Abstract
Background:
Spontaneous coronary artery dissection (SCAD) is a disease entity that often occurs in young, healthy women and can cause life-threatening ventricular arrhythmias and sudden cardiac arrest. However, the characteristics and outcomes of SCAD with cardiac arrest are not well characterized.
Methods:
This study investigated the baseline characteristics of SCAD patients with cardiac arrest using the National Inpatient Sample (NIS) database between 2016 and 2020. In addition, we also sought to determine the potential impact that implantable cardioverter defibrillator (ICD) therapy had on morbidity and mortality in SCAD patients presenting with cardiac arrest.
Results:
Our findings showed that the SCAD with cardiac arrest population had significantly higher comorbidities, including cardiac arrhythmias, congestive heart failure, pulmonary circulation disorders, liver diseases, solid tumors, coagulopathy, fluid disorders, chronic kidney disease (CKD), anemia secondary to deficiency, psychosis, neurological disorders, carotid artery disease, atrial fibrillation, ventricular arrhythmias (ventricular tachycardia (VT), ventricular fibrillation (VF)), and acute myocardial infarction (AMI), compared to the SCAD without cardiac arrest population. Likewise, for SCAD patients who did not have an ICD in place, we found increasing age, fluid and electrolyte disorders, uncomplicated diabetes, neurological disorders, peripheral vascular disease, pulmonary circulatory disorders, cardiac arrhythmias, and congestive heart failure to be associated with greater mortality.
Conclusions:
SCAD patients with certain comorbidities (e.g., pulmonary diseases, liver diseases, cancers, coagulopathy, and CKD) who presented with AMI or congestive heart failure should be monitored closely for ventricular arrhythmias as they have a higher chance of progressing to cardiac arrest. ICD therapy can be considered for these patients, but data on the success of this treatment option are limited, and more research needs to be performed to determine whether the benefits of this outweigh the risks.
Keywords: spontaneous coronary artery dissection, SCAD, cardiac arrest: ICD, ventricular arrhythmia
1. Introduction
Spontaneous coronary artery dissection (SCAD) is a heterogeneous condition that normally occurs in young, otherwise healthy women. Moreover, SCAD has received more attention recently as this disease process can cause life-threatening ventricular arrhythmias and cardiac arrest. A recent review article by Kaddoura and colleagues provided a comprehensive overview of this disease process. The authors discussed how the pathogenesis of SCAD is most likely secondary to an intimal tear of the epicardial coronary arteries, leading to intramural hematoma formation and subsequent occlusion of the epicardial lumen [1, 2, 3]. Patients with SCAD typically present with symptoms of acute coronary syndrome [4]. Diagnosis is usually made via coronary angiogram, along with intravascular ultrasound and optical coherence tomography being used as adjunct modalities [5]. Based on international guidelines, treatment generally favors early revascularization over medical therapy in unstable patients [6]. However, the characteristics and outcomes of SCAD with cardiac arrest are not yet well characterized. Likewise, the benefit of an implantable cardioverter defibrillator (ICD) in SCAD patients with cardiac arrest is unknown. This study investigated baseline characteristics in SCAD patients with cardiac arrest using the National Inpatient Sample (NIS) database between 2016 and 2020. We also evaluated the outcomes and their predictors in SCAD–cardiac arrest with or without an ICD.
2. Methods
2.1 Data Source
We queried the NIS database from 2016 to 2020. The NIS is the largest publicly available all-payer inpatient healthcare database that could produce U.S. regional and national estimates of inpatient utilization, access, cost, quality, and outcomes at both regional and national levels in the U.S. Its unweighted form includes information from approximately 7 million annual hospital stays. When weighted, it projects an estimation of about 35 million hospitalizations across the nation each year. The NIS encompasses data from states involved in the Healthcare Cost and Utilization Project (HCUP), representing over 97% of the United States population. It effectively approximates a 20% stratified sample of patient discharges from U.S. hospitals, excluding facilities specializing in rehabilitation and long-term acute care. In addition, given that the data contained within NIS are deidentified, our study did not require approval from the Institutional Review Board.
2.2 Study Population
From 2016 to 2020, the NIS contained up to 40 diagnoses and 25 procedures for each admission. Patients with a primary or secondary SCAD diagnosis were identified using the ICD-10-CM code I25.42. To ensure an appropriate diagnosis of SCAD, we selected patients who presented with acute myocardial infarction (AMI), defined as either non-ST-segment elevation or ST-segment elevation. Following that, we selected only patients with a procedural diagnosis of coronary angiography or percutaneous coronary intervention (PCI) and excluded any patients with concurrent diagnoses of accidental puncture or laceration to decrease the risk of coding errors. All adult hospitalizations (18 years of age) were included in our study. Hospitalization with missing data on age, gender, race, mortality, type of admission (elective vs. non-elective), median household income, and primary payment coverage were excluded from our study. Our methodology aligned with previously published literature and standards recommended by the Agency of Healthcare Research and Quality [7].
