Abstract
Background:
Although high serum levels of interleukin (IL)-17 and its producing cells have been found in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) in earlier research, it is still unclear how these findings relate to disease activity.
Objectives:
This study examines the link between serum levels of IL-17 and the activity of both RA and SLE.
Design:
This pilot case–control study included 100 patients with RA, 100 with SLE, and 100 healthy controls.
Methods:
The Disease Activity Score-28 (DAS28) scores assessed the activity of RA, whereas the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) scores assessed SLE activity. All participants’ data were compared and correlated.
Results:
Serum levels of IL-17 were significantly higher in RA and SLE patients compared with the controls (P < .001) and showed significantly positive correlations (P < .001) with rheumatoid factor titer, anti-cyclic citrullinated peptide (anti-CCP) and DAS28 score among the RA patients. Although among SLE patients, they were significantly positively correlated (P < .001) with anti-double-stranded DNA (anti-ds DNA) levels and the SLEDAI-2K scores, the best cut-off value of IL-17 for predicting moderate and high disease activity was > 175 pg/mL among RA patients and > 95 pg/mL among SLE patients.
Conclusions:
There is a significant correlation between RA and SLE activity and serum levels of IL-17. This discovery emphasizes IL-17 as a potential therapeutic target.
Keywords: Activity, DAS28, interleukin-17, rheumatoid arthritis, SLEDAI-2K, systemic lupus erythematosus
Introduction
The proinflammatory cytokine interleukin (IL)-17, a hallmark cytokine of T-helper 17 (Th17) cells, plays a significant role in host defense against infections, mainly against the extracellular pathogens, and in the pathogenesis of many autoimmune diseases. It is a pleiotropic cytokine that can induce the secretion of other proinflammatory factors.1-3 It also promotes the activation and recruitment of immune cells, stimulates stromal cells, and induces angiogenesis and osteoclastogenesis.4,5
Rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are common autoimmune disorders that cause disability, poor quality of life and low survival rates. Both disorders have genetic, environmental, and immunological etiology. 6 SLE is a heterogeneous disorder that can affect multiple organs such as skin, brain, joints, and kidneys due to the production of autoantibodies and the deposition of immune complexes. It is characterized by immune dysregulation with remissions and exacerbations. On the contrary, RA mainly affects the joints and can lead to deformities due to chronic inflammation, synovial hyperplasia, and autoantibody production. It can affect other body systems such as the cardiovascular and pulmonary systems.7,8 Previous studies have reported elevated serum levels of IL-17 and numbers of Th-17 cells in RA and SLE, but their association with disease activity is still uncertain.9,10 Early management of these disorders could improve disease outcomes and increase survival rates and quality of life. Several treatment approaches approved for psoriasis, psoriasis arthritis, and ankylosing spondylitis target the Th17 cells and IL-17 pathway.11,12 In an effort to identify a potential new target for treatment of RA and SLE, this study investigates the relationship between serum levels of IL-17 and the activity of both disorders.
Methodology
Ethics and participants selection
This pilot case–control study got ethical approval (# FMASUR10/2023) from the Research Ethics Committee, Faculty of Medicine, Ain Shams University. All participants or their legal guardians provided their written informed consent before the start. A total of 300 adults (100 healthy controls, 100 RA, and 100 SLE) participated in this study that was conducted over 6 months from March to August 2023. The RA and SLE patients were recruited from the Rheumatology Department of Ain-Shams University Hospital, whereas controls were recruited from the healthy sex- and age-matched attendees. The diagnosis of SLE followed the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria and the assessment of disease activity was done using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) scoring system, whereas RA diagnosis followed the 2010 American college of rheumatology (ACR)/European league against rheumatism (EULAR) criteria, and the assessment of disease activity was done using the Disease Activity Score-28 (DAS28) scoring system. 13 Patients with chronic infections, malignancies, and other autoimmune diseases were excluded from the study.
Clinical evaluation and data gathering
All participants were subjected to complete clinical evaluation and history-taking stressing on disease duration, the presence of comorbid conditions (eg, diabetes, hypertension), and body systems affection (eg, cardiac, pulmonary, renal, articular, cutaneous, vascular, oral, and central nervous systems). In addition, data were collected from patients’ medical reports, including routine lab investigations such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). As well as serum complement C3 and C4, anti-double-stranded DNA levels (anti-ds DNA) in SLE patients and rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) in RA patients.
