Abstract
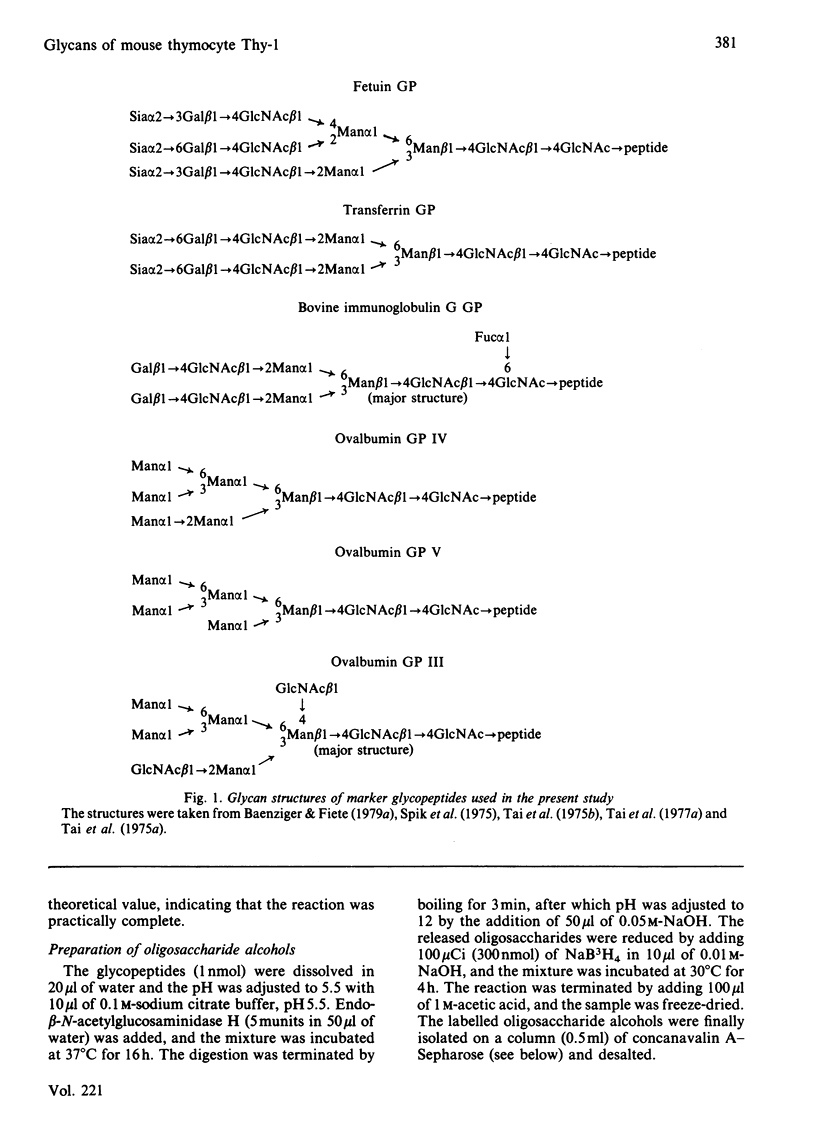
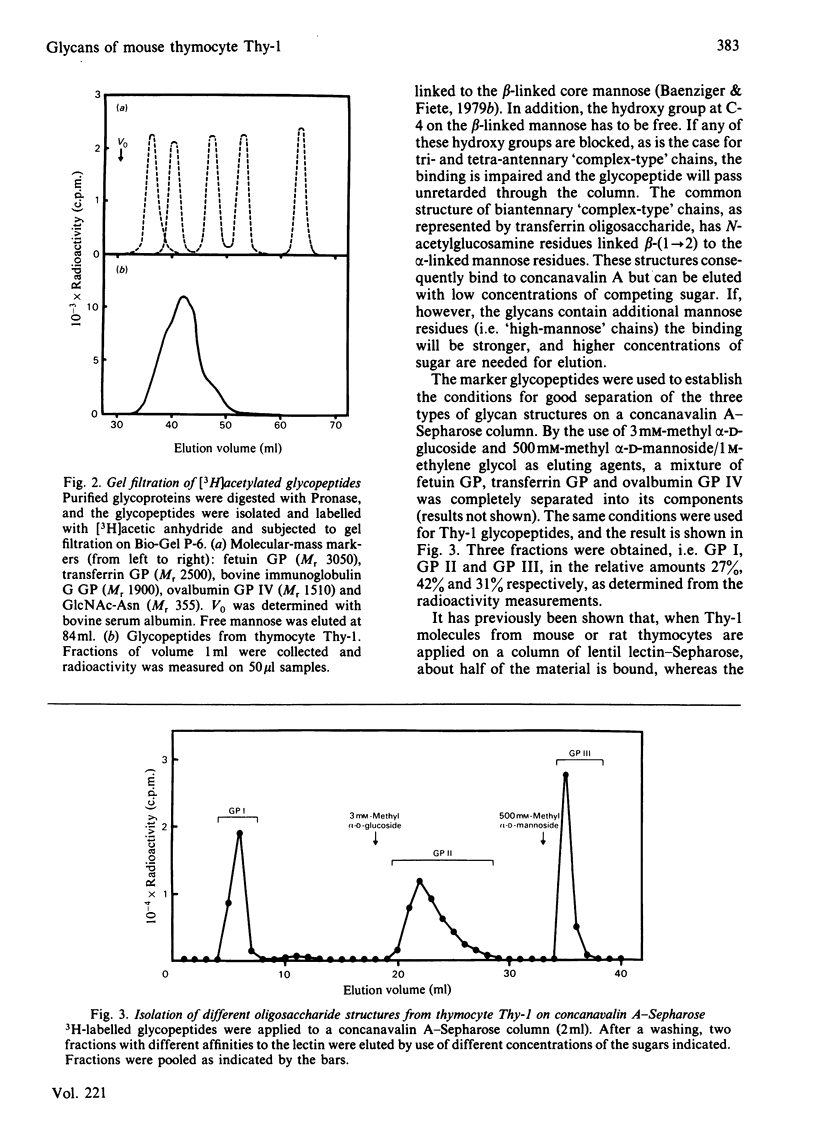
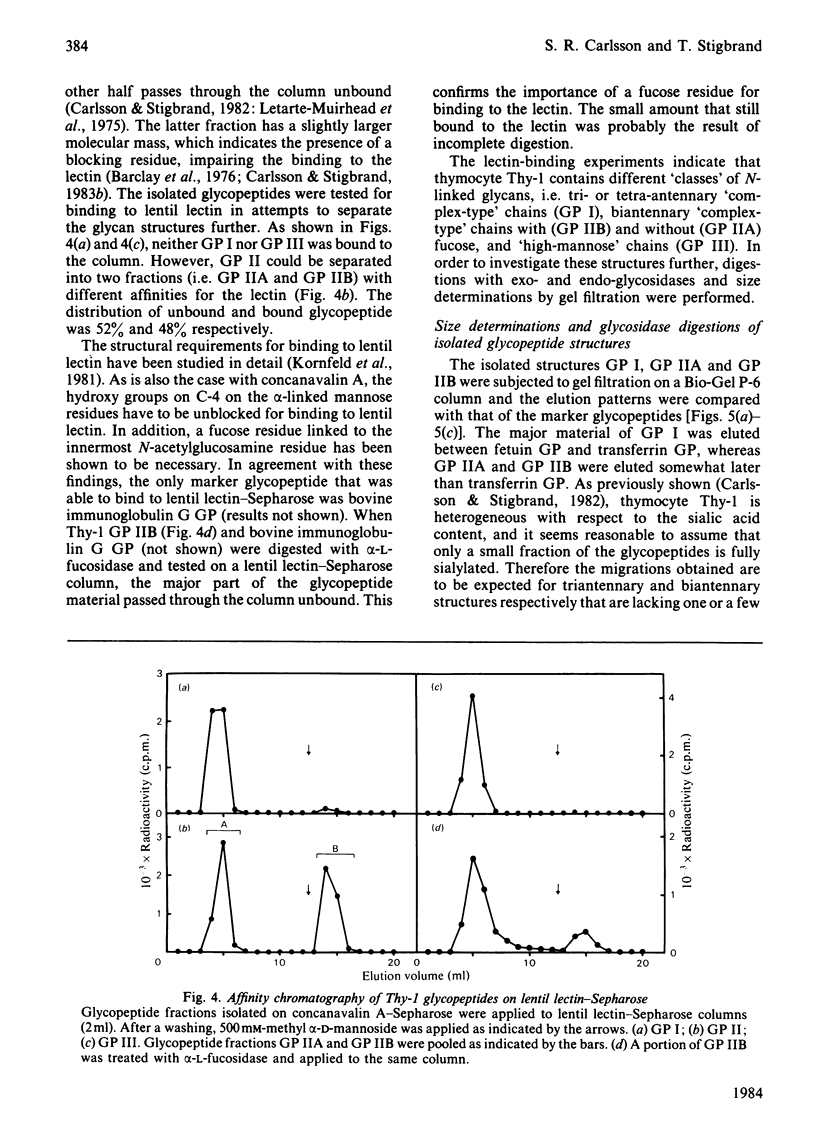
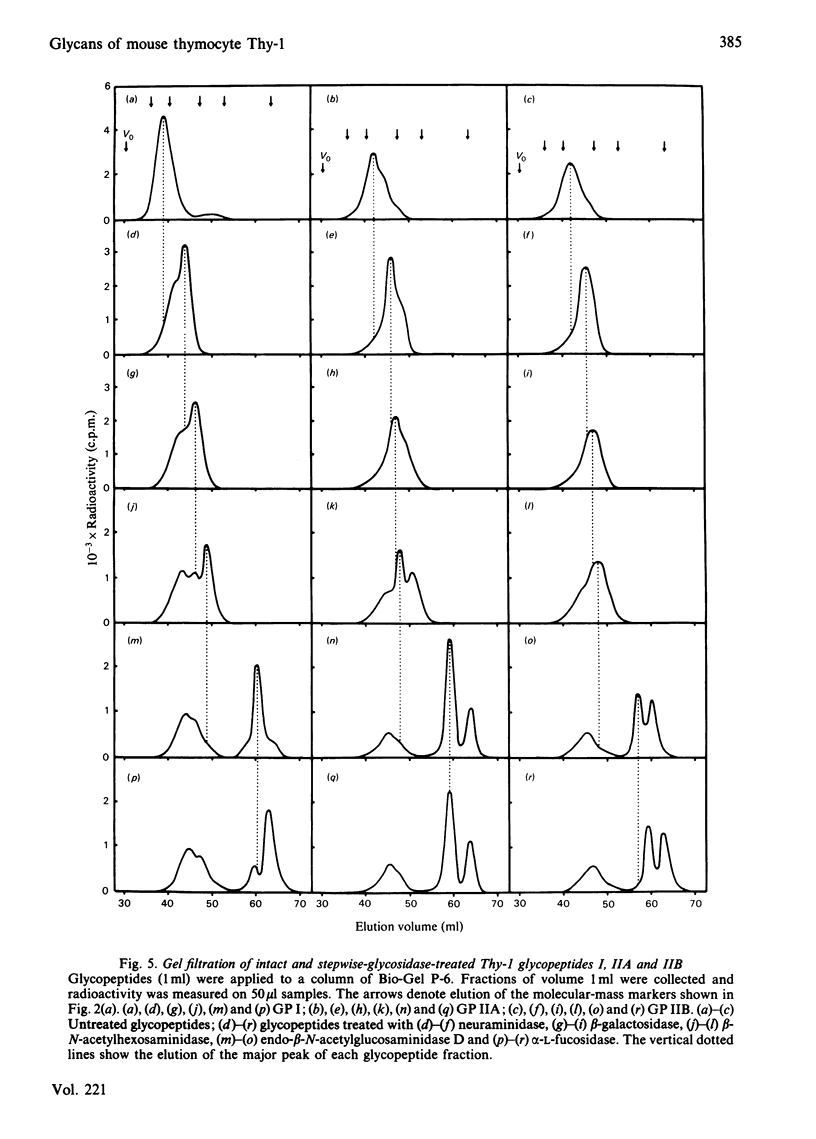
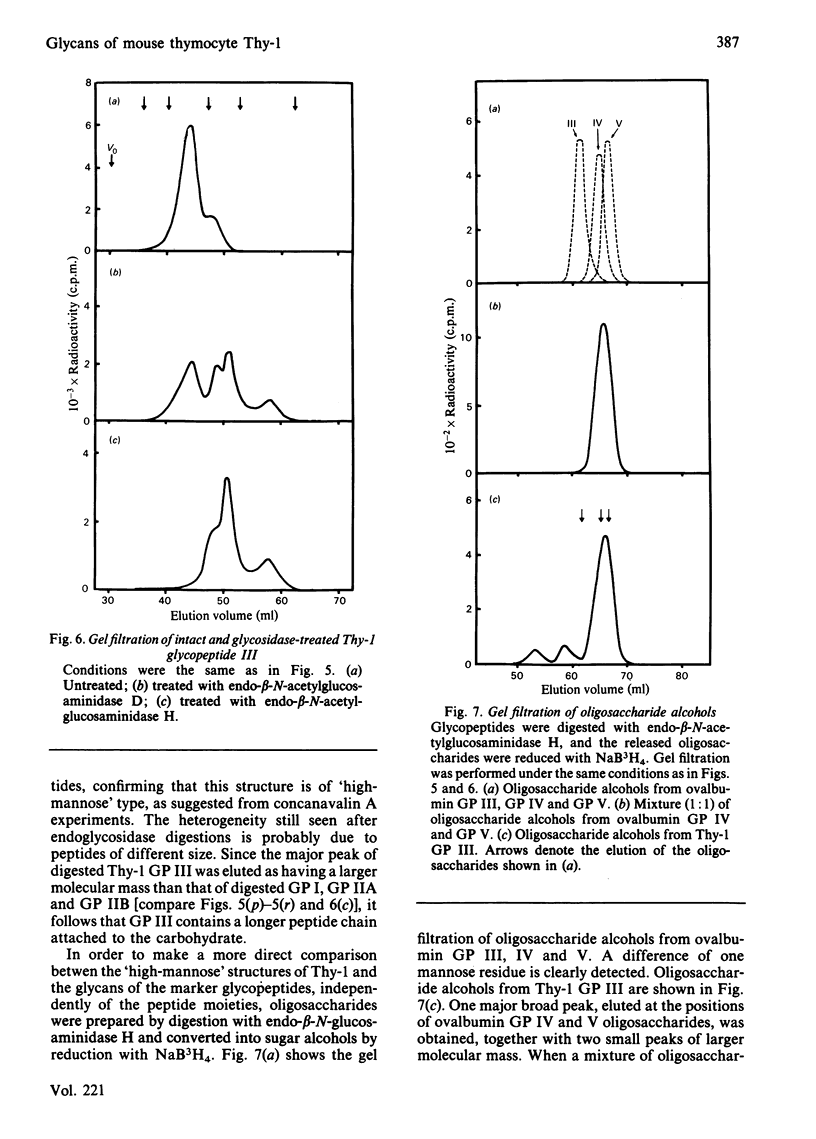
Four glycopeptides (I, IIA, IIB, III) with different oligosaccharide structures were isolated from purified mouse thymocyte Thy-1 glycoprotein. The glycoprotein was digested with Pronase, and the glycopeptide fraction was isolated by gel filtration and acetylated with [3H]acetic anhydride. The different glycan structures were separated by affinity chromatography on concanavalin A-Sepharose 4B and lentil lectin-Sepharose 4B. Size determinations of intact and exoglycosidase- and endoglycosidase-digested glycopeptides were performed by gel filtration on Bio-Gel P-6, calibrated with glycopeptides of known structure. On the basis of these experiments and on the behaviour of the glycopeptides on the lectin columns, the following structures of the oligosaccharide chains were proposed: I, triantennary 'complex-type' with terminal fucose; IIA, biantennary 'complex-type' without fucose; IIB, biantennary 'complex-type' with fucose; III, a mixture of 'high-mannose' chains containing either five or six mannose residues (approx. 