Abstract
Background
Bronchiectasis is a chronic respiratory condition characterised by airway and systemic inflammation with prevalence increasing with age. Given the median age of the patients, it is common to observe the presence of comorbidities, particularly cardiovascular diseases, which have been linked to adverse clinical outcomes. To investigate the pooled estimates of the association between bronchiectasis and coronary heart disease or stroke within this population, we conducted a systematic review and meta-analysis of the available scientific evidence.
Methods
Three investigators independently performed the search on PubMed and other sources and included studies published up to October 2023 according to predefined criteria. Relative measures of association between bronchiectasis and cardiovascular events were pooled and meta-analysed using a fixed-effects model. Studies were evaluated using the Newcastle-Ottawa Scale for assessing the quality of non-randomised studies in meta-analyses.
Results
A final pool of nine studies was included in the systematic review, with a total of 22 239 patients. Meta-analysis of three high-quality cohort studies showed a pooled hazard ratio of 1.42 (95% CI 1.30–1.57) for coronary heart disease and 1.71 (95% CI 1.55–1.89) for cerebrovascular stroke.
Conclusions
The increased cardiovascular risk among people with bronchiectasis underscores the critical need to raise awareness of this association and to develop preventive strategies accordingly. Further translational studies are imperative to gain a deeper understanding of the complex interplay between inflammation, the immune system and endothelial dysfunction in this patient group.
Shareable abstract
People with bronchiectasis have an increased risk for cardiovascular events, including coronary heart disease and cerebrovascular stroke https://bit.ly/4bgFvI1
Introduction
Bronchiectasis is a chronic respiratory condition characterised by abnormal and persistent dilation of the bronchi, accompanied by symptoms such as chronic cough, sputum production and a history of exacerbations [1]. Recent epidemiological studies have revealed an average prevalence ranging from 701 to 1200 per 100 000 population, with rates increasing with age [2–5]. The mean age of bronchiectasis patients typically falls between 60 and 70 years in European and American studies [6].
Given the median age of these patients, comorbidities are common and have been linked to worse clinical outcomes, including exacerbation frequency, lung function decline, diminished quality of life and increased mortality [7–10]. Cardiovascular (CV) diseases are among the most prevalent comorbidities in bronchiectasis patients, with factors associated with CV events, such as aortic stiffness and cardiac biomarkers, worsening as the disease progresses [11].
The increased CV risk in bronchiectasis patients has been attributed, in part, to advancing age. While the precise mechanisms underlying CV manifestations remain unclear, this association may also be attributable to the substantial burden of chronic systemic inflammation and oxidative stress observed in this population, as seen in other chronic conditions [11–14].
Some studies have reported a higher incidence of myocardial infarction and cerebrovascular stroke following severe pulmonary exacerbations or other lower respiratory tract infections, indicating increased mortality from CV events [15–20]. However, much of the evidence on CV risk is derived from a limited number of retrospective studies, and no study to date has systematically investigated the association between bronchiectasis and the risk of CV events [21]. A deeper understanding of this relationship could have significant implications for clinical monitoring and preventive strategies in this patient population.
Therefore, this systematic review aims to explore the association between bronchiectasis and coronary heart disease (CHD) or stroke within this population. We systematically collected and summarised observational cohort studies reporting the incidence and characteristics of CV events in bronchiectasis patients, conducting a systematic review and meta-analysis of the available scientific evidence.
Methods
Search methodology
After registration of the review protocol in the international prospective register PROSPERO (ID CRD42022330081), three investigators (AA, CC and IB) independently conducted an electronic search on PubMed and other databases (Cochrane Library, Embase, Ovid). Studies published from 1 January 2000 to 1 October 2023 were considered. The search string used for the electronic search was: (“bronchiectasis”[All Fields] OR “bronchiectasis”[MeSH Terms] OR “bronchiectasis”[All Fields] OR “bronchiectasis”[All Fields]) AND (“cardiovascular diseases”[MeSH Terms] OR (“cardiovascular”[All Fields] AND “diseases”[All Fields]) OR “cardiovascular diseases”[All Fields] OR (“cardiovascular”[All Fields] AND “disease”[All Fields]) OR “cardiovascular disease”[All Fields]). This systematic review was conducted according to the Preferred Reporting Items for Systematic Meta-Analyses (PRISMA) statement [22].
Study selection
Titles and abstracts were screened by two independent investigators (AG and IB). We also manually searched the references included in the full text of the eligible articles to identify any studies that might have been missed. In case of disagreement a final decision was taken by the principal investigator (AG). Records were excluded if 1) they were written in languages other than English, 2) they were case reports, case series or qualitative reviews, 3) they included paediatric patients (<18 years of age), 4) they included patients affected by cystic fibrosis or other genetically determined causes for bronchiectasis (e.g. primary ciliary dyskinesia) or 5) the full text was unavailable. Non-peer-reviewed papers were not selected owing to poor methodological reliability. The full text was then obtained for selected papers.