2.3 Study Covariates and Outcomes
To ensure a robust analysis and to minimize unrecognized confounders, we included a large number of covariates. Data on demographics (age, gender, insurance status, hospital bed size, hospital teaching status, elective admission, and race) were readily available within the database. Additional comorbidities included obesity, hypertension, ventricular arrhythmias, valvular heart diseases, pulmonary circulatory disorders, chronic lung diseases, liver diseases, diabetes mellitus, peripheral vascular diseases, lymphoma, metastatic cancer, solid tumors, rheumatological disorders, coagulopathy, fluid disorders, chronic kidney disease, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, acquired immunodeficiency syndrome (AIDS), prior myocardial infarction (MI), prior PCI, and prior coronary artery bypass graft (CABG), which were extracted according to their respective ICD-10-CM codes. Ventricular arrhythmias are defined as a composite of ventricular tachycardia or ventricular fibrillation. The category ‘other neurological disorders’ includes a range of neurological conditions, including ataxia, spastic paraplegia, spinocerebellar disease, chorea, multiple sclerosis, demyelinating diseases, epilepsy, seizures, convulsions, aphasia, Parkinson’s disease, neuroleptic malignant syndrome, and various other degenerative brain diseases not classified elsewhere.
Our study objective was to evaluate the demographics, clinical characteristics, and outcomes of SCAD patients stratified by the sudden cardiac arrest and ICD, respectively. Secondary outcomes included exploring the predictors of cardiac arrest and the ICD among patients with SCAD, as well as evaluating the predictors of mortality among patients who did not receive an ICD. We additionally assessed the temporal trend of incidence of sudden cardiac arrest among SCAD patients and in-hospital mortality among sudden cardiac arrest patients.
2.4 Statistical Analysis
The national weighted estimates were obtained using the discharge weight supplied by HCUP. Categorical variables were presented as counts and percentages and analyzed using the chi-square test. Alternatively, continuous variables were described using weighted means and standard deviations for those following a normal distribution or medians and interquartile ranges for those not normally distributed. Trends of sudden cardiac arrest and in-hospital mortality were summarized and analyzed using ogistic regression. We first obtained the absolute frequencies of each desired outcome, comparing those with and without cardiac arrest and the ICD, respectively. We rigorously adhered to the data use agreement for nationwide databases from the HCUP and avoided reporting any variable with a frequency of 10 and below, which was excluded given the risk of identifying individual patients [8]. We conducted a multivariate regression analysis using the multilevel mixed effect models to evaluate for predictors of cardiac arrest, ICD placement among SCAD patients, and predictors of mortality among patients who did not receive an ICD. Variables included in the model were any significant variables on univariate analysis using a liberal threshold of 0.2. All statistical analyses were conducted using Stata version 17.0 (StataCorp, College Station, TX, USA) and R software version 4.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
We analyzed 24,620 patients with spontaneous coronary artery dissection, 1125 of which suffered cardiac arrest and 23,495 who did not suffer cardiac arrest. Of the patients with SCAD who also suffered a cardiac arrest, the mean age was 62.4 years, with 57% of the patients being female and most patients being Caucasian. In comparison, of the patients with SCAD who did not have a cardiac arrest, the mean age was 59.8 years, with 59.6% of the patients being female and most patients being Caucasian. The SCAD with cardiac arrest population had significantly higher comorbidities, including ventricular arrhythmias, congestive heart failure, pulmonary circulatory disorders, liver diseases, solid tumors, coagulopathy, obesity, fluid disorders, chronic kidney disease (CKD), psychosis, and neurologic disorders, compared to the SCAD without cardiac arrest population (Table 1).
Table 1.
Baseline characteristics of SCAD patients with and without cardiac arrest.
