Abstract
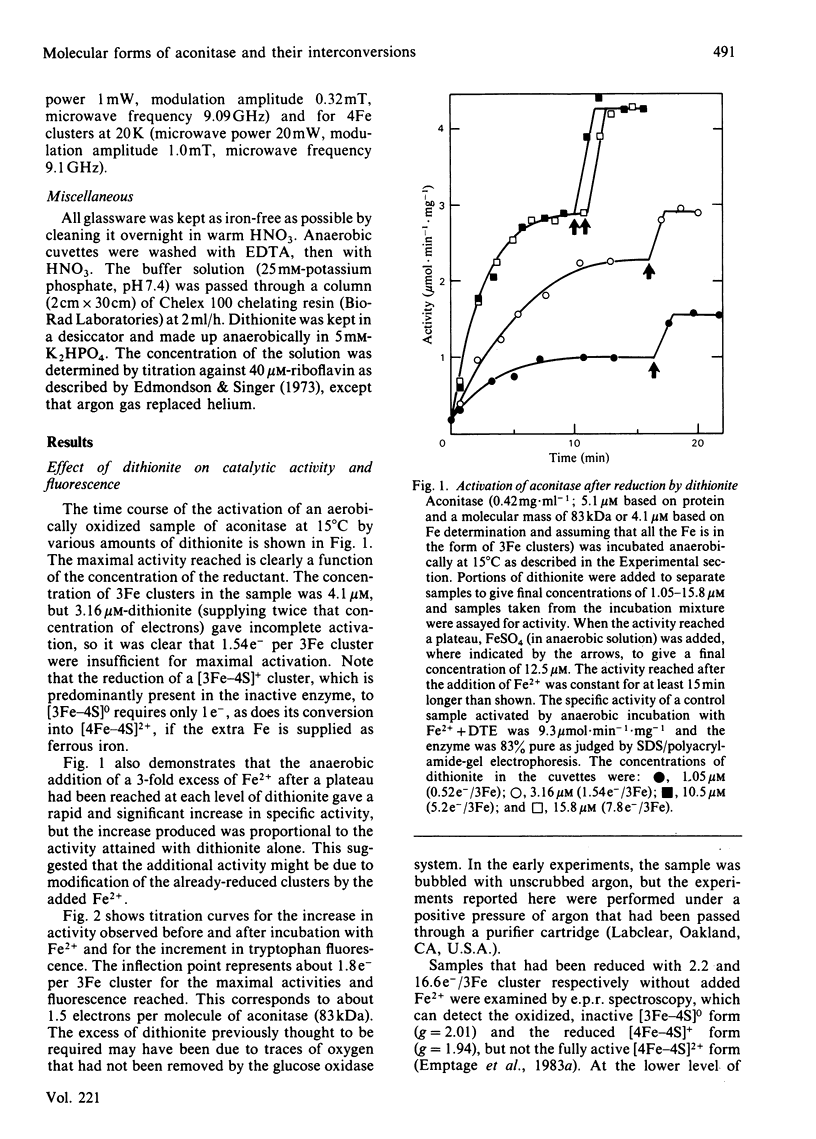
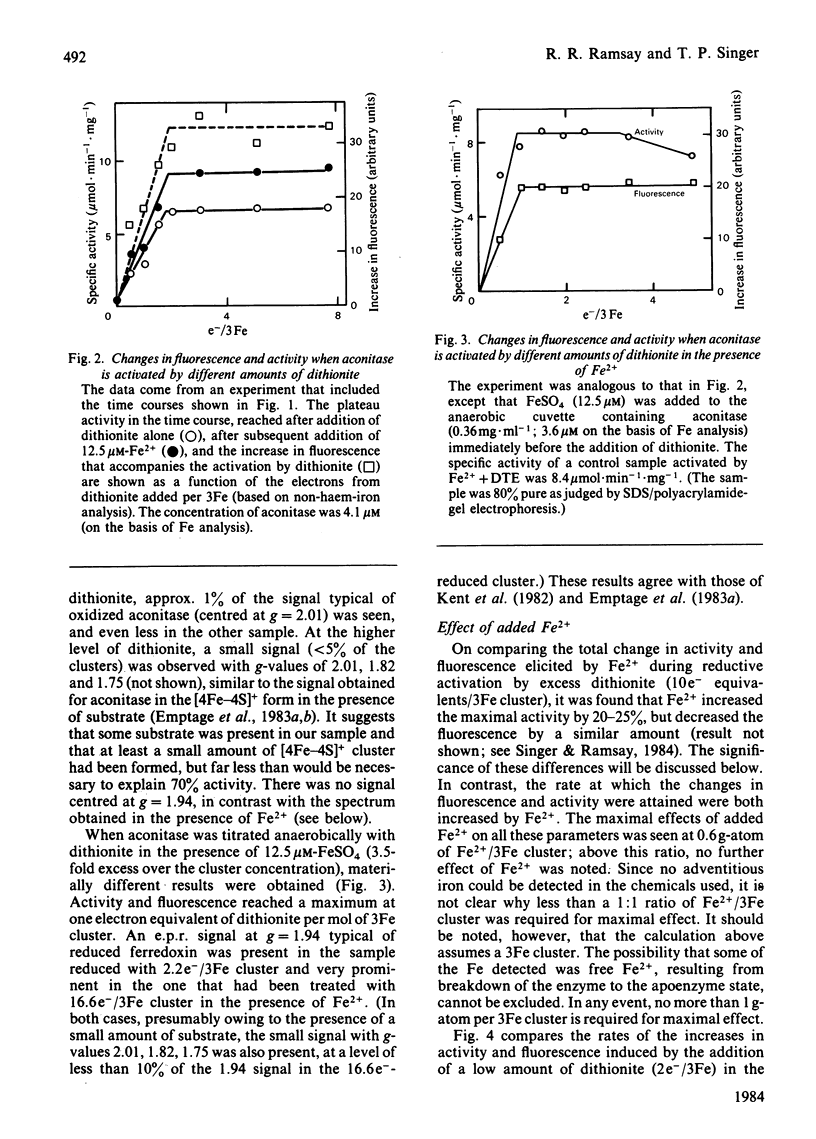
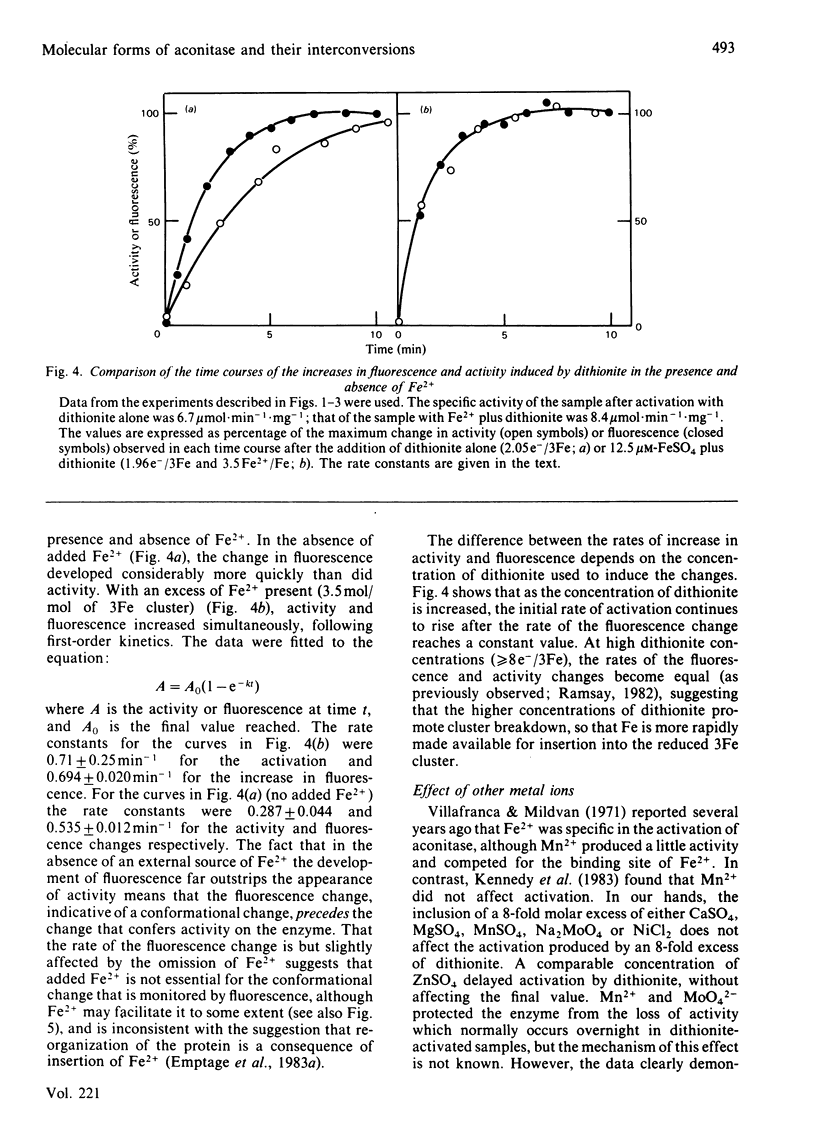
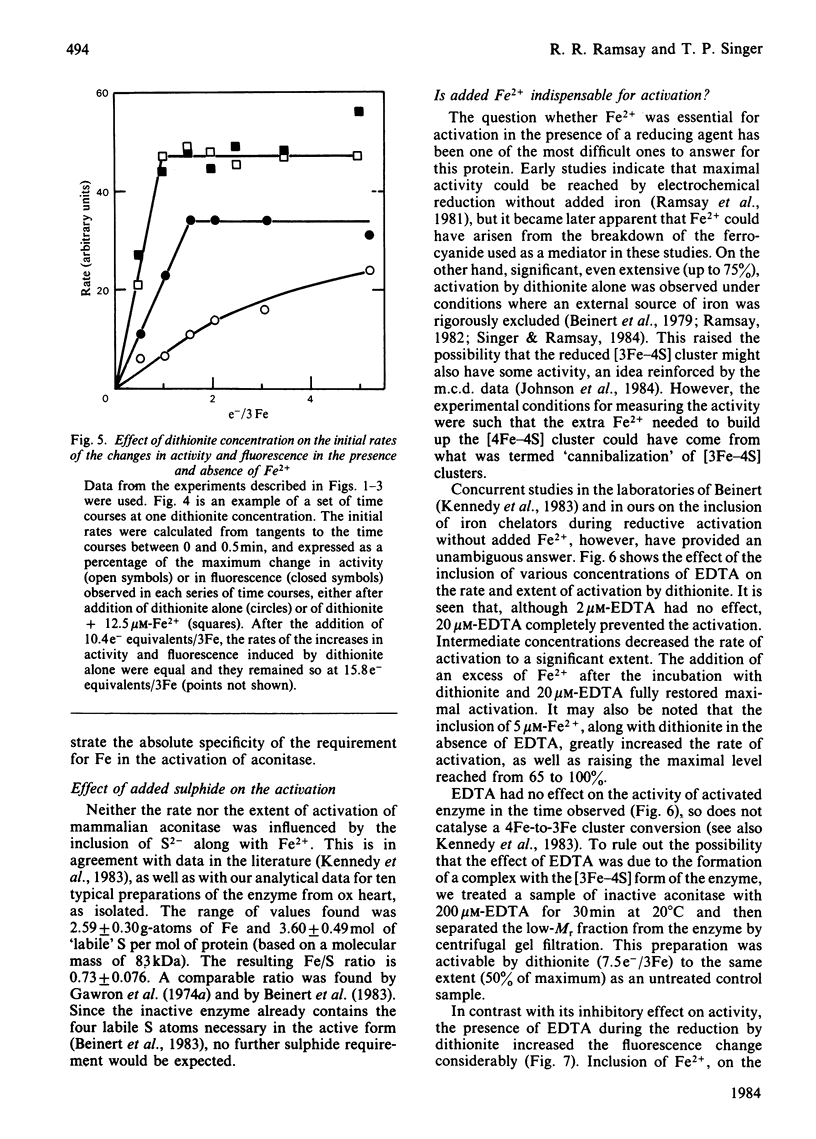
Aconitase, as isolated from mammalian mitochondria by traditional methods, is virtually inactive and contains an oxidized [3Fe-4S]+ cluster. The activation of the enzyme and attendant conformational change have been studied by monitoring the changes in activity, in tryptophan fluorescence, and in the electron paramagnetic resonance of the cluster on incubation with dithionite, with and without added Fe2+. Restoration of the full activity is achieved with one electron per 3Fe cluster and at least 0.6 g-atoms of Fe2+ per mol. The process involves building up of [4Fe-4S]2+ clusters. Other metal ions do not substitute for Fe2+. Reduction alone, in the absence of added Fe2+, yields up to 70% of the maximum activity, but requires approx. 