Data extraction
Following a comprehensive analysis of the full texts, relevant information was extracted from each paper. The extracted data included the year of publication, study design, number of included patients, type of controls (if any), participant age, bronchiectasis severity, study outcomes and main results. We searched each paper for any measure of association among odds ratio (OR), relative risk and hazard ratio (HR) and their corresponding 95% confidence intervals (CIs). Additionally, covariates employed for adjustments and collected risk factors associated with CV events were also collected.
Quantitative synthesis of the evidence
When at least two different studies provided a relative measure of association between bronchiectasis and CV events, the data were pooled using a meta-analytic approach. Specifically, a fixed-effects model was used to obtain a pooled estimate of the relative risk for the outcomes considered in the retrieved studies [23]. The pooled estimate was calculated as a weighted average of study-specific relative risk, for which the weights assigned to each study were the inverse of the study's variance.
Critical assessment of evidence quality
Studies included in the quantitative synthesis were evaluated using the Newcastle-Ottawa Scale for assessing the quality of non-randomised studies in meta-analyses (www.ohri.ca/programs/clinical_epidemiology/oxford.asp). This scale is based on a “star system” in which a study is judged on three perspectives: the selection of the study groups, the comparability of the groups and the ascertainment of the outcome of interest. Selection of the study groups can receive a maximum of four stars, comparability of the groups up to two stars and ascertainment of the study outcome up to three stars.
Results
Summary of evidence
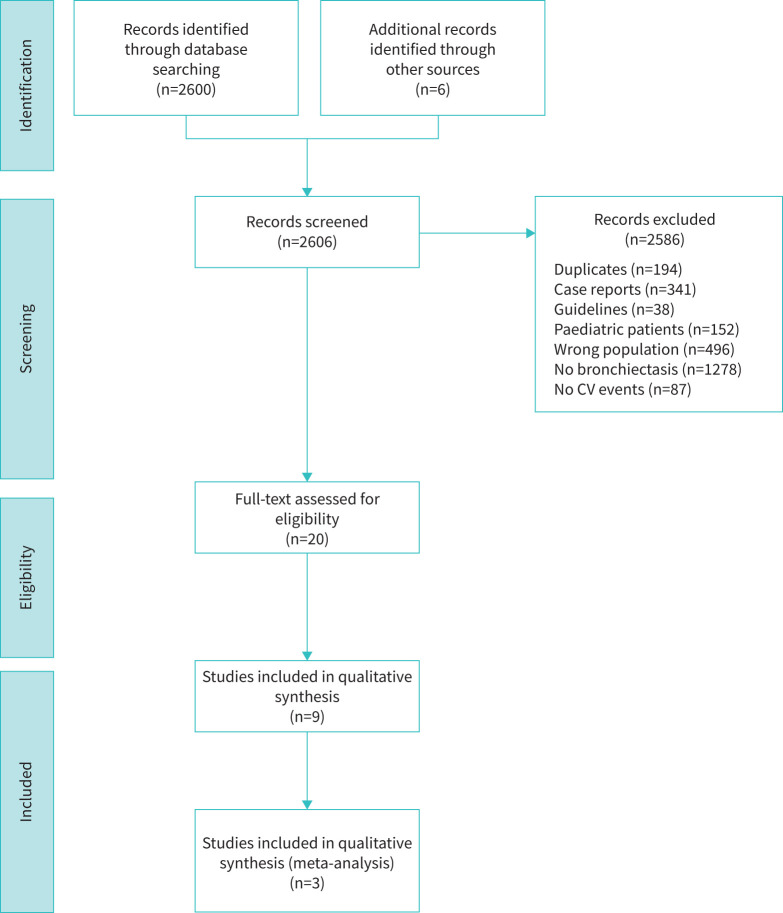
The electronic search initially identified 2600 records. The majority of the selected studies were excluded because they did not involve bronchiectasis patients (n=1278), focused on cystic fibrosis or other genetically determined causes of bronchiectasis (n=496) or involved paediatric patients (n=152). Additionally, 87 papers were excluded for not evaluating CV events. We also excluded 341 case reports and case series, along with 38 guidelines or recommendations. Six additional papers were identified through manual assessment of the references cited in the selected papers. After eliminating 194 duplicates, 20 papers were subjected to full-text analysis (figure 1). A final pool of nine papers were included in the qualitative analysis, with a total of 22 239 bronchiectasis patients. The selected studies were published between 2016 and 2022 (table 1).
FIGURE 1.
Flow chart of study selection. CV: cardiovascular.
TABLE 1.
