are lacking.
Potential biases in the review process
We identified all relevant studies but we could not obtain all relevant data. The number of vaccine doses which differed significantly between the studies, heterogeneous interventions, study location, healthcare environment and combining research across disparate geographical locations, may lead to bias.
Agreements and disagreements with other studies or reviews
We included no other studies.
Authors' conclusions
Implications for practice.
Overall, the level of evidence provided by the studies was low and we could not conclude that the immune responses elicited by the combined vaccine are equivalent to the separate vaccines. The data showed significantly less immunological response for HIB and tetanus and more local reactions to the injections. However, the differences rely mostly on one study each. In the case of HIB, the less immunological response is related to a cut‐off of 1.0 µg/ml, whose clinical relevance is questionable. It is not clear whether the results can be generalised to all vaccines. The results of this review should be viewed with caution, mostly as an indication that high quality data are lacking.
Implications for research.
Studies addressing clinical end points whenever possible, using correct methodology and a large enough sample size (and probably including DTPa components) should be conducted.
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2011 | New citation required but conclusions have not changed | The conclusions remain unchanged, although results differed slightly from the last publication of this review |
| 11 November 2011 | New search has been performed | Searches conducted. We included two new trials (Marshall 2010; Rao 2009) and excluded six new trials (Diaz‐Mitoma 2011; Gentile 2011; Halperin 2009; Kilpi 2009; Madhi 2011; Tregnaghi 2011). The two studies added to this update slightly changed the results. In anti‐T (tetanus) immunological responses, the combined vaccine achieved lower responses than the separate vaccines. This result changed from the last publication where in anti‐hepatitis B immunological responses, the combined vaccine achieved lower responses than the separately administered vaccines |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 6 October 2010 | Amended | Contact details updated. |
| 9 September 2010 | Amended | Contact details updated. |
| 5 August 2010 | Amended | Contact details updated. |
| 8 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge the support provided by the Cochrane Acute Respiratory Infections Group editorial team. The review authors wish to acknowledge the following peer referees in developing the protocol: Cheryl Flynn, Lize van der Merwe, Yuri Baidal, José Luis Ferrero Albert and Giovanni Gabutti. We thank the following people for commenting on the draft review: Barbara Loe Fisher, Durhane Wong‐Rieger, Giovanni Gabutti, Margie Andreae, Mark Jones and Chris Del Mar. And finally, we acknowledge the following people for commenting on this updated review: Reshma Carlo, Giovanni Gabutti, Conor Teljeur and Mieke van Driel
Appendices
Appendix 1. CENTRAL and MEDLINE search strategy
MEDLINE (OVID)
1 Diphtheria‐Tetanus‐Pertussis Vaccine/ 2 Diphtheria‐Tetanus‐acellular Pertussis Vaccines/ 3 (diphtheria and tetanus and pertussis).mp. 4 (dtp* or dtap*).tw. 5 1 or 2 or 3 or 4 6 exp Haemophilus Vaccines/ 7 exp Haemophilus influenzae type b/ 8 exp HAEMOPHILUS/ 9 (haemophilus or hemophilus).mp. 10 Hib.mp. 11 or/6‐10 12 exp Hepatitis B Vaccines/ 13 exp Hepatitis B/ 14 (hepatitis b or HBV).mp. 15 or/12‐14 16 5 and 11 and 15
Appendix 2. EMBASE.COM search strategy
#19. #15 AND #18 156 16 Mar 2011 #18. #16 OR #17 837,345 16 Mar 2011 #17. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti AND [embase]/lim 793,290 16 Mar 2011 #16. 'randomised controlled trial'/de OR 'single blind procedure'/de OR 'double blind procedure'/de OR 'crossover procedure'/de AND [embase]/lim 238,781 16 Mar 2011 #15. #5 AND #10 AND #14 576 16 Mar 2011 #14. #11 OR #12 OR #13 61,025 16 Mar 2011 #13. 'hepatitis b':ab,ti OR hbv:ab,ti AND [embase]/lim 48,241 16 Mar 2011 #12. 'hepatitis b'/de AND [embase]/lim 37,360 16 Mar 2011 #11. 'hepatitis b vaccine'/de AND [embase]/lim 11,421 16 Mar 2011 #10. #6 OR #7 OR #8 OR #9 18,750 16 Mar 2011 #9. haemophilus:ab,ti OR hemophilus:ab,ti OR hib:ab,ti AND [embase]/lim 17,035 16 Mar 2011 #8. 'haemophilus'/de AND [embase]/lim 1,545 16 Mar 2011 #7. 'haemophilus influenzae type b'/de AND [embase]/lim 3,371 16 Mar 2011 #6. 'haemophilus vaccine'/de AND [embase]/lim 242 16 Mar 2011 #5. #1 OR #2 OR #3 OR #4 6,261 16 Mar 2011 #4. dtp:ab,ti OR dtap:ab,ti AND [embase]/lim 1,255 16 Mar 2011 #3. diphtheria:ab,ti AND tetanus:ab,ti AND pertussis:ab,ti AND [embase]/lim 1,961 16 Mar 2011 #2. 'diphtheria pertussis tetanus haemophilus influenzae type b hepatitis b vaccine'/de OR 'diphtheria pertussis tetanus haemophilus influenzae type b vaccine'/de OR 'diphtheria pertussis poliomyelitis tetanus vaccine'/de OR 'diphtheria pertussis poliomyelitis tetanus haemophilus influenzae type b hepatitis b vaccine'/de AND [embase]/lim 916 16 Mar 2011 #1. 'diphtheria pertussis tetanus vaccine'/de AND [embase]/lim 4,736 16 Mar 2011
Data and analyses
Comparison 1. DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anti‐PRP titres below the assay cut‐off 0.15 µg/ml | 15 | 4272 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.98, 3.38] |
| 1.1 DTPa titres below the assay cut‐off 0.15 µg/ml | 8 | 2068 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.33, 5.08] |
| 1.2 DTPw titres below the assay cut‐off 0.15 µg/ml | 7 | 2204 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.10, 1.70] |
| 2 Anti‐PRP titres below the assay cut‐off 1.0 µg/ml | 15 | 5269 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.98, 2.10] |
| 2.1 DTPa titres below the assay cut‐off 1.0 µg/ml | 8 | 2060 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.48, 3.10] |
| 2.2 DTPw titre below the assay cut‐off 1.0 µg/ml | 7 | 3209 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.44, 1.58] |
| 3 Anti‐FHA (Filamentous haemagglutinin) | 8 | 1915 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.36] |
| 3.1 DTPa ‐ immunogenicity failure | 8 | 1915 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.36] |
| 4 Anti‐PRN (Pertactin) | 8 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.50] |
| 4.1 DTPa ‐ immunogenicity failure | 8 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.50] |
| 5 Anti‐HBV (Hepatitis B) | 19 | 5874 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.76, 2.01] |
| 5.1 DTPa ‐ immunogenicity failure | 9 | 2300 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.68, 3.34] |
| 5.2 DTPw ‐ immunogenicity failure | 10 | 3574 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.43, 2.16] |
| 6 Anti‐BPT (Pertussis) | 11 | 2928 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.28] |
| 6.1 DTPa ‐ immunogenicity failure | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.75, 1.25] |
| 6.2 DTPw ‐ immunogenicity failure | 9 | 2449 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.69, 2.57] |
| 7 Anti‐D (Diphtheria) | 17 | 4560 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.38] |
| 7.1 DTPa ‐ immunogenicity failure | 9 | 2172 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.73, 1.14] |
| 7.2 DTPw ‐ immunogenicity failure | 8 | 2388 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.39, 1.91] |
| 8 Anti‐T (Tetanus) | 18 | 4644 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.04, 8.95] |
| 8.1 DTPa ‐ immunogenicity failure | 9 | 2173 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.21, 4.06] |
| 8.2 DTPw ‐ immunogenicity failure | 9 | 2471 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.00, 9.94] |
| 9 DTPa Anti‐polio type 1 below the assay cut‐off 1:8 IU/mL | 5 | 1236 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.20, 7.56] |
| 10 DTPa Anti‐polio type 2 below the assay cut‐off 1:8 IU/mL | 5 | 1228 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.66, 5.12] |
| 11 DTPa Anti‐polio type 3 below the assay cut‐off 1:8 IU/mL | 5 | 1233 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.59, 5.94] |
| 12 Serious adverse events | 9 | 5232 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.58, 1.53] |
| 12.1 DTPa | 3 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.37] |
| 12.2 DTPw | 6 | 3934 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.64, 3.13] |
| 13 Pain | 18 | 19745 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.02, 1.16] |
| 13.1 DTPa | 8 | 10516 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.08, 1.34] |
| 13.2 DTPw | 10 | 9229 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.11] |
| 14 Redness | 18 | 19745 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.01, 1.18] |
| 14.1 DTPa | 8 | 10516 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.98, 1.27] |
| 14.2 DTPw | 10 | 9229 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.96, 1.17] |
| 15 Swelling | 18 | 19745 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.98, 1.11] |
| 15.1 DTPa | 8 | 10516 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.95, 1.18] |
| 15.2 DTPw | 10 | 9229 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
| 16 Fever | 14 | 17805 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.09] |
| 16.1 DTPa | 7 | 9811 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.24] |