| Variables | No cardiac arrest population | Cardiac arrest population | p-value | |||
| Number of patients | 23,495 | 1125 | ||||
| Age | 59.83 14.36 | 62.35 15.29 | 0.02 | |||
| n | % | n | % | |||
| Female | 14,005 | 59.6 | 645 | 57.3 | 0.50 | |
| Race | 0.34 | |||||
| White | 17,435 | 74.2 | 830 | 73.8 | ||
| Black | 2700 | 11.5 | 135 | 12.0 | ||
| Hispanic | 2090 | 8.9 | 85 | 7.6 | ||
| Asian or Pacific Islander | 520 | 2.2 | 15 | 1.3 | ||
| Native American | 110 | 0.5 | 15 | 1.3 | ||
| Other | 640 | 2.7 | 45 | 4.0 | ||
| Hospital bed size | 0.55 | |||||
| Small | 3295 | 14.0 | 185 | 16.4 | ||
| Medium | 6615 | 28.2 | 295 | 26.2 | ||
| Large | 13,585 | 57.8 | 645 | 57.3 | ||
| Hospital teaching status | 0.25 | |||||
| Rural | 1100 | 4.7 | 45 | 4.0 | ||
| Urban non-teaching | 4130 | 17.6 | 245 | 21.8 | ||
| Urban teaching | 18,265 | 77.7 | 835 | 74.2 | ||
| Admission | ||||||
| Elective | 1885 | 8.0 | 90 | 8.0 | 0.99 | |
| Primary payment coverage | 0.38 | |||||
| Medicare | 9130 | 38.9 | 515 | 45.8 | ||
| Medicaid | 2530 | 10.8 | 115 | 10.2 | ||
| Private insurance | 9870 | 42.0 | 420 | 37.3 | ||
| Self-pay | 1170 | 5.0 | 50 | 4.4 | ||
| No charge | - | - | - | - | - | |
| Other | 685 | 2.9 | 25 | 2.2 | ||
| Median household income | 0.50 | |||||
| 1–28,999 | 5990 | 25.5 | 315 | 28.0 | ||
| 29,000–35,999 | 5890 | 25.1 | 235 | 20.9 | ||
| 36,000–46,999 | 6495 | 27.6 | 310 | 27.6 | ||
| 47,000+ | 5120 | 21.8 | 265 | 23.6 | ||
| Hospital region | 0.06 | |||||
| Northeast | 4250 | 18.1 | 155 | 13.8 | ||
| Midwest | 5485 | 23.3 | 340 | 30.2 | ||
| South | 8755 | 37.3 | 420 | 37.3 | ||
| West | 5005 | 21.3 | 210 | 18.7 | ||
| Comorbidities | ||||||
| Congestive heart failure | 7245 | 30.8 | 575 | 51.1 | 0.001 | |
| Ventricular arrhythmias | 2955 | 12.6 | 760 | 67.6 | 0.001 | |
| Valvular heart diseases | 2505 | 10.7 | 135 | 12.0 | 0.52 | |
| Pulmonary circulatory disorders | 780 | 3.3 | 85 | 7.6 | 0.001 | |
| Peripheral vascular disease | 2585 | 11.0 | 140 | 12.4 | 0.50 | |
| Hypertension | 16,450 | 70.0 | 4303 | 67.6 | 0.43 | |
| Other neurologic disorders | 1385 | 5.9 | 280 | 24.9 | 0.001 | |
| Chronic lung disease | 4130 | 17.6 | 240 | 21.3 | 0.15 | |
| Diabetes mellitus | 5610 | 23.9 | 325 | 28.9 | 0.08 | |
| Hypothyroidism | 2705 | 11.5 | 90 | 8.0 | 0.11 | |
| CKD | 2845 | 12.1 | 215 | 19.1 | 0.00 | |
| Liver disease | 1025 | 4.4 | 120 | 10.7 | 0.001 | |
| Solid tumor | 335 | 1.4 | 40 | 3.6 | 0.01 | |
| Rheumatologic disorders | 550 | 2.3 | 45 | 4.0 | 0.11 | |
| Coagulopathy | 1635 | 7.0 | 200 | 17.8 | 0.001 | |
| Obesity | 5135 | 21.9 | 175 | 15.6 | 0.03 | |
| Weight loss | 575 | 2.4 | 30 | 2.7 | 0.84 | |
| Fluid and electrolyte disorders | 5225 | 22.2 | 530 | 47.1 | 0.001 | |
| Anemia | 875 | 3.7 | 40 | 3.6 | 0.90 | |
| Alcohol abuse | 595 | 2.5 | 45 | 4.0 | 0.18 | |
| Drug abuse | 955 | 4.1 | 45 | 4.0 | 0.96 | |
| Depression | 2510 | 10.7 | 120 | 10.7 | 0.99 | |
| Prior MI | 2855 | 12.2 | 120 | 10.7 | 0.50 | |
| Prior CABG | 980 | 4.2 | 40 | 3.6 | 0.65 | |
| Mortality | 310 | 1.3 | 1030 | 91.6 | 0.001 | |
| Cost of hospitalization | 31,436 35,012 | 52,922 47,636 | 0.001 | |||
| LOS | 4.68 6.05 | 8.36 10.57 | 0.001 | |||
CABG, coronary artery bypass graft; CKD, chronic kidney disease; LOS, length of stay; MI, myocardial infarction; SCAD, spontaneous coronary artery dissection.
Any variable with 10 patients was not reported per Agency for Healthcare Research and Quality (AHRQ) guidelines and appears as (-) in the table.