Serum IL-17 level assessment
Three milliliters of whole blood from all participants were collected under strict sterile conditions into a gel-containing vacutainer tube. The collected blood was allowed to clot entirely before the serum was separated by centrifugation at 4000 r/min for 15 min. According to the manufacturer manual, the collected sera were used for serum IL-17 level assay by ELISA (Bioassay Technology Laboratory, Shanghai, China, Code: E0142Hu). The kit detection range is 2 to 600 pg/mL, and the sensitivity is 1.06 pg/mL.
Statistical analysis
SPSS version 20 (International Business Machines Corporation, New York, 2010) was used to analyze the data. For comparisons, one-way analysis of variance (ANOVA), independent T-test, and Chi-square test were employed. Using Spearman’s approach for non-parametric data and Pearson’s approach for parametric data, correlation studies were carried out. Finally, the predictive power of serum IL-17 levels for moderate and high RA and SLE activity was examined using receiver operating characteristic (ROC) curve analysis. A statistically significant P-value is less than .05.
Results
This study was performed on 100 patients with RA, 100 with SLE, and 100 healthy matched controls in age and sex. RA and SLE patients did not differ according to their disease duration (P = .924; see Table 1).
Table 1.
Comparison of the demographic characteristics of the study groups.
| Groups | P-value | ||||
|---|---|---|---|---|---|
| Control | SLE | RA | |||
| Age (years) | Range | 16-61 | 15-58 | 18-57 | .630 a |
| Mean ± SD | 27.950 ± 8.959 | 28.980 ± 9.353 | 27.760±9.226 | ||
| Sex, n (%) | Male | 27 (27%) | 14 (14%) | 22 (22%) | .075 b |
| Female | 73 (73%) | 86 (86%) | 78 (78%) | ||
| Disease duration (years) | Range | — | 0.58-20 | 0.52-22 | .924 a |
| Mean ± SD | — | 4.833 ± 3.978 | 5.023±4.122 | ||
Abbreviations: RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
One-way analysis of variance (ANOVA) test.
Chi-square test.
The IL-17 levels differed significantly between the study groups (P < .001); they were significantly higher in RA and SLE patients than in the control group. The mean (±SD) serum level of IL-17 among the RA patients was the highest, 362.76 ± 140.30 pg/mL, ranging from 67 to 600 pg/mL. Although among the SLE patients, it was 127.208 ± 81.310 pg/mL and ranged from 31 to 360 pg/mL, the control group’s mean (±SD) serum level of IL-17 was 30.450 ± 9.537 pg/mL and ranged from 16 to 52 pg/mL (see Figure 1).
Figure 1.
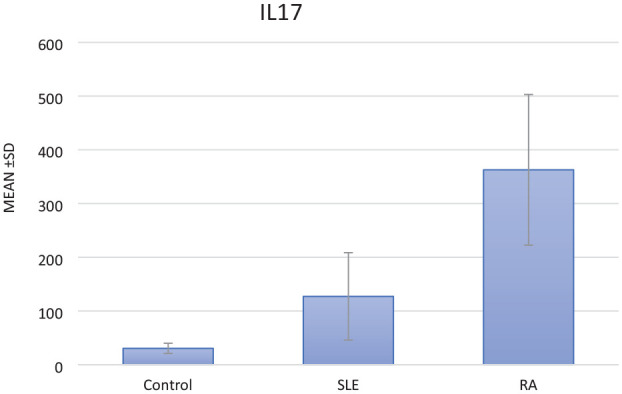
Box plot for comparison of serum levels of IL-17 (pg/mL) among the study groups (P < .001).
The DAS28 scores of the included RA patients ranged from 2.5 to 8.3 with a mean (±SD) of 4.967 ± 1.546. Of them, four patients were inactive, 11 had low, 42 had moderate, and 43 had high scores. On the contrary, according to the SLEDAI-2K scores of the included SLE patients, they ranged from 0 to 12 with a mean (±SD) of 5.360 ± 3.697. Of them, 51 patients had mild, 37 had moderate, and 12 had high scores. Serum levels of IL-17 differed significantly according to the DAS28 and the SLEDAI-2K scores; their levels increased significantly with higher scores (P < .001). Also, significantly higher serum levels of IL-17 among RA patients were associated with interstitial pulmonary fibrosis (P = .002). Although in SLE patients, IL-17 levels were significantly higher with serositis (P = .001), cerebritis (P < .001), thrombosis (P = .003), bleeding (P = .016), pulmonary complications (P < .001), consumed complement C3 and C4 (P < .001), arthritis (P = .025), and kidney affection in renal biopsy (P < .001; see Tables 2 and 3).