50% of each). Amino acid analysis of the glycopeptides showed that the predominant oligosaccharide at glycosylation-site Asn-23 was of 'high-mannose' type, whereas the other two sites (Asn-75 and Asn-99) were glycosylated with 'complex-type' chains. Both these sites were shown to be variably glycosylated. The major glycans linked to Asn-75 were of structures I and IIB, whereas all three 'complex-type' chains were represented at Asn-99. The results presented explain the previously reported carbohydrate heterogeneity of thymocyte Thy-1 glycoprotein.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton R. T., Morris R. J., Williams A. F. Estimation of the amount and tissue distribution of rat Thy-1.1 antigen. Eur J Immunol. 1974 Sep;4(9):598–602. doi: 10.1002/eji.1830040904. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structure of the complex oligosaccharides of fetuin. J Biol Chem. 1979 Feb 10;254(3):789–795. [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F., Faulkes R. A. Chemical characterisation of the Thy-1 glycoproteins from the membranes of rat thymocytes and brain. Nature. 1976 Oct 14;263(5578):563–567. doi: 10.1038/263563a0. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Stigbrand T. I. Alterations in expression and glycosylation pattern of the Thy-1 glycoprotein during maturation and transformation of mouse T lymphocytes. J Immunol. 1983 Apr;130(4):1837–1842. [PubMed] [Google Scholar]