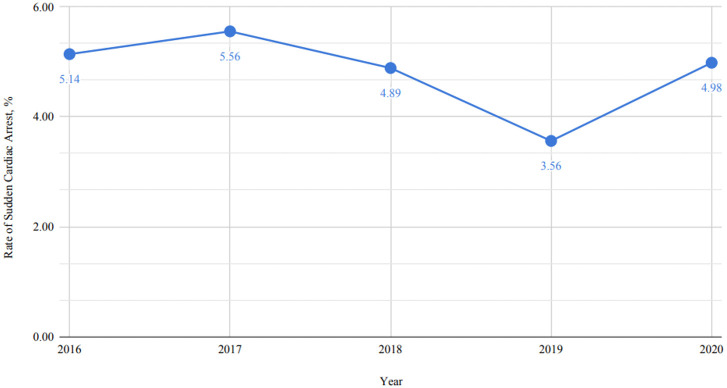
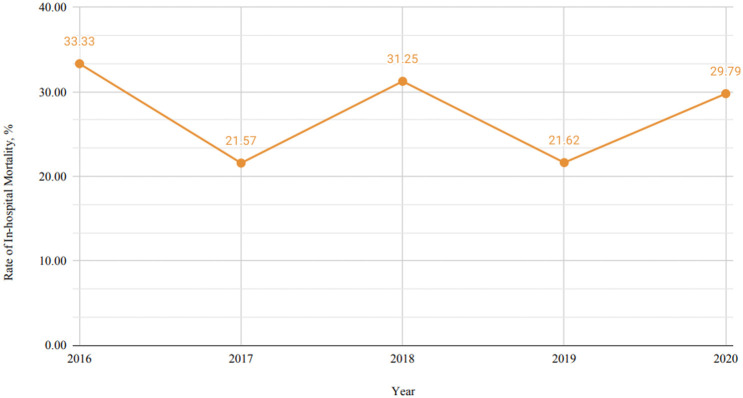
In addition, we constructed a model to assess which comorbidities were associated with a greater risk of cardiac arrest (Table 2), which according to our model were ventricular arrhythmias (OR 12.11 with 95% CI (8.78–16.70) p-value 0.0001), fluid and electrolyte disorders (OR 1.42 with 95% CI (1.01–2.01) p-value 0.045), and other neurological disorders (OR 2.55 with 95% CI (1.69–3.85) p-value 0.0001). Fig. 1 shows the temporal trend of sudden cardiac arrest in SCAD patients from 2016 to 2020. The rate of sudden cardiac arrest peaked in 2017 at 5.56%, then trended downwards over the next two years, with a low of 3.56% in 2019 before rising to 4.98% in 2020. Fig. 2 shows the temporal trend of in-hospital mortality among patients with SCAD who experienced sudden cardiac arrest from 2016 to 2019. There was high variability, as the in-hospital mortality in 2016 was 33.33% in 2016, 21.57% in 2017, 31.25% in 2018, 21.62% in 2019, and 29.79% in 2020. The trends of sudden cardiac arrest incidence in SCAD patients and in-hospital mortality among sudden cardiac arrest patients were analyzed; however, neither showed statistically significant changes over time (p-trend = 0.317 and 0.838, respectively).
Table 2.
Multivariate logistic regression for cardiac arrest prediction.
| Variables | Odds ratio | 95% CI | p-value | ||
| Lower limit | Upper limit | ||||
| Age | 1.01 | 0.99 | 1.02 | 0.36 | |
| Hospital region | |||||
| Northeast | Ref | ||||
| Midwest | 1.58 | 0.98 | 2.56 | 0.06 | |
| South | 1.24 | 0.77 | 2.00 | 0.38 | |
| West | 1.00 | 0.60 | 1.66 | 0.99 | |
| Congestive heart failure | 1.17 | 0.84 | 1.63 | 0.35 | |
| Ventricular arrhythmias | 12.11 | 8.78 | 16.70 | 0.001 | |
| Pulmonary circulatory disorders | 1.53 | 0.84 | 2.77 | 0.17 | |
| Other neurological disorders | 2.55 | 1.69 | 3.85 | 0.001 | |
| Chronic lung disease | 1.07 | 0.74 | 1.55 | 0.73 | |
| Diabetes mellitus | 1.41 | 0.98 | 2.02 | 0.06 | |
| Hypothyroidism | 0.75 | 0.45 | 1.25 | 0.27 | |
| CKD | 1.09 | 0.71 | 1.68 | 0.69 | |
| Liver disease | 0.79 | 0.43 | 1.43 | 0.43 | |
| Solid tumor | 1.99 | 0.85 | 4.67 | 0.11 | |
| Rheumatological disorders | 2.60 | 1.19 | 5.70 | 0.02 | |
| Coagulopathy | 1.43 | 0.90 | 2.27 | 0.13 | |
| Obesity | 0.66 | 0.44 | 1.01 | 0.06 | |
| Fluid and electrolyte disorders | 1.42 | 1.01 | 2.01 | 0.045 | |
| Alcohol abuse | 1.37 | 0.68 | 2.77 | 0.38 | |
CI, confidence interval; CKD, chronic kidney disease.