Table 2.
Serum levels of IL-17 according to the characteristics of the included RA patients (n = 100).
| Rheumatoid arthritis group | IL-17 (pg/mL) | P-value | ||
|---|---|---|---|---|
| n | Mean ± SD | |||
| Diabetes | Positive | 28 | 340.286 ± 129.389 | .320 a |
| Negative | 72 | 371.500 ± 144.244 | ||
| Hypertension | Positive | 38 | 340.447 ± 118.230 | .215 a |
| Negative | 62 | 376.435 ± 151.560 | ||
| Ischemic heart disease | Positive | 11 | 354.545 ± 132.331 | .838 a |
| Negative | 89 | 363.775 ± 141.941 | ||
| Interstitial pulmonary fibrosis | Positive | 26 | 435.000 ± 103.838 | .002 a |
| Negative | 74 | 337.378 ± 143.154 | ||
| DAS28 score | Inactive | 4 | 80.500 ± 15.588 | < .001 b |
| Low | 11 | 143.273 ± 19.764 | ||
| Moderate | 42 | 325.262 ± 54.448 | ||
| High | 43 | 481.791 ± 85.331 | ||
Abbreviations: DAS, Disease Activity Score; IL, interleukin; RA, rheumatoid arthritis.
Independent T-test.
One-way analysis of variance (ANOVA) test.
Bold P-value indicates statistical significance.
Table 3.
Serum levels of IL-17 according to the characteristics of the included SLE patients (n = 100).
| Systemic lupus erythematosus group | IL-17 (pg/mL) | P-value | ||
|---|---|---|---|---|
| n | Mean ± SD | |||
| Diabetes | Positive | 8 | 112.250 ± 50.514 | .590 a |
| Negative | 92 | 128.509 ± 83.515 | ||
| Hypertension | Positive | 38 | 121.474 ± 77.553 | .583 a |
| Negative | 62 | 130.723 ± 83.956 | ||
| Antiphospholipid syndrome | Positive | 52 | 125.169 ± 71.075 | .796 a |
| Negative | 48 | 129.417 ± 91.840 | ||
| Malar rash | Positive | 94 | 124.647 ± 80.232 | .214 a |
| Negative | 6 | 167.333 ± 95.475 | ||
| Oral ulcer | Positive | 84 | 121.438 ± 70.585 | .104 a |
| Negative | 16 | 157.500 ± 122.070 | ||
| Photosensitivity | Positive | 51 | 125.686 ± 83.623 | .850 a |
| Negative | 49 | 128.792 ± 79.665 | ||
| Alopecia | Positive | 61 | 121.311 ± 79.934 | .367 a |
| Negative | 39 | 136.431 ± 83.621 | ||
| Serositis | Positive | 26 | 171.685 ± 89.771 | .001 a |
| Negative | 74 | 111.581 ± 72.484 | ||
| Cerebritis | Positive | 15 | 209.067 ± 88.841 | < .001 a |
| Negative | 85 | 112.762 ± 71.194 | ||
| Thrombosis | Positive | 16 | 182.250 ± 109.444 | .003 a |
| Negative | 84 | 116.724 ± 70.893 | ||
| Bleeding | Positive | 10 | 133.676 ± 82.960 | .016 a |
| Negative | 90 | 69.000 ± 21.960 | ||
| Cardiac complications | Positive | 14 | 122.414 ± 75.346 | .813 |
| Negative | 86 | 127.988 ± 82.629 | ||
| Pulmonary complications | Positive | 16 | 192.125 ± 98.183 | < .001 a |
| Negative | 84 | 114.843 ± 71.948 | ||
| Complement C3 | Consumed | 34 | 191.353 ± 76.308 | < .001 a |
| Normal | 66 | 94.164 ± 62.067 | ||
| Complement C4 | Consumed | 29 | 178.345 ± 60.469 | < .001 a |
| Normal | 71 | 106.321 ± 79.761 | ||
| Joint affection | Negative | 4 | 107.500 ± 20.207 | .025 b |
| Arthralgia | 60 | 111.013 ± 70.664 | ||
| Arthritis | 36 | 156.389 ± 94.224 | ||
| Renal biopsy | Normal | 68 | 108.985 ± 70.550 | < .001 b |
| Class 2 | 6 | 112.500 ± 37.891 | ||
| Class 3 | 10 | 119.000 ± 30.441 | ||
| Class 4 | 8 | 152.633 ± 102.129 | ||
| Class 5 | 8 | 288.000 ± 45.981 | ||
| SLEDAI-2K score | Mild | 51 | 70.251 ± 25.736 | < .001 b |
| Moderate | 37 | 169.081 ± 74.495 | ||
| High | 12 | 240.167 ± 58.913 | ||
Abbreviations: IL, interleukin; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
Independent T-test.