| 16.2 DTPw | 7 | 7994 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 17 Fussiness or restlessness | 9 | 12183 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.09] |
| 17.1 DTPa | 7 | 9811 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 17.2 DTPw | 2 | 2372 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.90, 1.23] |
| 18 Drowsiness | 11 | 12011 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.09] |
| 18.1 DTPa | 6 | 6830 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
| 18.2 DTPw | 5 | 5181 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 19 Irritability or tenderness | 9 | 8273 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 19.1 DTPa | 2 | 1761 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.74, 1.44] |
| 19.2 DTPw | 7 | 6512 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.01] |
| 20 Poor appetite | 11 | 13922 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.08] |
| 20.1 DTPa | 6 | 9229 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.18] |
| 20.2 DTPw | 5 | 4693 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 21 Vomiting | 7 | 8281 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.90, 1.23] |
| 21.1 DTPa | 4 | 7262 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 21.2 DTPw | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.64, 1.81] |
| 22 Diarrhoea | 6 | 5761 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.32] |
| 22.1 DTPa | 3 | 4742 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.34] |
| 22.2 DTPw | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.69, 1.77] |
| 23 Unusual crying | 6 | 5890 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.05] |
| 23.1 DTPa | 2 | 3591 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.27, 7.84] |
| 23.2 DTPw | 4 | 2299 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.37, 1.10] |
| 24 Sleeping more than usual | 4 | 6563 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.11] |
| 24.1 DTPa | 4 | 6563 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aristegui 2003.
| Methods | Open, randomised, comparative phase IIIb, multi‐centre trial | |
| Participants | Healthy male and female infants; age 8.7 (± 0.8) weeks | |
| Interventions | Combined DTPa‐HBV‐IPV‐Hib compared to separate DTPa‐IPV/HIB + HBV in 3 doses at 2, 4 and 6 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | Study supported by a grant from SmithKline Beecham SA, Madrid, Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was conducted in full‐term healthy infants recruited in 9 Spanish centres |
| Allocation concealment (selection bias) | Unclear risk | Randomised trial ‐ two groups of healthy infants no details of randomisation method |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 71 out of 241 completed |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
| Other bias | High risk | The limited number of subjects (71 out of 235) from whom immunogenicity data are available does not allow any conclusions to be drawn |
Avdicova 2002.
| Methods | Open, randomised trial | |
| Participants | Healthy male and female infants; age 13.2 weeks, range 8 to 12 weeks | |
| Interventions | DTPa‐HBV‐IPV/HIB compared to DTPa‐IPV/HIB and HBV in separate injections; 3 doses between 11 and 17 weeks of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | The study was supported by a grant from GlaskoSmithKline Biologicals, Rixensart, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was conducted in healthy infants in 8 regions in Slovakia |
| Allocation concealment (selection bias) | Unclear risk | Randomised study without details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 309 out of 312 completed |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
| Other bias | Low risk | |
Bravo 1998.
| Methods | Open, randomised clinical trial | |
| Participants | Healthy male and female infants; no age reported | |
| Interventions | DTPw‐HBV‐HIB and separate DTP‐HBV and HIB when received hepatitis B vaccine at birth; 3 doses given at 6, 10 and 14 weeks of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | Funding for this study was provided by SmithKline Beecham Biologicals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was conducted in healthy infants with an Apgar score of 7 or higher at birth |
| Allocation concealment (selection bias) | Unclear risk | Randomised trial ‐ no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 8 out of 148 did not complete |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
| Other bias | Low risk | |
Faingezicht 2002.
| Methods | Phase III, observed‐blind, prospective RCT | |
| Participants | Healthy male and female infants; age 8.8 (SD = 0.9) weeks | |
| Interventions | DTPw‐HBV/HIB pentavalent combination after extemporaneous mixing of the liquid DTPw‐HBV with lyoHIB compared to DTPw‐HBV vaccine and HIB vaccine reconstituted with its own diluent. 3 doses given at 2, 4 and 6 months of age and booster at 15 to 18 months old | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This study was funded by GlaskoSmithKline Biologicals, Rixensart, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was conducted in healthy infants in a single vaccination centre in Costa Rica |
| Allocation concealment (selection bias) | Low risk | RCT detailed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Observer‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 out of 207 not completed |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
| Other bias | Low risk | |
Gabutti 2004.
| Methods | Open, phase III, randomised, multi‐centre study | |
| Participants | Healthy male and female infants mean age 13.3 weeks, range 9 to 17 weeks | |
| Interventions | DTPa‐HBV‐IPV/HIB compared to separate DTPa ‐ HBV ‐ IPV + HIB. 3 doses given at 3, 5 and 11 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This research was supported by a grant from GSK Biologicals, Rixensart, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was conducted in healthy infants in 24 centres in Germany and Italy ‐ no details |
| Allocation concealment (selection bias) | Unclear risk | Randomised study ‐ children were randomly allocate (1:1 ratio) to the 2 study groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26 out 440 did not complete the study |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
Gabutti 2005.
| Methods | Open, randomised, multi‐centre trial | |
| Participants | Healthy male and female infants aged 13 and 13.1 weeks | |
| Interventions | DTPa‐HBV‐HIB compared with two separate or mixed injection. 3 doses given at 3, 5 and 11 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This study was supported by a grand from GSK Biologicals, Rixensart, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was conducted in healthy infants in 12 Italian centres ‐ no details |
| Allocation concealment (selection bias) | Unclear risk | Randomised to two study groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 out 360 did not complete the study |
| Selective reporting (reporting bias) | Unclear risk | No details how the adverse events were evaluated |
| Other bias | Low risk | |
Greenberg 2000.
| Methods | Randomisation equally to 3 groups | |
| Participants | Healthy male and female infants; age 6 to 12 weeks at the time of the first vaccination | |
| Interventions | DTPa, HBV and PRP‐T (HIB). OPV was given concurrently. 3 doses given at 2, 4, 6 months of age and booster combined vaccine to ages 11 to 15 months | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This study was supported by a grant from SmithKline Beecham Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy infants were recruited from two Kaiser Permanente, Southern California Region medical centres |
| Allocation concealment (selection bias) | Unclear risk | Randomised equally to three groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parents and study personnel were not blinded. Laboratory personnel were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54 out of 405 did not complete the study |
| Selective reporting (reporting bias) | Low risk | Parents mailed the completed diary cards to office. Reserch personnel collected severe adverse event data from parents by telephone 1 and 3 days after each immunisation and from parents and medical records at each visit |
Mallet 2000.
| Methods | Open‐label, multi‐centre, prospective, comparative trial | |
| Participants | Healthy male and female infants; age 63 days ± 7 days | |
| Interventions | DTPa‐IPV‐HBV‐HIB compared to separate DTPa‐IPV‐HIB and HBV vaccine. 3 doses given at 2, 4 and 6 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This study was supported by a grant from Avintis Pasteur MSD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study was conducted in healthy infants born after 36 weeks of pregnancy with birth weight of > 2500 g by 70 paediatricians located in the Haute‐Normandie, Provence‐Alpes‐Cote d'azur and Rhone‐Alpes regions of France |
| Allocation concealment (selection bias) | Low risk | Randomly allocated according to a centralised list |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 166 out of 833 did not complete the study |
| Selective reporting (reporting bias) | Low risk | The reactogenicity profile was determined by describing the rates of immediate reactions, the rates of local and systemic adverse events within 15 min to 72 hours and the frequency of adverse events requiring a medical visit within 1 month of vaccination |
Marshall 2010.