The model was constructed based on univariate regression, with a threshold of 0.2.
The bolded p-value in Table 2 indicates that cardiac arrest higher in those with ventriuclar arrtyhmias like ventriuclar tachycardia or ventricular fibrillation, neurological disorders (which included disorders like stroke, multiple sclerosis), rheumatological disorders (like lupus), and fluid/electrolyte disorders (like hypokalemia, hyponatremia, etc).
Fig. 1.
Temporal trends of cardiac arrest among patients with spontaneous coronary artery dissection (SCAD).
Fig. 2.
Temporal trend of in-hospital mortality among spontaneous coronary artery dissection (SCAD) patients with cardiac arrest.
Among 24,620 SCAD patients, 0.6% underwent ICD placement, and 99.4% did not. The mean age was 61.8 years among SCAD patients who received an ICD, while the mean age was 59.9 years among SCAD patients who did not receive an ICD. Compared with SCAD patients without an ICD, those with an ICD were associated with congestive heart failure and cardiac arrhythmias (p 0.05). In addition, compared with SCAD patients without an ICD, those with an ICD had a significantly higher prevalence of pulmonary circulatory disorders, neurologic disorders, liver disease, fluid and electrolyte disorders, and alcohol abuse (p 0.05) (Table 3).
Table 3.
Baseline characteristics of SCAD patients with and without ICD placement.
| Variables | No ICD | ICD | p-value | |||
| Number of patients | 24,480 | 140 | ||||
| Age | 59.93 14.40 | 61.82 15.00 | 0.506 | |||
| n | % | n | % | |||
| Female | 14,580 | 59.56 | 70 | 50.00 | 0.3041 | |
| Race | ||||||
| White | 18,150 | 74.14 | 115 | 82.14 | 0.001 | |
| Hospital bed size | 0.6797 | |||||
| Small | 3455 | 14.11 | 25 | 17.86 | ||
| Medium | 6880 | 28.10 | 30 | 21.43 | ||
| Large | 14,145 | 57.78 | 85 | 60.71 | ||
| Hospital teaching status | 0.3033 | |||||
| Rural | - | - | - | - | ||
| Urban non-teaching | - | - | - | - | ||
| Urban teaching | 18,975 | 77.51 | 125 | 89.29 | ||
| Primary payment coverage | 0.8493 | |||||
| Medicare | 9600 | 39.22 | 45 | 32.14 | ||
| Medicaid | 2625 | 10.72 | 20 | 14.29 | ||
| Private insurance | 10,225 | 41.77 | 65 | 46.43 | ||
| Self-pay | - | - | - | - | ||
| No charge | - | - | - | - | ||
| Other | - | - | - | - | ||
| Median household income | 0.7919 | |||||
| 1–28,999 | 6260 | 25.57 | 45 | 32.14 | ||
| 29,000–35,999 | 6100 | 24.92 | 25 | 17.86 | ||
| 36,000–46,999 | 6765 | 27.63 | 40 | 28.57 | ||
| 47,000+ | 5355 | 21.88 | 30 | 21.43 | ||
| Hospital region | 0.0148 | |||||
| Northeast | 4350 | 17.77 | 55 | 39.29 | ||
| Midwest | 5790 | 23.65 | 35 | 25.00 | ||
| South | 9135 | 37.32 | 40 | 28.57 | ||
| West | - | - | - | - | ||
| Congestive heart failure | 7710 | 31.50 | 110 | 78.57 | 0.001 | |
| Ventricular arrhythmias | 3590 | 14.67 | 125 | 89.29 | 0.001 | |
| Valvular heart diseases | 2615 | 10.68 | 25 | 17.86 | 0.2216 | |
| Pulmonary circulatory disorders | 845 | 3.45 | 20 | 14.29 | 0.002 | |
| Peripheral vascular disease | 2705 | 11.05 | 20 | 14.29 | 0.5865 | |
| Hypertension | 17,125 | 69.96 | 85 | 60.71 | 0.2892 | |
| Diabetes mellitus | 5920 | 24.18 | 15 | 10.71 | 0.0966 | |
| Other neurologic disorders | 1640 | 6.70 | 25 | 17.86 | 0.0191 | |
| Chronic lung disease | 4355 | 17.79 | 15 | 10.71 | 0.3287 | |
| Hypothyroidism | 2770 | 11.32 | 25 | 17.86 | 0.276 | |
| CKD | 3045 | 12.44 | 15 | 10.71 | 0.7824 | |
| Liver disease | 1110 | 4.53 | 35 | 25.00 | 0.001 | |
| Obesity | 5280 | 21.57 | 30 | 21.43 | 0.9857 | |
| Weight loss | 585 | 2.39 | 20 | 14.29 | 0.0001 | |
| Fluid and electrolyte disorders | 5680 | 23.20 | 75 | 53.57 | 0.0001 | |
| Alcohol abuse | 625 | 2.55 | 15 | 10.71 | 0.0069 | |
| Prior MI | 2955 | 12.07 | 20 | 14.29 | 0.7197 | |
| Cost of hospitalization | 32,015 35,369 | 102,137 61,756 | 0.001 | |||
| LOS | 4.80 6.30 | 14.71 9.95 | 0.001 | |||
| Mortality | - | - | - | - | ||
CKD, chronic kidney disease; LOS, length of stay; MI, myocardial infarction; SCAD, spontaneous coronary artery dissection; ICD, implantable cardioverter defibrillator.