One-way analysis of variance (ANOVA) test.
Bold P-value indicates statistical significance.
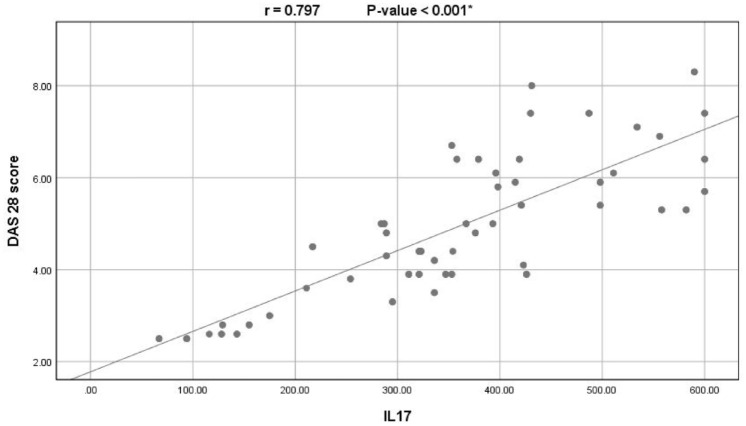
Among the RA patients, IL-17 levels showed significantly positive correlations with ESR (r: 0.588; P < .001), CRP (r: 0.553; P < .001), rheumatoid factor titer (r: 0.507; P < .001), anti-CCP (r: 0.575; P < .001), and DAS28 score (0.797; P < .001; see Figure 2).
Figure 2.
Correlation between serum levels of IL-17 and the DAS28 scores among the RA patients.
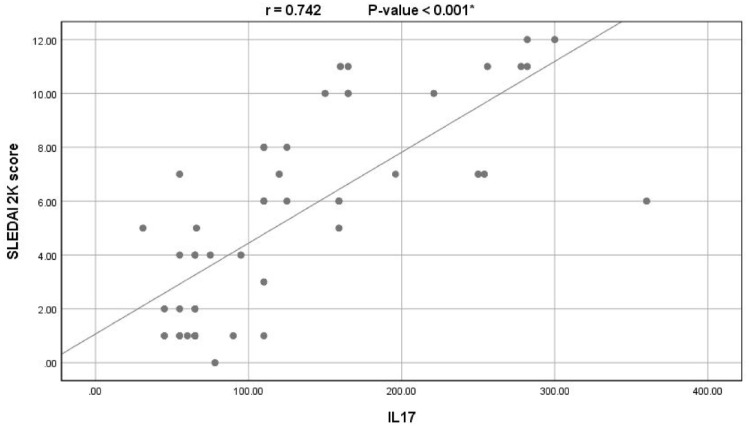
Among the SLE patients, serum IL-17 levels showed significantly positive correlations with ESR (r: 0.200; P = .046), anti-ds DNA levels (r: 0.490; P < .001), and the SLEDAI-2K scores (r: 0742; P < .001; see Figure 3).
Figure 3.
Correlation between serum levels of IL-17 and the SLEDAI-2K scores among the SLE patients.