| Methods | Open‐label, randomised comparative trial | |
| Participants | Healthy infants of either sex aged 2, 4 and 6 months of age | |
| Interventions | Combined DTPa‐HBV/HIB or separate injections of DTPa‐HBV and HIB in opposite thighs at 2, 4 and 6 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This study was supported by a grant from GlaskoSmithKline Biologicals, Rixensart, Belgium Conflict of interest statement reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was conducted in healthy infants in two centres in Australia ‐ no details |
| Allocation concealment (selection bias) | Unclear risk | Randomised to two study groups ‐ no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 out of 328 were excluded from the according‐to‐protocol (APT) analyses |
| Selective reporting (reporting bias) | High risk | Serious adverse events reported generally with no specification per study arm |
Nolan 2001.
| Methods | Randomised, double‐blind series of 3 studies | |
| Participants | Male and female infants in good health from community maternal and child health clinics in greater Melbourne and from the maternity service of the Royal Women's Hospital in Melbourne, Australia No age reported |
|
| Interventions | DTP‐HIB (PRP‐OMPC)‐HBV in three studies + OPV DTP‐liqHIB‐HBV + placebo (group A). DTP‐HBV + liqHIB (group B). HBV‐liqHIB + DTP (group C). DTP+lyoHIB+HBV (group D). Monovalent HBV at birth and DTP‐liqHIB‐HBV (group E). 3 and 4 doses (including booster) given at 2, 4, 6 and 18 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This study was supported by a grant to the Royal Children's Hospital Research Foundation from CSL Ltd. and Merck and Co., Inc. PRP‐OMPC : the Haemophilus influenzae capsular polysaccharide‐outer membrane protein conjugate, PRP‐OMPC (PedvaxHIB) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants in good health were recruited through community maternal and child health clinics in greater Melbourne and from the maternity service of the Royal Women's Hospital in Melbourne |
| Allocation concealment (selection bias) | Low risk | The principal study was randomised. Computer‐generated with stratification by consent for immunogenicity testing and blocks of varying size |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For the principal study, all study staff and parents/guardians were blinded to the vaccine administered for the duration of the study. The control group was an open study but parents were not aware of which vaccines were being administered in particular limbs. The third study was an open study with regard to administration of the pentavalent vaccine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Unclear risk | No details |
Omenaca 2001.
| Methods | Open, randomised, multi‐centre, comparative phase III clinical trial | |
| Participants | Healthy male and female infants; age 9.3 ± 1.4 weeks (range 5 to 16) | |
| Interventions | DTPa‐HBV‐HIB and separate DTPa‐HBV and HIB with OPV simultaneously. 3 doses given at 2, 4 and 6 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | Study supported by a grant from GlaskoSmithKline Biologicals, Rixensart, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy infants were recruited in 11 centres from Greece, Spain and Switzerland |
| Allocation concealment (selection bias) | Unclear risk | The randomizations was made using an algorithm of pseudo‐random numbers. Subjects were allocated to the two groups according to a 3:1 ratio |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 out of 885 did not complete the full vaccination course |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
| Other bias | High risk | The immunogenicity subset comprised 95 infants. The limited sample size of the immunogenicity results places a limitation on the conclusions that can be drawn |
Ortega‐Barria 2007.
| Methods | 4 separate phase III trials which assessed the immunogenicity and reactogenicity of DTPw‐HBV/HIB 2.5 in comparison with DTPw‐HBV + Hiberix™ (10µg PRP) given as separate or mixed injections (3 trials) or with or without HBV vaccine at birth (1 trial) | |
| Participants | Healthy male and female infants; age 2 to 14 weeks | |
| Interventions | DTPw‐HBV mixed with HIB 2.5 (lot A, lot B, lot C) compared with DTPw‐HBV and Hiberix™ either given as separate injections (HIB‐078) or as mixed injections (HIB‐079, HIB‐080) administered at either 2, 4 and 6 months of age (HIB‐078, HIB‐079); at 3, 4 and 5 months of age (HIB‐080); or at 6, 10 and 14 weeks of age (HIB‐081) | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | These studies were supported by grants from GSK Biologicals, Rixensart, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy infants born after a normal gestation period |
| Allocation concealment (selection bias) | Unclear risk | Randomised trials, Phase III. Groups within studies were well matched for age and gender distribution |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 144 out of 1803 completed |
| Selective reporting (reporting bias) | Low risk | Diary cards completed during the 4‐day follow‐up period after each vaccination |
Pichichero 1997.
| Methods | Prospective, randomised multi‐centre trial. 3 to 1 to group 1 and 2 respectively, comparing combined injections with three separate simultaneous injections | |
| Participants | Healthy male and female infants; age 6 to 12 weeks | |
| Interventions | DTPa‐HBV‐PRP‐T and booster of HIB. OPV was administered to all vaccinees in both groups concurrently at 2, 4 and 6 months of age. 3 doses given at 2, 4 and 6 months of age and booster of PRP conjugate vaccine to group 1 (combined) with low levels of antibody at 9 to 13 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This work was supported by SmithKline Beecham Biologicals (Philadelphia) and the National Institute of Health (AI45248) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy infants were recruited for a multi‐centre (3 groups in NY, Pittsburgh and VA), prospective, randomised trial |
| Allocation concealment (selection bias) | Unclear risk | Infants were randomised three to one to groups 1 and 2 respectively |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data |
| Selective reporting (reporting bias) | Unclear risk | No data |
Ramkissoon 2001.
| Methods | Open, randomised comparative study | |
| Participants | Healthy male and female infants; aged 6 weeks (not reported) in Durban, South Africa | |
| Interventions | DTPw‐HBV mixed with HIB compared with DTPw‐HBV and HIB separate. 3 doses given at 6, 10 and 14 weeks of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | No support reported. SmithKline Beecham Biologicals, Belgium address given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy male and female infants were enrolled ‐ no details |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised into two groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
Rao 2009.
| Methods | 10 centres across India recruited participants for a single‐blind, randomised, comparative, non‐inferiority 3‐arm study | |
| Participants | Healthy infants in the age group 6 to 8 weeks, born to mothers proven seronegative for HBV after a normal gestation period between 36 and 42 weeks | |
| Interventions | DTPw‐HBV‐HIB tetanus toxoid conjugate liquid pentavalent combination vaccine ‐ group 1, DTPw‐HBV‐HIB Pentavalent combination vaccine liquid form ‐ group 2, TritanrixHBTM + HibrixTM Pentavalent combination vaccine (Liquid + Lyophilised) ‐ group 3 | |
| Outcomes | Immune response and safety of DTPw‐HBV‐HIB tetanus toxoid conjugate vaccine when administered according to a 6‐10‐14 week Expanded Program on Immunization (EPI) Schedule compared to Easy five and TritanrixHBTM = HiberixTM | |
| Notes | This work was funded by Shantha Biotechnics Limited Competing interests reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 10 centres across India recruited healthy infants in the age group 6 to 8 weeks, born to mothers proven seronegative for HBV after a normal gestation period between 36 and 42 weeks |
| Allocation concealment (selection bias) | Low risk | Randomly assigned to one of the three study groups in a 2:1:1 ratio. The randomizations code was generated using the SAS version 8.2 software package |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 35 out of 365 were excluded from efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Parents/guardians recorded adverse events on diary cards for 3 days and 30 days respectively following vaccination |
| Other bias | Low risk | Safety analysis was based on ITT population |
Riedemann 2002.