*Any variable with 10 patients was not reported per Agency for Healthcare Research and Quality (AHRQ) guidelines and appears as (-) in the table.
+Data on other races are not reported as this contained a cell count of 10.
Similarly, we conducted a model to predict which comorbidities were associated with a greater risk of ICD placement (Table 4), which according to our model were congestive heart failure (OR 3.99 with 95% CI (1.46–10.87) p-value 0.01), ventricular arrhythmias (OR 33.94 with 95% CI (9.20–125.18) p-value 0.001), pulmonary circulatory disorders (OR 4.40 with 95% CI (1.34–14.44) p-value 0.02), and alcohol abuse (OR 4.41 with 95% CI (1.22–15.94) p-value 0.02).
Table 4.
Multivariate logistic regression for ICD prediction.
| Variables | Odds ratio | 95% CI | p-value | |
| Lower limit | Upper limit | |||
| Congestive heart failure | 3.99 | 1.46 | 10.87 | 0.01 |
| Ventricular arrhythmias | 33.94 | 9.20 | 125.18 | 0.001 |
| Pulmonary circulatory disorders | 4.40 | 1.34 | 14.44 | 0.02 |
| Diabetes mellitus | 0.29 | 0.08 | 1.02 | 0.05 |
| Other neurological disorders | 0.65 | 0.22 | 1.94 | 0.44 |
| Liver disease | 2.39 | 0.78 | 7.39 | 0.13 |
| Weight loss | 3.13 | 0.87 | 11.33 | 0.08 |
| Fluid and electrolyte disorders | 1.21 | 0.49 | 2.97 | 0.68 |
| Alcohol abuse | 4.41 | 1.22 | 15.94 | 0.02 |
CI, confidence interval; ICD, implantable cardioverter defibrillator.
The model was constructed based on univariate regression, with a threshold of 0.2.
The bolded p-value in Table 4 indicates that Ventricular arrhythmias like ventricular tachycardia or ventricular fibrillation, pulmonary circulation disorders like pulmonary embolism, and abusing alcohol (more than 3 drinks a day for men and 2 drinks per women) resulted in a greater likelihood of having an ICD placed.
We further evaluated the predictors of mortality among SCAD patients who did not receive an ICD. Based on our model, increasing age (OR 1.06 with 95% CI (1.04–1.07) p-value 0.001), fluid and electrolyte disorders (OR 2.34 with 95% CI (1.73–3.16) p-value 0.001), diabetes (OR 1.64 with 95% CI (1.21–2.23) p-value 0.001), neurological disorders (OR 2.42 with 95% CI (1.63–3.59) p-value 0.001), peripheral vascular disease (OR 1.48 with 95% CI (1.04–2.10) p-value 0.03), pulmonary circulatory disorders (OR 2.01 with 95% CI (1.20–3.39) p-value 0.01), ventricular arrhythmias (OR 3.25 with 95% CI (2.36–4.48) p-value 0.001), and congestive heart failure (OR 1.57 with 95% CI (1.18–2.10) p-value 0.001) were associated with increased risk of mortality (Table 5). Notably, given the small sample size (n = 140), we did not evaluate the predictors of mortality among SCAD patients who received an ICD.
Table 5.