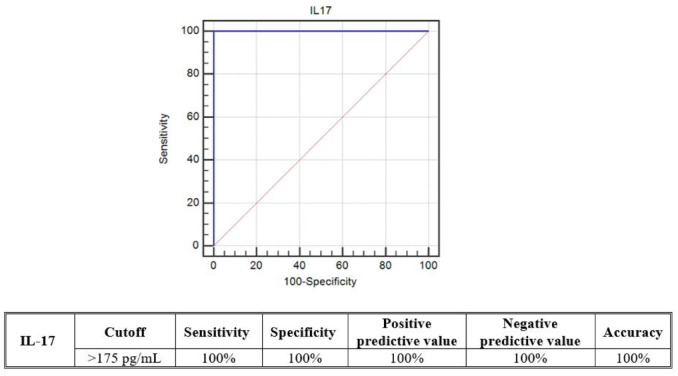
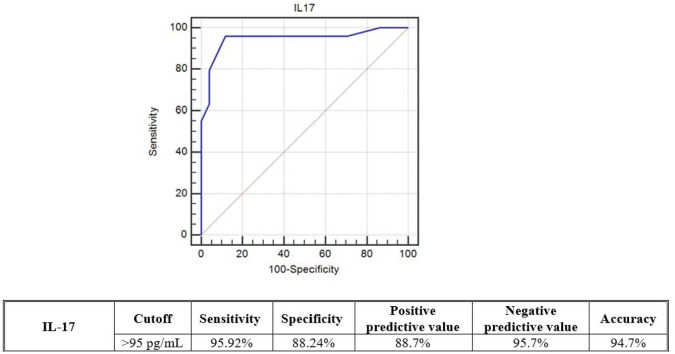
The predictive ability of serum IL-17 levels for the severity of RA and SLE was tested using ROC curve analyses. Among the RA patients, serum IL-17 at a cut-off > 175 pg/mL had 100% diagnostic sensitivity, 100% specificity, and 100% accuracy for predicting moderate to high score disease. On the contrary, among the SLE patients, serum IL-17 at a cut-off > 95 pg/mL had 95.92% diagnostic sensitivity, 88.24% specificity, and 94.7% accuracy for predicting moderate to high score disease (see Figures 4 and 5).
Figure 4.
ROC curve analysis for the predicting ability of serum IL-17 levels between (moderate + high) and (inactive + mild) DAS28 scores among the RA patients.
Figure 5.
ROC curve analysis for the predicting ability of serum IL-17 levels between (moderate + high) and (mild) SLEDAI-2K scores among the SLE patients.
Discussion
Biologic therapy for rheumatologic diseases targets immune system molecules and offers an alternative to disease-modifying anti-rheumatic medicines and other immunosuppressive pharmaceuticals. They are not widely used as first-line pharmaceuticals due to their high costs, intravenous delivery, and side effects. 14
This work aimed to investigate the association between serum levels of IL-17 and the activity of both RA and SLE to find a possible novel target for early intervention, better disease prognosis, and patient outcomes. We found that serum levels of IL-17 were significantly higher in RA and SLE patients compared with the controls. These elevated levels were significantly positively correlated with disease activity biomarkers and scores. This finding can propose IL-17 as an effective treatment target for these disorders as it might be essential in the pathogenesis of both diseases. In addition to its proinflammatory effects, IL-17 recruits monocytes and neutrophils by increasing chemokine production, facilitates T-cell infiltration into the inflamed tissues and its activation by enhancing expression of adhesion molecules, and boosts the immunological response by triggering the synthesis of other cytokines, such as IL-6. The IL-17 also synergizes the interplay between several proinflammatory cytokines. Its receptor is widely distributed; thus, IL-17 can impact the function of many cell types, including non-immune cells such as endothelium and epithelial cells. 15 The primary source of IL-17 is Th17 cells, which could be produced by the effect of IL-6, IL-1, and transforming growth factor β. These cells have the orphan nuclear receptor RORγt as a key transcription factor and IL-23 as a major stabilization and maturation factor. 16 IL-17 could also be produced by various innate cells, including macrophages, and natural killer cells. 17
Previous reports of the relationship between serum levels of IL-17 and the activity of autoimmune diseases have been inconsistent. Similar to this study, several other studies reported elevated IL-17 levels or IL-17-producing cells in the blood or the inflamed joints of RA patients. They also reported that these elevated levels were associated with disease activity and the extent of joint destruction. 4 ,18-20 Similarly, Chabaud et al 21 reported that IL-17 and Th17 cells were present in the synovial tissue of RA lesions, indicating their role in the pathogenesis of RA. In addition, Kirkham et al 19 reported that IL-17 is predictive of joint destruction progression and poor outcome. Studies also found that introducing IL-17 to stromal cells, such as synoviocytes from RA patients, enhanced the production of IL-6 and IL-8. 22 Also, Meng et al reported that IL-17 is closely related to the occurrence of RA. They also reported that using IL-17 in relation to other cytokines could be more suitable in reflecting the treatment effectiveness of RA. 23 Furthermore, Pratama and Widyowati 24 found that IL-17 inhibition has an anti-RA potential. Although there is compelling evidence that IL-17 plays a part in aggravating human RA, not all studies completely agree, and there is no solid evidence that IL-17 plays a part in osteoarthritis. 25