| Methods | Open randomised, parallel‐group design, randomised study | |
| Participants | Healthy male and female infants; age 9.9 weeks. No country reported | |
| Interventions | DTPw‐HBV/HIB compared with DTPw‐HBV and HIB separate in opposite deltoids. 3 doses given at 2, 4 and 6 months old and booster at 18 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | The research described is this manuscript was funded by GlaskoSmithKline Biologicals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy male and female infants, aged 6 to 12 weeks |
| Allocation concealment (selection bias) | Unclear risk | RCT. Participants were randomly allocated to one of two groups in a ratio of 1:1 |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 out of 101 were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | The study nurses used diary cards to record local and systemic signs and symptoms for the day of each vaccination and the 3 following days. At each subsequent visit the investigator transcribed information from the diary cards onto the Case Report Form and asked about any other adverse experiences that occurred after the period covered by the diary card |
| Other bias | Unclear risk | The results of this study cannot be generalised to combinations other than that of Hiberix with Trianrix HBV which are both (including the combination), WHO approved |
Santos 2002.
| Methods | Open, multi‐centre, randomised (1:1), parallel‐group design | |
| Participants | Healthy male and female infants; age 8 to 15 weeks | |
| Interventions | DTPw‐HBV mixed with HIB compared with DTPw‐HBV and HIB separate in opposite thighs. 3 doses given at 2, 4 and 6 months and booster at 18 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | This study was funded by SmithKline Beecham Biologicals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy male and female infants from centres in Mexico, Brazil, Panama, Venezuela and the Dominician Republic |
| Allocation concealment (selection bias) | Unclear risk | Randomised (1:1), parallel‐group design |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 382 out of 400 completed the primary vaccination phase |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days. At each subsequent visit the investigator transcribed information from the diary cards onto the Case Report Form and asked about any other adverse experiences that occurred after the period covered by the diary card |
Schmitt 2000.
| Methods | Open, multi‐centre, randomised trial | |
| Participants | Healthy male and female infants; age 8 to 16 weeks | |
| Interventions | DTPa‐HBV‐IPV/HIB compared to separate DTPa ‐ HBV ‐ IPV + HIB. 3 doses given to 2, 4 and 6 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | Supported by SmithKline Biologicals, Rixensart, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy infants from 12 private paediatric offices in Kiel, Germany |
| Allocation concealment (selection bias) | Unclear risk | Randomised |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 out of 359 failed to complete the study |
| Selective reporting (reporting bias) | Low risk | Parents documented the reactions for 4 days |
Tregnaghi 2006.
| Methods | Double‐blind design of three different production lots in the studies | |
| Participants | Healthy infants; age 8 ± 1.8 weeks with a male:female ratio of 1:1 | |
| Interventions | DTPw‐HBV/HIB compared with separate vaccines | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | No support reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthy infants from 4 centres in Central and Latin America: Argentina, Columbia, the Dominican Republic and Nicaragua |
| Allocation concealment (selection bias) | Unclear risk | Randomised in a balanced 1:1:1 allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Three different production lots of the combined DTPw‐HBV/HIB vaccine were used in a double‐blind manner. The control group received 2 separate injections; blinding could not be maintained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76 out of 1000 did not complete the primary vaccination study |
| Selective reporting (reporting bias) | Low risk | Details of adverse events were collected on diary cards. Reactogenicity data were collected during a 4‐day follow‐up period after each vaccination |
| Other bias | Unclear risk | The use of the new DTPw‐HBV/HIB vaccine in 'field' conditions was not assessed in this trial |
Win 1997.
| Methods | Open, randomised and controlled with 2 groups of healthy neonates | |
| Participants | Healthy male and female infants; age 5 to 8 weeks | |
| Interventions | DTPw‐HBV‐HIB and separate DTPw‐HBV and HIB. 3 doses given at 1.5, 3 and 5 months of age | |
| Outcomes | Immunogenicity (antibody concentrations by serological analysis) and adverse events ‐ reactogenicity | |
| Notes | Clinical Research and Development, SmithKline Beecham Biologicals, Rixensart, Belgium address reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Healthy neonates from Central Women's Hospital, Yangon, Myanmar. Neonates with an Apgar score of 7 or higher 5 minutes after birth and who had no concomitant administration of immunoglobulins were enrolled |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned to one of two groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 37 out of 286 did not complete the full vaccination course |
| Selective reporting (reporting bias) | Low risk | Demographic and reactogenicity data were collected from diary cards completed by parents or a study nurse on the day of vaccination and during the 3 days following each dose and transferred to a Standad Case Report Form by the investigator |
| Other bias | High risk | Recommendation should not be generalised, however, as the data from this study apply only to the 2 specified vaccines, TritanrixTM and HiberixTM alternative vaccines should be investigated with regards to non‐interference before use as syringe mixes |
DTPw: diphtheria, tetanus, whole cell pertussis HBV: hepatitis B virus HIB: H. influenzae type B IPV: inactivated polio virus ITT: intention‐to‐treat liqHIB: liquid H. influenzae type B lyoHIB: lyophilised HIB OPV: oral polio vaccine PRP‐T: polyribsylribitolphosphate, (vaccine conjugated to tetanus toxoid) RCT: randomised controlled trial SD: standard deviation WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aristegui 1998 | Comparison of three lots of HIB: DTPa‐HBV (lot no. 16707B2) + HIB (002A44); DTPa‐HBV (16708B2) + HIB (001A41); and DTPa‐HBV (16710A2) + HIB (003A41) |
| Aristegui 2001 | Compares DTPa/HIB with DTPw + HIB as booster. No HBV. Reactogenicity and safety only |
| Bavdekar 2007 | Evaluates the immunogenicity of the HBV and HIB components and the overall safety and reactogenicity of the DTPw‐HBV/HIB vaccine. No comparison of combined and separate vaccines |
| Botet‐Asensi 2003 | DTPw/HIB vaccine compared to separate injections of DTPw+ HIB. No HBV |
| Calbo 2002 | Comparative trial to assess the reactogenicity of the DTPa vaccine + HIB and DTPw + HIB administered in single injection as a booster dose. No HBV |
| Clemens 2003 | Immunogenicity and safety of a novel DTPw/HIB Brazilian combination compared to a licensed DTPw/HIB European combination |