Articles retrieved by the electronic search
| Study | Study design | Population | Controls | Median age | Median follow-up | Outcome | Results | Study quality assessment# |
|---|---|---|---|---|---|---|---|---|
| Navaratnam et al. 2017 [24] | Retrospective cohort | 10 942 patients included in the electronic primary care data from the CPRD (UK) | 3 884 770 individuals included in the CPRD without BE | 47 years | 5.6 years | Cross-sectional analysis: existing diagnoses of CHD¶ and stroke+ prior to the index date Historical cohort analysis: first-time diagnoses of CHD and stroke after the index date were considered as incident events |
Cross-sectional analysis: BE was associated with increased odds for CHD (OR 1.33, 95% CI 1.25–1.41), stroke (OR 1.92, 95% CI 1.85–2.01), angina (OR 1.33, 95% CI 1.24–1.43), CABG (OR 1.87, 95% CI 1.65–2.17) and MI (OR 1.11, 95% CI 1.01–1.22) Historical cohort analysis: crude rates of first CHD event in people with and without BE were 6.6 per 1000 person-years (95% CI 5.9–7.5 per 1000 person-years) and 2.2 per 1000 person-years, respectively The adjusted HR was 1.44 (95% CI 1.27–1.63) The estimate was adjusted for sex, age, smoking, diabetes, hypertension, hyperlipidaemia and family history of CVD |
Selection: ★★★★ Comparability: ★★ Outcome: ★★★ |
| Chen et al. 2017 [25] | Retrospective cohort | 1295 patients retrieved from the NHI Research Database (Taiwan) | 6475 individuals, frequency-matched by age and sex, selected from the general population without BE | 62 years | 4.9 and 5.4 years for patients with BE and controls, respectively¶ | Incidence and risk of ischaemic stroke | Higher risk of ischaemic stroke in patients with BE as compared to controls (HR 1.74, 95% CI 1.28–2.35) The estimate was adjusted for age, sex and comorbidities |
Selection: ★★★★ Comparability: ★★ Outcome: ★★★ |
| Evans et al. 2017 [26] | Retrospective cohort | 400 patients attending the BE service Edinburgh (UK) | None | 66 years | Not reported | Prevalence of CVD and risk factors | 45 patients (11.3%) developed vascular disease after the diagnosis of BE BSI was associated with increased odds of CVD (OR for scores 5–8: 3.92, 95% CI 1.21–12.71; OR for scores ≥9: 8.12, 95% CI 2.44–27.0) |
Selection: ★★☆☆ Comparability: ☆☆ Outcome: ★★★ |
| Menéndez et al. 2017 [27] | Prospective cohort | 265 patients attending the specialised outpatient clinics of two tertiary care university hospitals, Spain | None | 68.4 years | 1 year | To evaluate factors associated with exacerbations requiring hospital admission | HF at baseline was associated with exacerbation requiring hospital admission (OR 5.47, 95% CI 1.36–37.23), while MI was not (OR 0.72, 95% CI 0.13–6.06) The estimates were adjusted for FACED score Similar estimates were obtained when adjusting for BSI |
Selection: ★★★★ Comparability: ☆☆ Outcome: ☆☆☆ |
| Navaratnam et al. 2017 [24] | Retrospective cohort | 895 patients selected from a larger cohort of 26 518 individuals included in the electronic primary care data from the CPRD, who had both a first CV event and at least one RTI during the study observation period (UK) |
Self-controlled cases | Age categories: <45 years: 16.3% 45–55 years: 12.7% 56–65 years: 20.9% 66–75 years: 27.7% >75 years: 22.4% |
Incidence rate ratios of a first CV event evaluated at different time points up to 91 days after RTI | First record of a CV event, a composite outcome of first recorded diagnosis of MI or stroke | Compared to a baseline period (before RTI) the incidence rate ratios were: 2.39 (95% CI 1.21–5.62) during the first 3 days post RTI, 2.01 (1.22–2.78) 4–7 days after, 1.73 (1.09–2.13) 8–14 days after, 1.16 (0.77–2.19) 15–28 days after and 1.08 (0.69–1.53) 29–91 days after The estimates were adjusted for age and season |
Selection: ★★★★ Comparability: ★★ Outcome: ★★★ |
| Hung et al. 2018 [28] | Retrospective cohort | 7156 patients included in the Longitudinal Health Insurance Database 2000, a national database comprising data of 1 million randomly selected beneficiaries of the NHI programme in 2000 (Taiwan) |
14 084 individuals without BE, selected from the general population and frequency-matched according to sex, age and entry year |
63.3 years | 2.4 person-years for patients with BE and 5.2 person-years among controls§ | The primary outcome was an ACS event | ACS incidence was higher in the BE cohort than in the comparison cohort (13.49 versus 9.07 per 1000 person-years) Adjusted HR 1.40 (95% CI 1.20–1.61) The estimate was adjusted for age, sex and comorbidities |
Selection: ★★★★ Comparability: ★★ Outcome: ★★★ |
| Chen et al. 2020 [29] | Retrospective cohort | 603 inpatients diagnosed with BE in the Affiliated Yancheng Hospital of Southeast University Medical College (Jiangsu, China) |
None | 62 years among patients without CV comorbidities and 65.4 years among patients with CV comorbidities | Prevalence of CV comorbidities was evaluated only at baseline | CV comorbidity was defined as a composite outcome of having a history of CHD (ACS, chronic coronary artery disease), cerebrovascular events (including ischaemic stroke, haemorrhagic stroke or TIA), PAD or HF |
199 patients (33.0%) had a history of CV event Main CV event registered: ACS: 81 (13.4%), CAD: 23 (3.8%), ischaemic stroke: 37 (6.1%), haemorrhagic stroke: 8 (1.3%), TIA: 58 (9.6%), PAD: 24 (4.0%), HF: 29 (4.8%) |