Furthermore, numerous investigations have documented higher serum IL-17 levels in SLE patients compared with controls; these elevated levels were significantly positively correlated with anti-ds DNA titer and disease activity.26-28 These findings are similar to our own. On the contrary, Vincent et al reported that despite serum IL-17 being significantly elevated among SLE patients compared with controls, its concentration correlated poorly with disease activity. However, IL-17 elevation was significantly associated with central nervous system involvement among their included SLE patients. 9 In addition, Lovato et al 29 reported that anti-IL-17 medications may be useful for treating cutaneous lupus erythematosus (CLE) lesions, as IL-17 expression was considerably greater in CLE/SLE patients. Also, Behiry et al found elevated levels of serum IL-17 in SLE cases. They suggested that IL-17 may have a role in the pathogenesis of SLE. However, they did not find any statistically significant correlations between IL-17 and lupus nephritis nor the SLEDAI score. 30 On the contrary, IL-17 serum levels and SLE activity were not related as well, according to other research.31,32
The discrepancy between studies could be attributable to the difference in sample sizes, ethnicity, the age of the included participants, the sensitivity of IL-17 detection methods, the immunosuppressive treatment regimen, and the disease duration. In addition, the production of IL-17 could be localized into the inflamed organs only, like the brain or joints; thus, measuring peripheral blood IL-17 level might not accurately indicate its true levels in the affected organs.9,33
Based on the available data, it has been demonstrated that treating autoimmune diseases such as psoriasis, psoriatic arthritis, and ankylosing spondylitis with monoclonal antibodies that target the IL-17 cytokine (secukinumab, ixekizumab) or its receptor (brodalumab) can effectively block the IL-17 secreted by auto-reactive Th17 cells and inhibit IL-17 signaling, leading to better treatment outcomes. 2 ,34-36 However, because IL-17 and neutrophils are linked, medications that target IL-17 may have unintended consequences that impact acute immune responses. Furthermore, IL-17 can be produced by cells other than TH17, and IL-17 from these cells may have unique (local) functions that make therapies targeting IL-17 less effective. 16 Therefore, a more thorough understanding of the mechanism of action of IL-17 and its inhibitors will help the development of an efficient, targeted therapy with fewer side effects. On the contrary, other studies reported that since several cytokines are implicated in the pathogenesis of autoimmune diseases, a single molecule suppression strategy—such as blocking IL-17—is unlikely to help treat the full range of clinical symptoms. Future research ought to determine which autoimmune disease patients qualify for medication that targets Th17.37,38
This study has several limitations being a single-center study with no close follow-up of the included patients. The study also did not look into the different patterns of other cytokines and their localization and did not consider other confounding factors that could have affected our results. Future studies, with proper sample size calculation, should get beyond these obstacles and produce more thorough and therapeutically relevant results by addressing these constraints through careful study design, exacting methodology, addressing clinical and laboratory features at the moment of enrollment, citing concomitant therapies, and cooperation with other research centers.
Conclusions
Despite not taking into consideration the established treatment protocol for each included RA and SLE patient, this study found a strong association between serum levels of IL-17 and the activity of these autoimmune diseases. This finding highlights IL-17 as a viable therapeutic target for those conditions. For further proof and greater therapeutic benefits, larger-scale research and clinical trials utilizing IL-17-targeting medicines are required.
Acknowledgments
None.
Footnotes
ORCID iD: Sara I. Taha  https://orcid.org/0000-0001-8224-8701
https://orcid.org/0000-0001-8224-8701
Declarations
Ethics approval and consent to participate: This study got ethical approval (# FMASUR10/2023) from the Research Ethics Committee, Faculty of Medicine, Ain Shams University. All participants or their legal guardians provided their written informed consent before the start.