| Denoel 2007 | Not a RCT: report of primary and booster‐based paediatric clinical trials |
| Diaz‐Mitoma 2011 | Compares different formulas of combined vaccines: diptheria‐tetanus‐pertussis‐polio‐HIB + HBV vaccine compared to 1 of 3 double‐blind investigational formulations |
| Gatchalian 2005 | Compares 2 combined vaccines: DTPw‐HBV/HIB containing 2.5 micro PRP compared to GSK Biologicals' licensed Tritanrix HepB/Hiberix containing 10 micro PRP |
| Gentile 2011 | Assesses DTPw‐HBV‐HIB combination vaccine in infants who had or had not received a birth dose of HBV vaccine |
| Gylca 2001 | DTPa‐HBV‐IPV + HIB vaccine compared to DTPw‐IPV/HIB + HBV vaccine (diphtheria, tetanus, pertussis, polioviruses, PRP antigens + HBsAg (HBV) vaccine) |
| Halperin 2009 | Compared 4 formulations of a liquid, hexavalent DTPa‐IPV‐HIB‐HBV vaccine |
| Hla 2006 | A randomised, dose‐ranging trial to asses the combined vaccine content (no comparison to separate vaccines) |
| Hogg 2003 | Assesses the immunogenicity of oral poliomyelitis vaccine under current and possible new conditions (different objective) |
| Huang 1998 | Combined DTP/HIB and separate DTP + HIB vaccination without HBV |
| Kalies 2004 | No RCT: follow‐up of case surveillance and vaccine uptake |
| Kanra 2006 | Combined DTPw‐HBV‐HIB compared with separately administered DTPw‐HIB and HBV vaccines |
| Kilpi 2009 | Evaluates 2 commercial DTPa‐HBV‐IPV/HIB combination vaccines |
| Knuf 2006 | Hexavalent diphtheria‐tetanus‐acellular pertussis‐hepatitis B‐inactivated polio virus‐H. influenzae type b vaccine concomitantly with PCV7 (DTPa‐HBV‐IPV‐HIB and PCV7) compared with DTPa‐HBV‐IPV/HIB |
| Lagos 2005 | Comparison of Lot‐to‐Lot consistency of combined vaccine and not comparison of combined and separate vaccines |
| Lim 2007 | Comparison of combined DTPa‐IPV/HIB + HBV vaccines with DTPa‐HBV‐IPV/HIB vaccine |
| Lopez 2002 | Not a RCT: no control group |
| Madhi 2011 | Compares DTPa‐IPV‐HBV‐PRP‐T with DTPw‐HIB, HBV and OPV or DTPa‐IPV‐HBV‐PRP‐T vaccine with HBV vaccine at birth |
| Meriste 2006 | Comparison of combined DTPa‐HBV‐IPV with DTPa‐HBV and IPV separate vaccines |
| Mills 1998 | Comparison between a 5‐component pertussis combination vaccine (CPDT‐IPV/PRP‐T) to that of whole cell pertussis combination vaccine (DPT‐IPV/PRP‐T) |
| Nolan 2004 | Only data on antibody persistence (immunogenicity) of plain PRP and conjugate PRP‐T was provided |
| Pichichero 1999 | Avidity maturation of antibody to HIB after immunisation with DTPa/HIB/HBV |
| Pichichero 2007 | Compares the DTPa‐HBV‐IPV vaccine co‐administered with PCV7 and HIB vaccine to separate vaccines concurrently or staggered (delayed) administration of PCV7 vaccine |
| Poolman 2001 | Not RCT: 2 studies in Germany and USA reported to show that the nature and function of the antibody are the same in combined and separate DTPa‐HBV‐IPV/HIB vaccination |
| Saenger 2005 | 2 studies reported elsewhere, while only data of safety is provided |
| Scheifele 2005 | Evaluation of a fourth dose of DTPa‐IPV/PRP‐T and not compared with separate vaccines |
| Scheifele 2006 | Concurrently administered PCV7, DTPa‐IPV/PRP‐T and HBV compared with separate injections |
| Tichmann 2005 | Comparison of 2 combined vaccines |
| Tichmann‐Schumann 2005 | DTPa‐HBV‐IPV/HIB vaccine and 7vPn conjugate vaccine compared with the administration of the hexavalent DTPa‐HBV‐IPV/HIB vaccine given alone |
| Tregnaghi 2011 | Compares DTPa‐IPV‐HBV‐PRP‐T vaccine with Pentaxim and Engerix B Pediatrico (HBV monovalent) vaccine in infants born to HBV surface antigen seronegative mothers |
| Trollfors 2005 | Study of the effect of pertussis toxoid on the immunogenicity of DT during a trial of an Pa vaccine |
| Usonis 1999a | The target is to ensure that separate, concomitant vaccination does not interfere with the PRP response nor negatively influence the reactogenicity profiles of the vaccines when used with an Pa based combination. In the trial, HIB immunisation performed concomitantly with a candidate DTPa‐HBV‐IPV in order to compare the local reactogenicity and immunogenicity of 4 commercial HIB vaccines |
| Usonis 1999b | Evaluation of the immunogenicity and reactogenicity of a new combined DTPw‐HBV/HIB. Comparison of HIB Lot 001A44 to HIB Lot 002A41 |
| Zepp 1997 | A study of memory B‐cell induction and the immune response to the combined DTPa‐HBV‐HIB vaccine (no comparison) |
| Zepp 2004 | 2 studies report of safety and reactogenicity of infant primary immunisation with the simultaneous administration of six vaccines in a single injection (DTPa‐IPV/HIB) to the administration of the same vaccine‐antigens given as 2 separate injections with widely used licensed products |
DT: diphtheria and tetanus toxoids DTPa: diphtheria, tetanus, acellular pertussis DTPw: diphtheria, tetanus, whole cell pertussis HBV: hepatitis B virus HIB: H. influenzae type B IPV: inactivated polio virus Pa: acellular pertussis PCV7: pneumococcal 7‐valent conjugate vaccine PRP: polyribsylribitolphosphate PRP‐T: polyribsylribitolphosphate vaccine conjugated to tetanus toxoid RCT: randomised controlled trial 7vPn: pneumococcal 7‐valent conjugate vaccine
Characteristics of ongoing studies [ordered by study ID]
Sanofi‐Aventis 2011.
| Trial name or title | PENTAXIM™ Vaccine Versus TETRAXIM™ Vaccine Given With ACTHIB™ Vaccine in South Korean Infants |
| Methods | |
| Participants | This study is currently recruiting participants |
| Interventions | Immunogenicity and safety of the Sanofi Pasteur's DTacP‐IPV//PRP˜T combined vaccine (PENTAXIM™) versus Sanofi Pasteur's DTacP‐IPV combined vaccine (TETRAXIM™) given simultaneously at separate sites with PRP˜T conjugate vaccine (ACTHIB™) as a three‐dose primary vaccination at 2, 4 and 6 months of age in South Korean infants |
| Outcomes | |
| Starting date | October 1, 2010 |
| Contact information | Public Registry Sanofi Pasteur RegistryContactUs@sanofipasteur.com |
| Notes | This study is designed to assess the immunogenicity and safety of PENTAXIM™ combined vaccine versus TETRAXIM™ vaccine to support registration of PENTAXIM™ in South Korea |
Differences between protocol and review
The separate vaccine immunogenicity analysis of two types of pertussis vaccination: acellular pertussis (Pa) and whole cell pertussis (Pw) was added after the protocol was written.
Contributions of authors
Edna Bar‐On (EB): was responsible for the reference searches, article retrieval, study inclusion and exclusion, data extraction, analysis, interpretation of results and writing and updating the review. Abigail Fraser (AF): assisted with writing the protocol. Sarah Hellmann (SH): has assisted with writing the protocol, analysing and updating the review. Goldberg Elad (GE): was responsible for the reference searches, article retrieval, study inclusion and exclusion, data extraction and analysis and interpretation of results of the review and update. Liat Vidal (LV): assisted with the search terms for the protocol. Leonard Leibovici (LL): was responsible for study inclusion and exclusion, analysis, interpretation of results and writing of the review and update.
Sources of support
Internal sources
Rabin Medical Center, Beilinson Campus, Israel.
External sources
The National Institute for Health Policy and Health Services Research, Israel.