Selection: ★★☆☆ Comparability: ☆☆ Outcome: ★☆☆ |
| Huang et al. 2020 [21] | Longitudinal cohort | 433 patients | None | 67 years | 61.4 months per participant | All-cause and CV mortality | Increasing serum desmosine concentrations were associated with increasing all-cause mortality (HR 2.30, 95% CI 1.85–2.84; p<0.0001) Serum desmosine was associated with increased cardiovascular mortality (HR 2.21, sd 95% CI 1.60–3.05; p<0.0001) |
Selection: ★★★ Comparability: ☆☆ Outcome: ★☆☆ |
| Méndez et al. 2022 [30] | Post hoc retrospective analysis of a prospective observational study | 250 patients enrolled at two tertiary care hospitals (Spain) | None | 72 years | 35 months | CV events were defined as any ACS, new or worsening HF, new or recurrent arrhythmia requiring hospital admission or emergency department care, or cerebrovascular accident (stroke or TIA) | 74 patients (29.6%) had a CV event | Selection: ★★ Comparability: ☆☆ Outcome: ★★★ |
ACS: acute coronary syndrome (defined as acute MI or unstable angina); BE: bronchiectasis; CAD: coronary artery disease; CHD: coronary heart disease; CI: confidence interval; CABG: coronary artery bypass graft; CPRD: Clinical Practice Research Datalink; CVD: cardiovascular disease; CV: cardiovascular; HF: heart failure; MI: myocardial infarction; NHI: National Health Insurance; OR: odds ratio; PAD: peripheral artery disease; RTI: respiratory tract infection; TIA: transient ischaemic attack. #: studies were evaluated used the Newcastle-Ottawa Scale, which is based on a “star system” in which a study is judged on three perspectives: the selection of the study groups (maximum of four stars), the comparability of the groups (maximum of two stars) and the ascertainment of the outcome of interest (maximum of three stars). Each star awarded to a study is represented by a filled star, and the total of filled and unfilled stars indicates the maximum stars attainable in each domain; ¶: CHD was a composite outcome of having at least one recorded diagnosis of angina (including unstable angina), MI or CABG; +: stroke included ischaemic or haemorrhagic stroke, transient ischaemic attack and subarachnoid haemorrhage; §: computed from table 2 of the original manuscript by dividing the person-years by the number of patients.
Chen et al. [25] in 2017 conducted a prospective study on 1295 bronchiectasis patients enrolled between 2000 and 2008 and 6475 controls without bronchiectasis among 1 million randomly selected beneficiaries of the Taiwanese National Health Insurance Database. Subjects were followed up to the date of ischaemic stroke, censoring or the end of 2010. During follow-up, 163 ischaemic strokes were observed among bronchiectasis patients and 58 among controls, with a rate of 9.1 per 1000 person-years among bronchiectasis patients and 4.7 among controls. After adjusting for sex, age and comorbidities (hypertension, diabetes, hyperlipidaemia, chronic artery disease, myocardial infarction, ischaemic heart disease, angina pectoris, chronic heart failure, COPD and atrial fibrillation), bronchiectasis patients had a 74% excess risk of ischaemic stroke (HR 1.74, 95% CI 1.28–2.35). The excess risk was even higher among patients with at least one comorbidity at baseline (HR 2.66, 95% CI 1.85–3.84).
The retrospective study by Evans et al. [26] conducted in 2017 included 400 patients from a National Health Service Lothian bronchiectasis clinic in Edinburgh (UK) between May 2013 and September 2014. Their aim was to determine the prevalence of vascular diseases (such as ischaemic heart disease, cerebrovascular disease, peripheral vascular disease and atrial fibrillation) in bronchiectasis patients. Data from the study showed that 11% of patients had a diagnosis of vascular disease prior to bronchiectasis diagnosis, while an additional 11% developed CV disease in the following period, with an average onset occurring after 9.4 years. Independent risk factors for CV disease in bronchiectasis patients were male sex, arterial hypertension, long-term statin therapy and moderate-to-severe bronchiectasis, suggesting that the severity of bronchiectasis is independently linked to the onset of vascular disease.
The prospective study from Menéndez et al. [27] enrolled individuals from two tertiary care university hospitals in Spain during the period 2011–2015. Pulmonary exacerbations requiring the administration of antibiotics were tracked over a year. The study included 265 patients with 162 hospitalisations during the follow-up. Independent risk factors for hospital admission were age, prior bronchiectasis-related hospitalisations, proton pump inhibitor use, heart failure and disease severity according to validated scores (Bronchiectasis Severity Index (BSI) and FACED).
In 2017, Navaratnam et al. [24] published a cross-sectional study based on registry data. The authors examined CV risk factors and medication usage in individuals with and without bronchiectasis. A number of the 10 942 participants had bronchiectasis. This analysis showed differences in CV risk factors and medication use between bronchiectasis patients and the general population. The lower prevalence of chronic CV diseases suggested that other factors related to bronchiectasis may play a role in increasing the risk of CV events in this population.