Consent for publication: Not applicable.
Author contributions: Sara F Samaan: Conceptualization; Validation; Investigation; Formal analysis.
Sara I Taha: Writing – original draft; Methodology; Investigation; Supervision.
Fatma A Mahmoud: Writing – review & editing; Resources; Investigation; Formal analysis.
Yara Elsaadawy: Conceptualization; Investigation; Writing – review & editing; Resources.
Salma A Khalil: Conceptualization; Investigation; Writing – review & editing; Formal analysis.
Dalia M Gamal: Conceptualization; Investigation; Writing – review & editing; Formal analysis.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All the data needed to support the current findings will be available upon request.
References
- 1. Baioumy SA, Sallam DE, Abdalgeleel SA, Fouad SH, Khedr AS, Taha SI. Interleukin-17A serum levels in young patients with atopic dermatitis and food allergy. Eur Cytokine Netw. 2021;32:55-63. doi: 10.1684/ecn.2021.0470 [DOI] [Google Scholar]
- 2. Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond). 2012;122:487-511. doi: 10.1042/CS20110496 [DOI] [PubMed] [Google Scholar]
- 3. Raymond W, Ostli-Eilertsen G, Griffiths S, Nossent J. IL-17A levels in systemic lupus erythematosus associated with inflammatory markers and lower rates of malignancy and heart damage: evidence for a dual role. Eur J Rheumatol. 2017;4:29-35. doi: 10.5152/eurjrheum.2017.16059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taams LS. Interleukin-17 in rheumatoid arthritis: trials and tribulations. J Exp Med. 2020;217:e20192048. doi: 10.1084/jem.20192048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robert M, Miossec P. Interleukin-17 and lupus: enough to be a target? For which patients? Lupus. 2020;29:6-14. doi: 10.1177/0961203319891243 [DOI] [PubMed] [Google Scholar]
- 6. Pabón-Porras MA, Molina-Ríos S, Flórez-Suárez JB, Coral-Alvarado PX, Méndez-Patarroyo P, Quintana-López G. Rheumatoid arthritis and systemic lupus erythematosus: pathophysiological mechanisms related to innate immune system. SAGE Open Med. 2019;7:2050312119876146. doi: 10.1177/2050312119876146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ameer MA, Chaudhry H, Mushtaq J, et al. An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management. Cureus. 2022;14:e30330. doi: 10.7759/cureus.30330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fassbender HG, Meyer-Scholten C, Zorn K. Klinicka slika reumatoidnog artritisa—kompleks triju neovisnih mehanizama [The clinical picture of rheumatoid arthritis—the complex of three independent mechanisms]. Reumatizam. 2009;56:5-7. [PubMed] [Google Scholar]
- 9. Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R97. doi: 10.1186/ar4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robert M, Miossec P. IL-17 in rheumatoid arthritis and precision medicine: from synovitis expression to circulating bioactive levels. Front Med (Lausanne). 2019;5:364. doi: 10.3389/fmed.2018.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koga T, Ichinose K, Kawakami A, Tsokos GC. Current insights and future prospects for targeting IL-17 to treat patients with systemic lupus erythematosus. Front Immunol. 2021;11:624971. doi: 10.3389/fimmu.2020.624971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radi G, Campanati A, Diotallevi F, Bianchelli T, Offidani A. Novel therapeutic approaches and targets for treatment of psoriasis. Curr Pharm Biotechnol. 2021;22:7-31. doi: 10.2174/1389201021666200629150231 [DOI] [PubMed] [Google Scholar]
- 13. Taha SI, Samaan SF, Ibrahim RA, Moustafa NM, El-Sehsah EM, Youssef MK. Can complete blood count picture tell us more about the activity of rheumatological diseases? Clin Med Insights Arthritis Musculoskelet Disord. 2022;15:11795441221089182. doi: 10.1177/11795441221089182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013;11:88. doi: 10.1186/1741-7015-11-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nalbandian A, Crispín JC, Tsokos GC. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol. 2009;157:209-215. doi: 10.1111/j.1365-2249.2009.03944.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549-553. doi: 10.1038/nrrheum.2009.179 [DOI] [PubMed] [Google Scholar]