Declarations of interest
None to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aristegui 2003 {published data only}
- Aristegui J, Dal‐Re R, Diez‐Delgado J, Mares J, Casanovas JM, Garcia‐Corbeira P, et al. Comparison of the reactogenicity and immunogenicity of a combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio (DTPa‐HBV‐IPV) vaccine, mixed with the Haemophilus influenzae type b (Hib) conjugate vaccine and administered as a single injection, with the DTPa‐IPV/Hib and hepatitis B vaccines administered in two simultaneous injections to infants at 2, 4 and 6 months of age. Vaccine 2003;21(25‐6):3593‐600. [DOI] [PubMed] [Google Scholar]
Avdicova 2002 {published data only}
- Avdicova M, Prikazsky V, Hudeckova H, Schuerman L, Willems P. Immunogenicity and reactogenicity of a novel hexavalent DTPa‐HBV‐IPV/Hib vaccine compared to separate concomitant injections of DTPa‐IPV/Hib and HBV vaccines, when administered according to a 3, 5 and 11 month vaccination schedule. European Journal of Pediatrics 2002;161(11):581‐7. [DOI] [PubMed] [Google Scholar]
Bravo 1998 {published data only}
- Bravo L, Carlos J, Gatchalian S, Borja‐Tabora C, Bibera G, Willems P, et al. The new DTPw‐HBV‐Hib combination vaccine can be used at the WHO schedule with a monovalent dose of hepatitis B vaccine at birth. Southeast Asian Journal of Tropical Medicine and Public Health 1998;29(4):772‐8. [PubMed] [Google Scholar]
Faingezicht 2002 {published data only}
- Faingezicht I, Avila‐Aguerro ML, Cervantes Y, Fourneau M, Clemens SA. Primary and booster vaccination with DTPw‐HB/Hib pentavalent vaccine in Costa Rican children who had received a birth dose of hepatitis b vaccine. Pan American Journal of Public Health 2002;12(4):247‐57. [DOI] [PubMed] [Google Scholar]
Gabutti 2004 {published data only}
- Gabutti G, Zepp F, Schuerman L, Dentico P, Bamfi F, Soncini R, et al. Evaluation of the immunogenicity and reactogenicity of a DTPa‐HBV‐IPV combination vaccine co‐administered with a Hib conjugate vaccine either as a single injection of a hexavalent combination or as two separate injections at 3, 5 and 11 months of age. Scandinavian Journal of Infectious Diseases 2004;36(8):585‐92. [DOI] [PubMed] [Google Scholar]
Gabutti 2005 {published data only}
- Gabutti G, Bona G, Dentico P, Bamfi F, Hardt K, Majori S, et al. Immunogenicity and reactogenicity following primary immunisation with a combined DTaP‐HBV vaccine and a Haemophilus influenzae type B vaccine administered by separate or mixed injection. Clinical Drug Investigation 2005;25(5):315‐23. [DOI] [PubMed] [Google Scholar]
Greenberg 2000 {published data only}
- Greenberg DP, Wong VK, Partridge S, Chang SJ, Jing J, Howe BJ, et al. Immunogenicity of a Haemophilus influenzae type b‐tetanus toxoid conjugate vaccine when mixed with a diphtheria‐tetanus‐acellular pertussis‐hepatitis B combination vaccine. Pediatric Infectious Disease Journal 2000;19(12):1135‐40. [DOI] [PubMed] [Google Scholar]
Mallet 2000 {published data only}
- Mallet E, Fabre P, Pines E, Salomon H, Staub T, Schodel F, et al. Immunogenicity and safety of a new liquid hexavalent combined vaccine compared with separate administration of reference licensed vaccines in infants. Pediatric Infectious Disease Journal 2000;19(12):1119‐27. [DOI] [PubMed] [Google Scholar]
Marshall 2010 {published data only}
- Marshall H, McIntyre P, Roberton D, Dinan L, Hardt K. Primary and booster immunization with a diphtheria, tetanus, acellular pertussis, hepatitis B (DTPa‐HBV) and Haemophilus influenzae type b (Hib) vaccine administered separately or together is safe and immunogenic. International Journal of Infectious Diseases 2010;14(1):e41‐9. [PUBMED: 19467896] [DOI] [PubMed] [Google Scholar]
Nolan 2001 {published data only}
- Nolan T, Hogg G, Darcy MA, Skeljo M, Carlin J, Boslego J. A combined liquid Hib (PRP‐OMPC), hepatitis B, diphtheria, tetanus and whole‐cell pertussis vaccine: controlled studies of immunogenicity and reactogenicity. Vaccine 2001;19(15‐16):2127‐37. [DOI] [PubMed] [Google Scholar]
Omenaca 2001 {published data only}
- Omenaca F, Dal‐Re R, D'Apuzzo V, Kattamis C, Gnehm HP, Garcia‐Sicilia J, et al. Reactogenicity of DTPa‐HBV/Hib vaccine administered as a single injection vs DTPa‐HBV and Hib vaccines administered simultaneously at separate sites, to infants at 2, 4 and 6 months of age. Vaccine 2001;19(30):4260‐6. [DOI] [PubMed] [Google Scholar]
Ortega‐Barria 2007 {published data only}
- Ortega‐Barria E, Kanra G, Leroux G, Bravo L, Safary A, Levlevre I. The immunogenicity and reactogenicity of DTPw‐HBV/Hib 2.5 combination vaccine: Results from four phase III multicenter trials across three continents. Vaccine 2007;25(50):8432‐40. [DOI] [PubMed] [Google Scholar]
Pichichero 1997 {published data only}
- Pichichero ME, Passador S. Administration of combined diphtheria and tetanus toxoids and pertussis vaccine, hepatitis B vaccine, and Haemophilus influenzae type b (Hib) vaccine to infants and response to a booster dose of Hib conjugate vaccine. Clinical Infectious Diseases 1997;25(6):1378‐84. [DOI] [PubMed] [Google Scholar]
Ramkissoon 2001 {published data only}
- Ramkissoon A, Coovadia HM, Jugnundan P, Willems P, Clemens BR. A new combined DTP‐HBV‐Hib vaccine‐strategy for incorporation of Hib vaccination into childhood immunisation programmes. South African Medical Journal 2001;91(10):864‐9. [PubMed] [Google Scholar]
Rao 2009 {published data only}
- Rao R, Dhingra MS, Bavdekar S, Behera N, Daga SR, Dutta AK, et al. A comparison of immunogenicity and safety of indigenously developed liquid (DTwPHB‐Hib) pentavalent combination vaccine (Shan 5) with Easyfive (liq) and TritanrixHB + Hiberix (lyo) in Indian infants administered according to the EPI schedule. Human vaccines 2009;5(6):425‐9. [PUBMED: 19333002] [DOI] [PubMed] [Google Scholar]
Riedemann 2002 {published data only}
- Riedemann S, Reinhardt G, Jara J, Rios R, Wenzel MS, Willems P, et al. Immunogenicity and reactogenicity of combined versus separately administered DTPw‐HBV and Hib vaccines given to healthy infants at 2, 4, and 6 months of age, with a booster at 18 months. International Journal of Infectious Diseases 2002;6(3):215‐22. [DOI] [PubMed] [Google Scholar]
Santos 2002 {published data only}
- Santos JI, Martin A, Leon T, Rivera L, Gaitan ME, Rio C, et al. DTPw‐HB and Hib primary and booster vaccination: combined versus separate administration to Latin American children. Vaccine 2002;20(13‐14):1887‐93. [DOI] [PubMed] [Google Scholar]
Schmitt 2000 {published data only}
- Schmitt HJ, Knuf M, Ortiz E, Sanger R, Uwamwezi MC, Kaufhold A. Primary vaccination of infants with diphtheria‐tetanus‐acellular pertussis‐hepatitis B virus‐ inactivated polio virus and Haemophilus influenzae type b vaccines given as either separate or mixed injections. Journal of Pediatrics 2000;137(3):304‐12. [DOI] [PubMed] [Google Scholar]
Tregnaghi 2006 {published data only}
- Tregnaghi M, Lopez P, Rocha C, Rivera L, David M, Ruttimann R. A new DTPw‐HB/Hib combination vaccine for primary and booster vaccination of infants in Latin America. Pan American Journal of Public Health 2006;19(3):179‐88. [DOI] [PubMed] [Google Scholar]
Win 1997 {published data only}
- Win KM, Aye M, Htay‐Htay H, Safary A, et al. Comparison of separate and mixed administration of DTPw‐HBV and Hib vaccines: Immunogenicity and reactogenicity profiles. International Journal of Infectious Diseases 1997;2(2):79‐84. [Google Scholar]
References to studies excluded from this review
Aristegui 1998 {published data only}