Multivariate logistic regression for mortality prediction in patients without an ICD.
| Variables | Odds ratio | 95% CI | p-value | ||
| Lower limit | Upper limit | ||||
| Age | 1.06 | 1.04 | 1.07 | 0.001 | |
| Female | 1.24 | 0.92 | 1.66 | 0.16 | |
| Hospital bed size | |||||
| Small | Ref | ||||
| Medium | 0.67 | 0.42 | 1.09 | 0.11 | |
| Large | 0.90 | 0.59 | 1.37 | 0.63 | |
| Hospital region | |||||
| Northeast | Ref | ||||
| Midwest | 1.02 | 0.63 | 1.64 | 0.95 | |
| South | 1.27 | 0.83 | 1.95 | 0.27 | |
| West | 1.39 | 0.87 | 2.21 | 0.17 | |
| Elective | 1.09 | 0.69 | 1.72 | 0.71 | |
| Household median income | |||||
| 1–28,999 | Ref | ||||
| 29,000–35,999 | 0.68 | 0.47 | 0.98 | 0.04 | |
| 36,000–46,999 | 0.61 | 0.42 | 0.89 | 0.01 | |
| 47,000+ | 0.50 | 0.33 | 0.75 | 0.001 | |
| Diabetes | 1.64 | 1.21 | 2.23 | 0.001 | |
| Hypertension | 0.79 | 0.56 | 1.13 | 0.21 | |
| Ventricular arrhythmia | 3.25 | 2.36 | 4.48 | 0.001 | |
| Prior CABG | 1.15 | 0.67 | 1.96 | 0.62 | |
| Depression | 0.70 | 0.40 | 1.20 | 0.19 | |
| Fluid and electrolyte disorders | 2.34 | 1.73 | 3.16 | 0.001 | |
| Weight loss | 1.33 | 0.71 | 2.51 | 0.38 | |
| Obesity | 0.66 | 0.44 | 0.99 | 0.04 | |
| Coagulopathy | 1.61 | 1.08 | 2.40 | 0.02 | |
| Rheumatological disorders | 0.33 | 0.08 | 1.38 | 0.13 | |
| Liver disease | 3.28 | 2.08 | 5.15 | 0.001 | |
| CKD | 1.26 | 0.90 | 1.77 | 0.18 | |
| Chronic lung disease | 0.79 | 0.56 | 1.11 | 0.17 | |
| Other neurological disorders | 2.42 | 1.63 | 3.59 | 0.001 | |
| Paralysis | 0.58 | 0.17 | 1.97 | 0.38 | |
| Peripheral vascular diseases | 1.48 | 1.04 | 2.10 | 0.03 | |
| Pulmonary circulatory disorders | 2.01 | 1.20 | 3.39 | 0.01 | |
| Valvular heart diseases | 0.66 | 0.43 | 1.02 | 0.06 | |
| Congestive heart failure | 1.57 | 1.18 | 2.10 | 0.001 | |
CABG, coronary artery bypass graft; CI, confidence interval; CKD, chronic kidney disease; ICD, implantable cardioverter defibrillator.
The model was constructed based on univariate regression, with a threshold of 0.2.
+Data on other races are not reported as this contained a cell count of 10.
4. Discussion
In our study, we found that SCAD patients who suffered a cardiac arrest had higher comorbidities, such as congestive heart failure, pulmonary diseases, liver diseases, cancers, coagulopathy, and CKD, compared to SCAD patients who did not suffer a cardiac arrest. SCAD patients who underwent cardiac arrest were associated with AMI and ventricular arrhythmias (ventricular tachycardia (VT), ventricular fibrillation (VF)). We also found that the trend of cardiac arrest in SCAD patients has continually been trending downward, whereas the in-hospital mortality of these patients has remained quite variable. The decline in trends of cardiac arrest in SCAD patients is perhaps due to increased recognition of SCAD and improvement in medical therapy for SCAD.
One prior study also tried to answer the question of which characteristics are inherently present in SCAD patients with cardiac arrest. Phan and colleagues performed a retrospective cohort analysis of 208 SCAD patients from 2006 to 2016. Of those who suffered cardiac arrest, the investigators concluded that this subset was more likely to have coronary lesions involving the left main or left anterior descending artery (LAD) territory. This could explain the results of our findings, as patients who have ischemic disease are more likely to develop cardiac arrhythmia and heart failure [9, 10, 11]. In other words, in SCAD patients who presented with cardiac arrest, it seems they carried an increased risk of developing cardiac arrest secondary to underlying coronary artery disease. Interestingly, they also found that secondary prevention with an ICD did not significantly benefit this population. This brings into question whether ICD therapy is beneficial in SCAD patients with cardiac arrest. Sharma and colleagues also evaluated 102 patients presenting to Massachusetts General Hospital with SCAD. Comparing those presenting with cardiac arrest to those without cardiac arrest, they found that the cardiac arrest subset was more likely to smoke and present with ST elevation myocardial infarction (STEMI). Although these data are from a very small sample size [12], the findings echo our current findings. Both of these studies seem to stress that SCAD patients presenting with cardiac arrest occurred in the presence of underlying heart disease and comorbidities such as smoking.