- 17. Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311-321. doi: 10.1111/j.1365-2567.2009.03240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gullick NJ, Abozaid HS, Jayaraj DM, et al. Enhanced and persistent levels of interleukin (IL)-17+ CD4+ T cells and serum IL-17 in patients with early inflammatory arthritis. Clin Exp Immunol. 2013;174:292-301. doi: 10.1111/cei.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirkham BW, Lassere MN, Edmonds JP, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum. 2006;54:1122-1131. doi: 10.1002/art.21749 [DOI] [PubMed] [Google Scholar]
- 20. Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876-2885. doi: 10.1002/art.27622 [DOI] [PubMed] [Google Scholar]
- 21. Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092-1099. doi: 10.1006/cyto.2000.0681 [DOI] [PubMed] [Google Scholar]
- 22. Miossec P. Interleukin-17 in fashion, at last: ten years after its description, its cellular source has been identified. Arthritis Rheum. 2007;56:2111-2115. doi: 10.1002/art.22733 [DOI] [PubMed] [Google Scholar]
- 23. Meng Y, Cai XL, Cong S, et al. Role of platelet/lymphocyte, neutrophil/lymphocyte, and interleukin-37/interleukin-17 ratios in the occurrence and treatment of rheumatoid arthritis. Immunol Invest. 2024;53:464-474. doi: 10.1080/08820139.2023.2299687 [DOI] [PubMed] [Google Scholar]
- 24. Pratama RR, Widyowati R. Anti-rheumatoid arthritis potential of Acorus calamus L. extract by interleukin-17 inhibition: molecular insights through an in silico study. J Pharm Pharmacogn Res. 2024;12:628-646. [Google Scholar]
- 25. Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370. doi: 10.1007/s11926-009-0052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385-393. doi: 10.1016/j.clim.2008.01.019 [DOI] [PubMed] [Google Scholar]
- 27. Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589-593. doi: 10.1191/096120300678828703 [DOI] [PubMed] [Google Scholar]
- 28. Doreau A, Belot A, Bastid J, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778-785. [DOI] [PubMed] [Google Scholar]
- 29. Lovato BH, Fogagnolo L, Souza EM, et al. IL-1β and IL-17 in cutaneous lupus erythematous skin biopsies: could immunohistochemicals indicate a tendency towards systemic involvement. An Bras Dermatol. 2024;99:66-71. doi: 10.1016/j.abd.2023.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Behiry M, Wadie M, Mohamed NA, Farid R, Ramadan H. Assessment of interleukin 17 in Egyptian systemic lupus erythematosus patients as a biomarker in disease activity. Curr Rheumatol Rev 2024;20:534-543. doi: 10.2174/0115733971282065240123075748 [DOI] [PubMed] [Google Scholar]
- 31. Cheng F, Guo Z, Xu H, Yan D, Li Q. Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 2009;68:604-606. doi: 10.1136/ard.2008.097089 [DOI] [PubMed] [Google Scholar]
- 32. Zhao XF, Pan HF, Yuan H, et al. Increased serum interleukin 17 in patients with systemic lupus erythematosus. Mol Biol Rep. 2010;37:81-85. doi: 10.1007/s11033-009-9533-3 [DOI] [PubMed] [Google Scholar]
- 33. Shen HH, Fan Y, Wang YN, et al. Elevated circulating interleukin-17 levels in patients with systemic lupus erythematosus: a meta-analysis. Immunol Invest. 2020;49:662-675. doi: 10.1080/08820139.2019.1699107 [DOI] [PubMed] [Google Scholar]
- 34. Berry SPD, Dossou C, Kashif A, et al. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int Immunopharmacol. 2022;102:108402. doi: 10.1016/j.intimp.2021.108402 [DOI] [PubMed] [Google Scholar]
- 35. Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11. doi: 10.1016/j.jaut.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 36. Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72:ii116-ii123. doi: 10.1136/annrheumdis-2012-202371 [DOI] [PubMed] [Google Scholar]
- 37. Petrić M, Radić M. Is Th17-targeted therapy effective in systemic lupus erythematosus? Curr Issues Mol Biol. 2023;45:4331-4343. doi: 10.3390/cimb45050275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim M, Choe YH, Lee SI. Lessons from the success and failure of targeted drugs for rheumatoid arthritis: perspectives for effective basic and translational research. Immune Netw. 2022;22:e8. doi: 10.4110/in.2022.22.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]