- Aristegui J, Dal‐Re R, Garrote E, Gonzalez A, Arrate JP, Perez A. Assessment of the immunogenicity and reactogenicity of a quadrivalent diphtheria, tetanus, acellular pertussis and hepatitis B (DTPa‐HBV) vaccine administered in a single injection with Haemophilus influenzae type b conjugate vaccine, to infants at 2, 4 and 6 months of age. Vaccine 1998;16(20):1976‐81. [DOI] [PubMed] [Google Scholar]
Aristegui 2001 {published data only}
- Aristegui J, Garcia‐Corbeira P, De‐la‐Flor J, Dal‐Re R, Mares J, Moraga F, et al. Reactogenicity and safety of DTPa vaccine and Haemophilus influenzae type b conjugate vaccine (Hib) in a single injection vs DTPw and Hib as separate injections as a booster vaccination in 18‐month‐old children. Clinical Drug Investigation 2001;21(1):9‐16. [Google Scholar]
Bavdekar 2007 {published data only}
- Bavdekar SB, Maiya PP, Subba Rao SD, Bock HL. Immunogenicity and safety of combined diphtheria tetanus whole cell pertussis hepatitis B/ Haemophilus influenzae type b vaccine in Indian infants. Indian Pediatrics 2007;44(7):505‐10. [PubMed] [Google Scholar]
Botet‐Asensi 2003 {published data only}
- Botet‐Asensi FI, Veronese A, Carmen Otero M, Desamparados Tamarit Perez M, Hontangas Lopez JL, Viviani S. Immunogenicity and safety in infants of a DTwPHib full liquid vaccine. Acta Pediatrica 2003;92(5):541‐5. [DOI] [PubMed] [Google Scholar]
Calbo 2002 {published data only}
- Calbo F, Dal Re R, Diez Delgado J, Ona S, Sanchez Prados F, Garcia Corbeira P, et al. Comparative trial to assess the reactogenicity of the diphtheria‐tetanusacellular pertussis (DTPa) vaccine plus Haemophilus influenza type b (Hib) conjugate vaccine and that of the diphtheria‐tetanus‐whole cell pertussis (DTPw) vaccine plus Hib conjugate vaccine, administered in single injection as a booster dose to 14‐20 months‐old children. Medicina Clinica 2002;118(1):1‐4. [DOI] [PubMed] [Google Scholar]
Clemens 2003 {published data only}
- Clemens SC, Azevedo T, Homma A. Feasibility study of the immunogenicity and safety of a novel DTPw/Hib (PRP‐T) Brazilian combination compared to a licensed vaccine in healthy children at 2, 4, and 6 months of age. Revista da Sociedade Brasileira de Medicina Tropical 2003;36(3):321‐30. [DOI] [PubMed] [Google Scholar]
Denoel 2007 {published data only}
- Denoel PA, Goldblatt D, Vleeschauwer I, Jacquet JM, Pichichero ME, Poolman JT. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria‐tetanus‐acellular pertussis/Hib combination vaccines. Clinical and Vaccine Immunology 2007;14(10):1362‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz‐Mitoma 2011 {published data only}
- Diaz‐Mitoma F, Halperin SA, Tapiero B, Hoffenbach A, Zappacosta PS, Radley D, et al. Safety and immunogenicity of three different formulations of a liquid hexavalent diphtheria‐tetanus‐acellular pertussis‐inactivated poliovirus‐Haemophilus influenzae b conjugate‐hepatitis B vaccine at 2, 4, 6 and 12‐14 months of age. Vaccine 2011;29(6):1324‐31. [DOI] [PubMed] [Google Scholar]
Gatchalian 2005 {published data only}
- Gatchalian S, Reyes M, Bernal N, Lefevre I, David MP, Han HH, et al. A new DTPw‐HBV/Hib vaccine is immunogenic and safe when administered according to the EPI (Expanded Programme for Immunization) schedule and following hepatitis B vaccination at birth. Human Vaccines 2005;1(5):198‐203. [DOI] [PubMed] [Google Scholar]
Gentile 2011 {published data only}
- Gentile A, Umido V, Czerniuk P, Nacul J, Seigelchifer M, Hilbert AK, et al. Immunogenicity and reactogenicity of a combined fully liquid DTPw‐HepB‐Hib pentavalent vaccine in healthy infants: No clinically relevant impact of a birth dose of hepatitis B vaccine. International Journal of Infectious Diseases 2011;15(1):e24‐e9. [DOI] [PubMed] [Google Scholar]
Gylca 2001 {published data only}
- Gylca R, Gylca V, Benes O, Melnic A, Chicu V, Weisbecker C, et al. A new DTPa‐HBV‐IPV vaccine co‐administered with Hib, compared to a commercially available DTPw‐IPV/Hib vaccine co‐administered with HBV, given at 6, 10 and 14 weeks following HBV at birth. Vaccine 2001;19(7‐8):825‐33. [DOI] [PubMed] [Google Scholar]
Halperin 2009 {published data only}
- Halperin SA, Tapiero B, Diaz‐Mitoma F, Law B, Hoffenbach J, Zappacosta A, et al. Safety and immunogenicity of a hexavalent diphtheria‐tetanus‐acellular pertussis‐inactivated poliovirus‐Haemophilus influenzae b conjugate‐hepatitis B vaccine at 2, 3, 4, and 12‐14 months of age. Vaccine 2009;27(19):2540‐7. [DOI] [PubMed] [Google Scholar]
Hla 2006 {published data only}
- Hla KH, Thein SA, Aye A, Han HH, Bock HL, David MP, et al. Reactogenicity and immunogenicity profiles of a novel pentavalent diphtheria‐tetanus‐whole cell pertussis‐hepatitis B and Haemophilus influenzae type B vaccine: a randomised dose‐ranging trial of the Hib tetanus‐conjugate content. Pediatric Infectious Disease Journal 2006;25(8):706‐12. [DOI] [PubMed] [Google Scholar]
Hogg 2003 {published data only}
- Hogg K, Hogg G. The immunogenicity of oral poliomyelitis vaccine in a primary vaccination series at 2, 4 and 6 months given concurrently with Hib, hepatitis B and diphtheria, tetanus and whole‐cell pertussis vaccines administered as three separate injections or as a combination pentavalent vaccine. Vaccine 2003;21(21‐2):2906‐10. [DOI] [PubMed] [Google Scholar]
Huang 1998 {published data only}
- Huang LM, Chang PF, Lee PI, Chiu HH, Tsai SY, Lee CY. Immunogenicity and safety of Haemophilus influenzae type b conjugate vaccine (HibTITER) and a combination vaccine of diphtheria, tetanus, pertussis and HibTITER (TETRAMUNE) in two‐month‐old infants. Journal of Microbiology, Immunology, and Infection 1998;31(3):180‐6. [PubMed] [Google Scholar]
Kalies 2004 {published data only}
- Kalies H, Verstraeten T, Grote V, Meyer N, Siedler A, Schmitt HJ, et al. Four and one‐half‐year follow‐up of the effectiveness of diphtheria‐tetanus toxoids‐acellular pertussis/Haemophilus influenzae type b and diphtheria‐tetanus toxoids‐acellular pertussis‐inactivated poliovirus/H. influenzae type b combination vaccines in Germany. Pediatric Infectious Disease Journal 2004;23(10):944‐50. [DOI] [PubMed] [Google Scholar]
Kanra 2006 {published data only}
- Kanra G, Kara A, Demiralp O, Contorni M, Hilbert AK, Spyr C, et al. Safety and immunogenicity of a new fully liquid DTPw‐HepB‐Hib combination vaccine in infants. Human Vaccines 2006;2(4):155‐60. [DOI] [PubMed] [Google Scholar]
Kilpi 2009 {published data only}
- Kilpi T, Silfverdal M, Nilsson SA, Syrjanen L, Belloni R, Desole C, et al. Immunogenicity and reactogenicity of two diphtheria‐tetanus‐acellular pertussis‐hepatitis B‐inactivated polio virus‐Haemophilus influenzae type b vaccines administered at 3, 5 and 11‐12 months of age. Human Vaccines 2009;5(1):18‐25. [DOI] [PubMed] [Google Scholar]
Knuf 2006 {published data only}
- Knuf M, Habermehl P, Cimino C, Petersen G, Schmitt HJ, Habermehl P. Immunogenicity, reactogenicity and safety of a 7‐valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a DTPa‐HBV‐IPV/Hib combination vaccine in healthy infants. Vaccine 2006;24(22):4727‐36. [DOI] [PubMed] [Google Scholar]
Lagos 2005 {published data only}
- Lagos R, Hoffenbach A, Scemama M, Dupuy M, Schodel F, Hessel L, et al. Lot‐to‐lot consistency of a combined hexavalent diptheria‐tetanus‐acellular‐pertussis, hepatitis B, Inactivated polio and haemophilus B conjugate vaccine, administered to healthy Chilean infants at two, four and six months of age. Human Vaccines 2005;1(3):112‐7. [DOI] [PubMed] [Google Scholar]
Lim 2007 {published data only}
- Lim FS, Han HH, Jacquet JM, Bock HL. Primary vaccination of infants against hepatitis B can be completed using a combined hexavalent diphtheria‐tetanus‐acellular pertussis‐hepatitis B‐inactivated poliomyelitis‐Haemophilus influenzae type B vaccine. Annals of the Academy of Medicine, Singapore 2007;36(10):801‐6. [PubMed] [Google Scholar]