Navaratnam et al. [24] also analysed primary care electronic records from the Clinical Practice Research Datalink to conduct a cross-sectional study employing logistic regression to assess the association between bronchiectasis and CV events, defined as CHD or stroke. The findings revealed that bronchiectasis patients showed high prevalence of pre-existing CHD (OR 1.33, 95% CI 1.25–1.41) and stroke (OR 1.92, 95% CI 1.85–2.01) compared to those without bronchiectasis, even after adjusting for age, sex, smoking and other risk factors for CV disease. In addition, the incidence rate of CHD and stroke events was higher in bronchiectasis patients than in the comparison group (HR for CHD 1.44, 95% CI 1.27–1.63; HR for stroke 1.71, 95% CI 1.54–1.90).
In a case–control study from 2018, Hung et al. [28] enrolled 3521 bronchiectasis patients and matched with 14 084 general population participants based on sex, age and index year. Groups were followed from 2000 until the end of 2010 to assess the incidence of acute coronary syndrome (ACS). Data from the study highlighted that the overall risk of ACS was 40% higher in the bronchiectasis cohort (adjusted HR 1.40, 95% CI 1.20–1.62). The ACS risk was significantly elevated for those bronchiectasis patients with a higher incidence of respiratory infection-related emergency room visits or hospitalisations, showing a 5.46-fold and 8.15-fold increase, respectively.
The 2020 study from Chen et al. [29] retrospectively examined 603 consecutive inpatients with bronchiectasis at a hospital in China from 2014 to 2017. During their initial hospitalisation, symptoms, bacterial cultures, blood indicators and chest computed tomography scans were evaluated. During this period, 335 patients experienced at least one bronchiectasis exacerbation. The study identified independent risk factors for pulmonary exacerbations, including the presence of CV diseases, isolation of Pseudomonas aeruginosa and extension of radiological involvement.
Despite advancements in research on the topic, the risk factors that lead to an increased CV risk in patients with bronchiectasis are still largely unexplored. Some biomarkers have been studied to try and stratify patients with bronchiectasis based on CV risk. The longitudinal cohort study from Huang et al. [21] from 2020 found that serum desmosine predicted future mortality and particularly CV mortality in a cohort of 433 bronchiectasis patients enrolled in the TAYBRIDGE (Tayside Bronchiectasis Registry Integrating Datasets, Genomics, and Enrolment into Clinical Trials) bronchiectasis registry. This is in line with previous studies in COPD in which circulating desmosine concentrations were significantly higher in patients with a prior history of CV disease and were significantly associated with mortality [32]. The study also found that the combination of serum desmosine and BSI significantly improved the prediction over the BSI alone, especially among individuals classified as having severe disease.
In a retrospective analysis of a prospective study by Méndez et al. [30] including 250 bronchiectasis patients across two tertiary care hospitals in Spain, risk factors for CV events during and following pulmonary exacerbations were investigated. Over a median follow-up of 35 months, 29.6% experienced at least one CV event, and 37.2% ultimately died. Semi-competing risk analysis demonstrated that age, arterial hypertension, COPD and potentially severe exacerbations significantly increased the likelihood of CV events, as well as bronchiectasis patients and CV events showing a higher mortality rate.
Quantitative evaluation of the CV risk in patients with bronchiectasis
Figure 2 shows the pooled estimates of the association between bronchiectasis and CHD or stroke obtained from three studies. The pooled HR were 1.42 (95% CI 1.30–1.57) for CHD and 1.71 (95% CI 1.55–1.89) for stroke.
FIGURE 2.
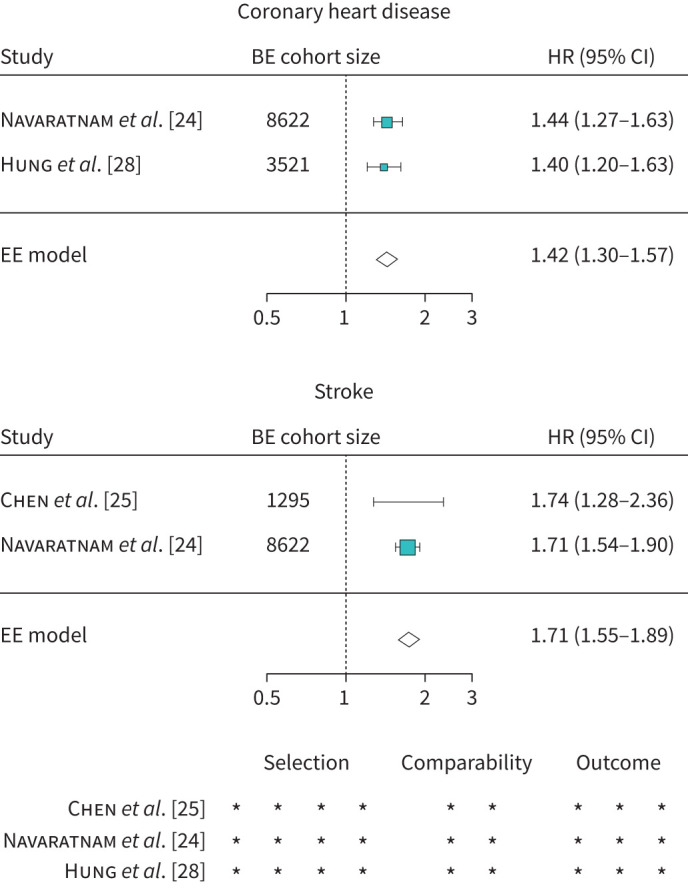
Pooled estimate of excess risk for coronary heart disease or stroke in patients with bronchiectasis (BE) and a quality assessment of the studies. The quality assessment was performed using the Newcastle-Ottawa Scale, with a maximum of four stars for group selection, two stars for group comparability and three stars for outcome ascertainment. HR: hazard ratio; CI: confidence interval.