Although SCAD patients are likely to be healthy and young, SCAD patients with cardiac arrest tend to have risk factors that would predispose them to cardiac arrest. These factors include coronary artery disease, congestive heart failure, and atrial or ventricular arrhythmia. In our prior study from a single healthcare system, we found that ventricular arrhythmia and atrial fibrillation were independently associated with in-hospital mortality in patients with SCAD [13]. The trend in SCAD patients experiencing cardiac arrest decreased from 2017 to 2019, which could be explained by better detection of SCAD in general. The in-hospital mortality variability for SCAD with sudden cardiac arrest (SCA) is probably a combination of factors, including the expertise of each hospital and physicians in treating SCAD patients.
Our study has certain limitations. Notable limitations include the small number of SCAD patients who received ICD. This potentially explained the relatively wide confidence intervals of certain ICD placement predictors, suggesting that these predictors may be imprecise; thus, the results are inconclusive. Other limitations include the lack of essential laboratory and medication data.
5. Conclusions
SCAD patients with certain comorbidities (e.g., pulmonary diseases, liver diseases, cancers, coagulopathy, and CKD) who presented with AMI or congestive heart failure should be monitored closely for ventricular arrhythmias as they have a higher chance of progressing to cardiac arrest. More research needs to be conducted to effectively determine how SCAD patients with cardiac arrest should be treated and managed going forward. ICD therapy can be considered for these patients, but data on the success of this treatment option are limited, and more research needs to be performed to determine whether the benefits of this outweigh the risks.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
Not applicable.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
CK designed the research study. CK, YKQ, ZW, SPA, MA, SS, HJ performed the research. SPA analyzed the data. ZW, MA performed validation of analyses. CK, YKQ, ZW, SPA, MA, SS, HJ wrote, reviewed and edited the manuscript. CK, SS, MA, HJ supervision. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Given that the data contained within NIS are deidentified, our study did not require approval from the Institutional Review Board. The participants provided written informed consents before enrollment.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. Hani Jneid is serving as one of the Editorial Board members of this journal. We declare that Hani Jneid had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Giuseppe Boriani.
References
- [1].Kaddoura R, Cader FA, Ahmed A, Alasnag M. Spontaneous coronary artery dissection: an overview. Postgraduate Medical Journal . 2023;99:1226–1236. doi: 10.1093/postmj/qgad086. [DOI] [PubMed] [Google Scholar]
- [2].Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation . 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, et al. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. Journal of the American College of Cardiology . 2020;76:961–984. doi: 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- [4].Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circulation. Cardiovascular Interventions . 2014;7:645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- [5].Buccheri D, Zambelli G, Alfonso F, Cortese B. Pulse on Spontaneous Coronary Artery Dissections: Experience-Based Survey. JACC. Cardiovascular Interventions . 2017;10:1469–1471. doi: 10.1016/j.jcin.2017.05.039. [DOI] [PubMed] [Google Scholar]
- [6].Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation . 2022;145:e4–e17. doi: 10.1161/CIR.0000000000001039. [DOI] [PubMed] [Google Scholar]
- [7].Mahmoud AN, Taduru SS, Mentias A, Mahtta D, Barakat AF, Saad M, et al. Trends of Incidence, Clinical Presentation, and In-Hospital Mortality Among Women With Acute Myocardial Infarction With or Without Spontaneous Coronary Artery Dissection: A Population-Based Analysis. JACC. Cardiovascular Interventions . 2018;11:80–90. doi: 10.1016/j.jcin.2017.08.016. [DOI] [PubMed] [Google Scholar]
- [8].Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS) 2023. [(Accessed: 12 December 2023)]. Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp .
- [9].Phan D, Clare R, Duan L, Kim C, Moore N, Lee MS. Characteristics and outcomes of patients with spontaneous coronary artery dissection who suffered sudden cardiac arrest. Journal of Interventional Cardiac Electrophysiology . 2021;60:77–83. doi: 10.1007/s10840-019-00695-9. [DOI] [PubMed] [Google Scholar]
- [10].Berg DD, Wiviott SD, Braunwald E, Guo J, Im K, Kashani A, et al. Modes and timing of death in 66 252 patients with non-ST-segment elevation acute coronary syndromes enrolled in 14 TIMI trials. European Heart Journal . 2018;39:3810–3820. doi: 10.1093/eurheartj/ehy556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chaudhry MA. Heart Failure. Current Hypertension Reviews . 2019;15:7. doi: 10.2174/157340211501190129144451. [DOI] [PubMed] [Google Scholar]
- [12].Sharma S, Rozen G, Duran J, Mela T, Wood MJ. Sudden Cardiac Death in Patients With Spontaneous Coronary Artery Dissection. Journal of the American College of Cardiology . 2017;70:114–115. doi: 10.1016/j.jacc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- [13].Krittanawong C, Kumar A, Wang Z, Johnson KW, Baber U, Palazzo A, et al. Clinical features and prognosis of patients with spontaneous coronary artery dissection. International Journal of Cardiology . 2020;312:33–36. doi: 10.1016/j.ijcard.2020.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.