Lopez 2002 {published data only}
- Lopez P, Rubiano L, Pilar Rubio M, David MP, Safary A. Immunogenicity and reactogenicity of DTPw‐HB/Hib vaccine administered to Colombian infants after a birth dose of hepatitis B vaccine. Expert Review of Vaccines 2002;1(3):277‐83. [DOI] [PubMed] [Google Scholar]
Madhi 2011 {published data only}
- Madhi SA, Mitha I, Cutland C, Groome M, Santos‐Lima E. Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatric Infectious Disease Journal 2011;30(4):e68‐74. [DOI] [PubMed] [Google Scholar]
Meriste 2006 {published data only}
- Meriste S, Lutsar I, Tamm E, Willems P. Safety and immunogenicity of a primary course and booster dose of a combined diphtheria, tetanus, acellular pertussis, hepatitis B and inactivated poliovirus vaccine. Scandinavian Journal of Infectious Diseases 2006;38(5):350‐6. [DOI] [PubMed] [Google Scholar]
Mills 1998 {published data only}
- Mills E, Gold R, Thipphawong J, Barreto L, Guasparini R, Meekison W, et al. Safety and immunogenicity of a combined five‐component pertussis‐diphtheria‐tetanus‐inactivated poliomyelitis‐Haemophilus B conjugate vaccine administered to infants at two, four and six months of age. Vaccine 1998;16(6):576‐85. [DOI] [PubMed] [Google Scholar]
Nolan 2004 {published data only}
- Nolan T, Altmann A, Skeljo M, Streeton C, Schuerman L. Antibody persistence, PRP‐specific immune memory, and booster responses in infants immunised with a combination DTPa‐HBV‐IPV/Hib vaccine. Vaccine 2004;23(1):14‐20. [DOI] [PubMed] [Google Scholar]
Pichichero 1999 {published data only}
- Pichichero ME, Voloshen T, Zajac D, Passador S. Avidity maturation of antibody to Haemophilus influenzae type b (Hib) after immunization with diphtheria‐tetanus‐acellular pertussis‐hib‐hepatitis B combined vaccine in infants. Journal of Infectious Diseases 1999;180(4):1390‐3. [DOI] [PubMed] [Google Scholar]
Pichichero 2007 {published data only}
- Pichichero ME, Bernstein H, Blatter MM, Schuerman L, Cheuvart B, Holmes SJ. Immunogenicity and safety of a combination diphtheria, tetanus toxoid, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine co‐administered with a 7‐valent pneumococcal conjugate vaccine and a Haemophilus influenzae type b conjugate vaccine. Journal of Pediatrics 2007;151(1):43‐9; e1‐2. [DOI] [PubMed] [Google Scholar]
Poolman 2001 {published data only}
- Poolman J, Kaufhold A, Grave D, Goldblatt D. Clinical relevance of lower Hib response in DTPa‐based combination vaccines. Vaccine 2001;19(17‐9):2280‐5. [DOI] [PubMed] [Google Scholar]
Saenger 2005 {published data only}
- Saenger R, Maechler G, Potreck M, Zepp F, Knuf M, Habermehl P, et al. Booster vaccination with hexavalent DTPa‐HBV‐IPV/Hib vaccine in the second year of life is as safe as concomitant DTPa‐IPV/Hib + HBV administered separately. Vaccine 2005;23(9):1135‐43. [DOI] [PubMed] [Google Scholar]
Scheifele 2005 {published data only}
- Scheifele DW, Halperin SA, Rubin E, Tapiero B, Guasparini R, Meekison W, et al. Safety and immunogenicity of a pentavalent combination vaccine (diphtheria, tetanus, acellular pertussis, polio and haemophilus influenzae type B conjugate) when administered as a fourth dose at 15 to 18 months of age. Human Vaccines 2005;1(5):180‐6. [DOI] [PubMed] [Google Scholar]
Scheifele 2006 {published data only}
- Scheifele DW, Halperin SA, Smith B, Ochnio J, Meloff K, Duarte‐Monteiro D. Assessment of the compatibility of co‐administered 7‐valent pneumococcal conjugate, DTaP.IPV/PRP‐T Hib and hepatitis B vaccines in infants 2‐7 months of age. Vaccine 2006;24(12):2057‐64. [DOI] [PubMed] [Google Scholar]
Tichmann 2005 {published data only}
- Tichmann I, Preidel H, Grunert D, Habash S, Schult R, Maier R, et al. Comparison of the immunogenicity and reactogenicity of two commercially available hexavalent vaccines administered as a primary vaccination course at 2, 4 and 6 months of age. Vaccine 2005;23(25):3272‐9. [DOI] [PubMed] [Google Scholar]
Tichmann‐Schumann 2005 {published data only}
- Tichmann‐Schumann I, Soemantri P, Behre U, Disselhoff J, Mahler H, Maechler G, et al. Immunogenicity and reactogenicity of four doses of diphtheria‐tetanus‐three‐component acellular pertussis‐hepatitis B‐inactivated polio virus‐Haemophilus influenzae type b vaccine co‐administered with 7‐valent pneumococcal conjugate vaccine. Pediatric Infectious Disease Journal 2005;24(1):70‐7. [DOI] [PubMed] [Google Scholar]
Tregnaghi 2011 {published data only}
- Tregnaghi MW, Zambrano B, Santos‐Lima E. Immunogenicity and safety of an investigational hexavalent diphtheria‐tetanus‐acellular pertussis‐inactivated poliovirus‐hepatitis B‐Haemophilus influenzae B conjugate combined vaccine in healthy 2‐, 4‐, and 6‐month‐old Argentinean infants. Pediatric Infectious Disease Journal 2011;30(6):e88‐96. [DOI] [PubMed] [Google Scholar]
Trollfors 2005 {published data only}
- Trollfors B, Taranger J, Lagergard T, Sundh V. Reduced immunogenicity of diphtheria and tetanus toxoids when combined with pertussis toxoid. Pediatric Infectious Disease Journal 2005;24(1):85‐6. [DOI] [PubMed] [Google Scholar]
Usonis 1999a {published data only}
- Usonis V, Bakasenas V. Does concomitant injection of a combined diphtheria‐tetanus‐acellular pertussis ‐ Hepatitis B virus ‐ Inactivated polio virus vaccine influence the reactogenicity and immunogenicity of commercial Haemophilus influenzae type b conjugate vaccines?. European Journal of Pediatrics 1999;158(5):398‐402. [DOI] [PubMed] [Google Scholar]
Usonis 1999b {published data only}
- Usonis V, Bakasenas V. Evaluation of the immunogenicity and reactogenicity of a new combined diphtheria, tetanus, whole‐cell Bordetella pertussis and Hepatitis B vaccine and Haemophilus influenzae type b vaccine in children at 3, 4.5 and 6 months of age. Acta Medica Lituanica 1999;T6:Nr 3. [Google Scholar]
Zepp 1997 {published data only}
- Zepp F, Schmitt HJ, Kaufhold A, Schuind A, Knuf M, Habermehl P, et al. Evidence for induction of polysaccharide specific B‐cell‐memory in the 1st year of life: plain Haemophilus influenzae type b‐PRP (Hib) boosters children primed with a tetanus‐conjugate Hib‐DTPa‐HBV combined vaccine. European Journal of Pediatrics 1997;156(1):18‐24. [DOI] [PubMed] [Google Scholar]
Zepp 2004 {published data only}
- Zepp F, Knuf M, Heininger U, Jahn K, Collard A, Habermehl P, et al. Safety, reactogenicity and immunogenicity of a combined hexavalent tetanus, diphtheria, acellular pertussis, hepatitis B, inactivated poliovirus vaccine and Haemophilus influenzae type b conjugate vaccine, for primary immunization of infants. Vaccine 2004;22(17‐8):2226‐33. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Sanofi‐Aventis 2011 {published data only (unpublished sought but not used)}
- Sanofi‐Aventis ICMJE Investigators. Study of PENTAXIM™ Vaccine Versus TETRAXIM™ Vaccine Given With ACTHIB™ Vaccine in South Korean Infants. www.clinicaltrials.gov First Received on October 1, 2010. Last Updated on April 4, 2011.
Additional references
Ball 2001
- Ball LK, Falk LA, Horne D, Finn TM. Evaluating the immune response to combination vaccines. Clinical Infectious Diseases 2001;33(Suppl 4):299‐305. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
WHO 1998
- World Health Organization. Global Programme for Vaccines and Immunization: The WHO position paper on Haemophilus influenza type b conjugate vaccines. Weekly Epidemiological Record 1998;73(10):64‐71. [PubMed] [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94:41‐7. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bar‐On 2009
- Bar‐On ES, Goldberg E, Fraser A, Vidal L, Hellmann S, Leibovici L. Combined DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD005530.pub2] [DOI] [PubMed] [Google Scholar]