- Carlsson S. R., Stigbrand T. I. Purification and characterization of the mouse thymocyte Thy-1 glycoprotein. Biochem J. 1983 Jun 1;211(3):641–647. doi: 10.1042/bj2110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S., Stigbrand T. Carbohydrate complexity of the mouse thymocyte Thy-1 glycoprotein as demonstrated by lectin affinity and isoelectric focusing. Eur J Biochem. 1982 Mar;123(1):1–7. doi: 10.1111/j.1432-1033.1982.tb06490.x. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droege W., Zucker R. Lymphocyte subpopulations in the thymus. Transplant Rev. 1975;25:3–25. doi: 10.1111/j.1600-065x.1975.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Finne J., Krusius T. Preparation and fractionation of glycopeptides. Methods Enzymol. 1982;83:269–277. doi: 10.1016/0076-6879(82)83020-6. [DOI] [PubMed] [Google Scholar]
- Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J Biol Chem. 1982 Oct 25;257(20):11966–11970. [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Kornfeld R. Structure of the oligosaccharides of three glycopeptides from calf thymocyte plasma membranes. Biochemistry. 1978 Apr 18;17(8):1415–1423. doi: 10.1021/bi00601a009. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The structural basis of the different affinities of two types of acidic N-glycosidic glycopeptides for concanavalin A--sepharose. FEBS Lett. 1976 Nov 15;72(1):117–120. doi: 10.1016/0014-5793(76)80911-8. [DOI] [PubMed] [Google Scholar]
- Letarte-Muirhead M., Barclay A. N., Williams A. F. Purification of the Thy-1 molecule, a major cell-surface glycoprotein of rat thymocytes. Biochem J. 1975 Dec;151(3):685–697. doi: 10.1042/bj1510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T., Yamashita K., Fujikawa K., Kisiel W., Kobata A. The carbohydrate of bovine prothrombin. Occurrence of Gal beta 1 leads to 3GlcNAc grouping in asparagine-linked sugar chains. J Biol Chem. 1979 Jul 25;254(14):6419–6425. [PubMed] [Google Scholar]
- Moriuchi T., Chang H. C., Denome R., Silver J. Thy-1 cDNA sequence suggests a novel regulatory mechanism. Nature. 1983 Jan 6;301(5895):80–82. doi: 10.1038/301080a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Spik G., Bayard B., Fournet B., Strecker G., Bouquelet S., Montreuil J. Studies on glycoconjugates. LXIV. Complete structure of two carbohydrate units of human serotransferrin. FEBS Lett. 1975 Feb 15;50(3):296–299. doi: 10.1016/0014-5793(75)80513-8. [DOI] [PubMed] [Google Scholar]
- Tai T., Ito S., Yamashita K., Muramatsu T., Kobata A. Asparagine-linked oligosaccharide chains of IgG: a revised structure. Biochem Biophys Res Commun. 1975 Aug 4;65(3):968–974. doi: 10.1016/s0006-291x(75)80480-3. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ito S., Kobata A. Structures of the carbohydrate moiety of ovalbumin glycopeptide III and the difference in specificity of endo-beta-N-acetylglucosaminidases CII and H. J Biol Chem. 1977 Oct 10;252(19):6687–6694. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Kobata A. The substrate specificities of endo-beta-N-acetylglucosaminidases CII and H. Biochem Biophys Res Commun. 1977 Sep 9;78(1):434–441. doi: 10.1016/0006-291x(77)91273-6. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Takasaki S., Mizuochi T., Kobata A. Hydrazinolysis of asparagine-linked sugar chains to produce free oligosaccharides. Methods Enzymol. 1982;83:263–268. doi: 10.1016/0076-6879(82)83019-x. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Tsuji T., Matsumoto I., Osawa T. Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry. 1981 Sep 29;20(20):5894–5899. doi: 10.1021/bi00523a037. [DOI] [PubMed] [Google Scholar]