Quality assessment of the included studies
The quality of the included studies was generally high. Patients included were selected from national electronic databases or specialised tertiary hospitals, thus ensuring they were representative of the bronchiectasis population. The main critical point was the absence of a comparison group in five of the nine studies included in this systematic review [21, 26, 27, 29, 30], which prevented us from including them in the quantitative assessment of the excess CV risk in this population. Among the remaining four studies, the first one published in 2014 by Navaratnam et al. [31] was excluded from the meta-analysis because data on a larger cohort selected from the same primary care database were published in 2017 [24]. After the exclusion of this study, the three studies were all considered in the quantitative evaluation and received the maximum score in every domain of the Newcastle-Ottawa Scale, indicating that the pooled estimates of excess risk for CV events in the bronchiectasis population were derived from high-quality studies.
Discussion
We present here the first systematic evaluation of CV events in individuals with bronchiectasis. Our study found high incidence of various CV events with pooled risk being 42% higher for CHD and 71% higher for stroke in bronchiectasis patients.
CV events have been previously reported to affect disease progression and clinical outcomes in several chronic respiratory diseases, with the majority of data derived from patients with COPD [33]. A landmark meta-analysis by Chen et al. [33] reported a 2–5-fold higher risk of ischaemic heart disease, arrhythmias and large artery disease in patients with COPD compared to controls. Pathophysiological mechanisms between COPD and CV disease might include shared risk factors (e.g. smoking), respiratory factors (lung hyperinflation and chronic hypoxemia) and systemic inflammation [34]. On the other hand, the coexistence of bronchiectasis and CV diseases has been addressed to date by a small number of studies with only fragmentary data on the prevalence and incidence of CV events. However, this association is biologically plausible and can be attributed to several underlying mechanisms. Many studies showed that local and systemic inflammation are increased in bronchiectasis patients [21]. Low-grade inflammation has been demonstrated to contribute to the development and progression of atherosclerosis, from endothelial dysfunction and formation of mature atherosclerotic plaques to plaque rupture and thrombosis [35]. Consistent with this, in a recent retrospective study Williams et al. [36] described high prevalence of coronary artery calcification in bronchiectasis patients, with more extensive coronary artery calcification in the group with severe bronchiectasis. Several studies showed a relationship between inflammation and vascular dysfunction in this population [35]. Inflammation plays an important role in stiffening of large arteries, endothelial dysfunction and vascular calcification, thus explaining the increased vascular risk in several inflammatory disorders. In 2012 Gale et al. [13] demonstrated increased pulse wave velocity as a validated technique for aortic stiffness in bronchiectasis patients. These findings were confirmed by other studies, which observed a significant correlation between carotid and femoral pulse wave velocity with disease severity and frequency of pulmonary exacerbations [11, 37, 38].
Pulmonary exacerbations may also play a role in this field and lead to a transient increase in the risk of CV events [39–42]. Again, most of data come from COPD research; in a recently published meta-analysis, for example, Müllerová et al. [43] found an increased risk of coronary syndrome or stroke within a relatively short period of time following a COPD exacerbation.
Extending the rationale to bronchiectasis patients, it is to be noted that most of the studies considered in this analysis involved patients during and after a bronchial exacerbation.
This study has both strengths and limitations. To our knowledge this is the first systematic evaluation of an increased risk of CV events in bronchiectasis patients both in terms of a qualitative review and quantification of the risk. The limited number of the studies included in the quantitative analysis is a major limitation for this study. It prevented us from stratifying participants based on clinical characteristics such as frequent exacerbations or disease severity and did not allow us to evaluate possible sources of heterogeneity across different studies conducted in various settings and countries.
Conclusions
The coexistence between bronchiectasis and the risk of CV events underlines the importance of CV screening in this population. In terms of clinical implications, interventions aimed at increasing awareness of this association should be implemented in order to develop preventive strategies. Future research may investigate the mechanisms underlying the excess CV disease in patients with bronchiectasis. Large cohort studies are needed to provide accurate and precise estimates of the occurrence of CV events in this population. Although CV events are influenced by low-grade chronic inflammation and oxidative stress, further translational studies are needed to better clarify the interaction between respiratory and CV health.
Acknowledgement
Results from this study were presented at the ERS International Congress 2023.
Provenance: Submitted article, peer reviewed.
Author contributions: A. Gramegna and F. Blasi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A. Gramegna, I. Barone, S. Aliberti and F. Blasi contributed to the study design. A. Gramegna, I. Barone, A. Arcadu, C. Colombo, M. Vicenzi and F. Blasi contributed to data collection. I. Barone, G. Alicandro and G. Sotgiu contributed to data analysis. All the authors contributed to interpretation. A. Gramegna, I. Barone and C. Colombo contributed to the writing of the manuscript.
Conflict of interest: All authors have nothing to disclose.
Support statement: This study was supported by the Italian Ministry of Education and Research (MUR) Dipartimenti di Eccellenza Program 2023–2027, and the Department of Pathophysiology and Transplantation, University of Milan. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Aliberti S, Goeminne PC, O'Donnell AE, et al. . Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med 2022; 10: 298–306. doi: 10.1016/S2213-2600(21)00277-0. [DOI] [PubMed] [Google Scholar]
- 2.Henkle E, Chan B, Curtis JR, et al. . Characteristics and health-care utilization history of patients with bronchiectasis in US Medicare enrollees with prescription drug plans, 2006 to 2014. Chest 2018; 154: 1311–1320. doi: 10.1016/j.chest.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 3.Quint JK, Millett ER, Joshi M, et al. . Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. doi: 10.1183/13993003.01033-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diel R, Ewig S, Blaas S, et al. . Incidence of patients with non-cystic fibrosis bronchiectasis in Germany - a healthcare insurance claims data analysis. Respir Med 2019; 151: 121–127. doi: 10.1016/j.rmed.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Aliberti S, Sotgiu G, Lapi F, et al. . Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med 2020; 20: 15. doi: 10.1186/s12890-020-1050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellelli G, Chalmers JD, Sotgiu G, et al. . Characterization of bronchiectasis in the elderly. Respir Med 2016; 119: 13–19. doi: 10.1016/j.rmed.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 7.McDonnell MJ, Aliberti S, Goeminne PC, et al. . Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016; 4: 969–979. doi: 10.1016/S2213-2600(16)30320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olveira C, Olveira G, Gaspar I, et al. . Depression and anxiety symptoms in bronchiectasis: associations with health-related quality of life. Qual Life Res 2013; 22: 597–605. doi: 10.1007/s11136-012-0188-5 [DOI] [PubMed] [Google Scholar]
- 9.Chung WS, Lin CL, Lin CL, et al. . Bronchiectasis and the risk of cancer: a nationwide retrospective cohort study. Int J Clin Pract 2015; 69: 682–688. doi: 10.1111/ijcp.12599 [DOI] [PubMed] [Google Scholar]
- 10.Hurst JR, Elborn JS, De Soyza Aet al. . COPD-bronchiectasis overlap syndrome. Eur Respir J 2015; 45: 310–313. doi: 10.1183/09031936.00170014 [DOI] [PubMed] [Google Scholar]
- 11.Saleh AD, Kwok B, Brown JS, et al. . Correlates and assessment of excess cardiovascular risk in bronchiectasis. Eur Respir J 2017; 50: 1701127. doi: 10.1183/13993003.01127-2017 [DOI] [PubMed] [Google Scholar]
- 12.Emerging Risk Factors Collaboration , Kaptoge S, Di Angelantonio E, et al. . C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012; 367: 1310–1320. doi: 10.1056/NEJMoa1107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gale NS, Bolton CE, Duckers JM, et al. . Systemic comorbidities in bronchiectasis. Chron Respir Dis 2012; 9: 231–238. doi: 10.1177/1479972312459973 [DOI] [PubMed] [Google Scholar]
- 14.Patel AR, Kowlessar BS, Donaldson GC, et al. . Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188: 1091–1099. doi: 10.1164/rccm.201306-1170OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SC, Son KJ, Hoon Han C, et al. . Cardiovascular and cerebrovascular-associated mortality in patients with preceding bronchiectasis exacerbation. Ther Adv Respir Dis 2022; 16: 17534666221144206. doi: 10.1177/17534666221144206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scioscia G, Alcaraz-Serrano V, Méndez R, et al. . Factors associated with one-year mortality in hospitalised patients with exacerbated bronchiectasis. Arch Bronconeumol 2022; 58: 773–775. doi: 10.1016/j.arbres.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Aliberti S, Amir A, Peyrani P, et al. . Incidence, etiology, timing, and risk factors for clinical failure in hospitalized patients with community-acquired pneumonia. Chest 2008; 134: 955–962. doi: 10.1378/chest.08-0334 [DOI] [PubMed] [Google Scholar]
- 18.Aliberti S, Ramirez J, Cosentini R, et al. . Acute myocardial infarction versus other cardiovascular events in community-acquired pneumonia. ERJ Open Res 2015; 1: 00020-2015. doi: 10.1183/23120541.00020-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes LF, Restrepo MI, Hinojosa CA, et al. . Severe pneumococcal pneumonia causes acute cardiac toxicity and subsequent cardiac remodeling. Am J Respir Crit Care Med 2017; 196: 609–620. doi: 10.1164/rccm.201701-0104OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aliberti S, Ramirez JA. Cardiac diseases complicating community-acquired pneumonia. Curr Opin Infect Dis 2014; 27: 295–301. doi: 10.1097/QCO.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 21.Huang JT, Kuzmanova E, Dicker AJ, et al. . Serum desmosine is associated with long-term all-cause and cardiovascular mortality in bronchiectasis. Am J Respir Crit Care Med 2020; 202: 897–899. doi: 10.1164/rccm.202002-0434LE [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 23.Borenstein M, Hedges LV, Higgins JP, et al. . A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1: 97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 24.Navaratnam V, Millett ER, Hurst JR, et al. . Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax 2017; 72: 161–166. doi: 10.1136/thoraxjnl-2015-208188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YF, Lin HH, Lin CS, et al. . Bronchiectasis and increased risk of ischemic stroke: a nationwide population-based cohort study. Int J Chron Obstruct Pulmon Dis 2017; 12: 1375–1383. doi: 10.2147/COPD.S126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans IE, Bedi P, Quinn TM, et al. . Bronchiectasis severity is an independent risk factor for vascular disease in a bronchiectasis cohort. Chest 2017; 151: 383–388. doi: 10.1016/j.chest.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 27.Menéndez R, Méndez R, Polverino E, et al. . Factors associated with hospitalization in bronchiectasis exacerbations: a one-year follow-up study. Respir Res 2017; 18: 176. doi: 10.1186/s12931-017-0659-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung CT, Li SF, Chung WS. Increased risk of acute coronary syndrome in patients with bronchiectasis: a population-based cohort study. Respirology 2018; 23: 828–834. doi: 10.1111/resp.13298 [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Qiu A, Tao Z, et al. . Clinical impact of cardiovascular disease on patients with bronchiectasis. BMC Pulm Med 2020; 20: 101. doi: 10.1186/s12890-020-1137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Méndez R, Feced L, Alcaraz-Serrano V, et al. . Cardiovascular events during and after bronchiectasis exacerbations and long-term mortality. Chest 2022; 161: 629–636. doi: 10.1016/j.chest.2021.10.013 [DOI] [PubMed] [Google Scholar]
- 31.Navaratnam V, Millett E, Hurst J, et al. . S17 Cardiovascular risk factors in people with bronchiectasis: a cross sectional study. Thorax 2014; 69: A11–A12. doi: 10.1136/thoraxjnl-2014-206260.23 [DOI] [Google Scholar]
- 32.Rabinovich RA, Miller BE, Wrobel K, et al. . Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. Eur Respir J 2016; 47: 1365–1373. doi: 10.1183/13993003.01824-2015 [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Thomas J, Sadatsafavi M, et al. . Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015; 3: 631–639. doi: 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 34.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons. Eur Respir Rev 2018; 27: 180057. doi: 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozos I, Malainer C, Horbańczuk J, et al. . Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol 2017; 8: 1058. doi: 10.3389/fimmu.2017.01058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams MC, van Beek EJR, Hill AT, et al. . Coronary artery calcification on thoracic computed tomography is an independent predictor of mortality in patients with bronchiectasis. J Thorac Imaging 2021; 36: 166–173. doi: 10.1097/RTI.0000000000000553 [DOI] [PubMed] [Google Scholar]
- 37.Gao YH, Cui JJ, Wang LY, et al. . Arterial stiffness in adults with steady-state bronchiectasis: association with clinical indices and disease severity. Respir Res 2018; 19: 86. doi: 10.1186/s12931-018-0790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao YH, Liu SX, Cui JJ, et al. . Subclinical atherosclerosis in adults with steady-state bronchiectasis: a case-control study. Respir Med 2018; 134: 110–116. doi: 10.1016/j.rmed.2017.11.024 [DOI] [PubMed] [Google Scholar]
- 39.Clayton TC, Capps NE, Stephens NG, et al. . Recent respiratory infection and the risk of myocardial infarction. Heart 2005; 91: 1601–1602. doi: 10.1136/hrt.2004.046920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier CR, Jick SS, Derby LE, et al. . Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet 1998; 351: 1467–1471. doi: 10.1016/s0140-6736(97)11084-4 [DOI] [PubMed] [Google Scholar]
- 41.Smeeth L, Thomas SL, Hall AJ, et al. . Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004; 351: 2611–2618. doi: 10.1056/NEJMoa041747 [DOI] [PubMed] [Google Scholar]
- 42.Donaldson GC, Hurst JR, Smith CJ, et al. . Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010; 137: 1091–1097. doi: 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 43.Müllerová H, Marshall J, de Nigris E, et al. . Association of COPD exacerbations and acute cardiovascular events: a systematic review and meta-analysis. Ther Adv Respir Dis 2022; 16: 17534666221113647. doi: 10.1177/17534666221113647 [DOI] [PMC free article] [PubMed] [Google Scholar]