1.8 electrons of reductant per cluster. The results presented are consistent with the view that activation without added Fe2+ involves the destruction of some of the [3Fe-4S] clusters and the incorporation of the Fe so liberated into other clusters to yield a tetra-nuclear one. In particular, the effect of EDTA and of other iron chelators in inhibiting activation by dithionite alone is in accord with this view, although recent magnetic-circular-dichroism studies do not support this interpretation. The rates of increase in activity and tryptophan fluorescence are the same when Fe2+ is present, but in its absence, activation is very much slower than the increase in fluorescence, suggesting that the protein conformational change triggered by reduction of the Fe-S clusters precedes the insertion of the iron. Consistent with this view is the observation that iron chelators inhibit activation by dithionite, but not the increase in fluorescence and, hence, the conformational change. The results are discussed in light of data in the literature on the forms of the cluster and its possible function in catalysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Emptage M. H., Dreyer J. L., Scott R. A., Hahn J. E., Hodgson K. O., Thomson A. J. Iron-sulfur stoichiometry and structure of iron-sulfur clusters in three-iron proteins: evidence for [3Fe-4S] clusters. Proc Natl Acad Sci U S A. 1983 Jan;80(2):393–396. doi: 10.1073/pnas.80.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. E., Singer T. P. Oxidation-reduction properties of the 8 alpha-substituted flavins. J Biol Chem. 1973 Dec 10;248(23):8144–8149. [PubMed] [Google Scholar]
- Emptage M. H., Dreyers J. L., Kennedy M. C., Beinert H. Optical and EPR characterization of different species of active and inactive aconitase. J Biol Chem. 1983 Sep 25;258(18):11106–11111. [PubMed] [Google Scholar]
- Emptage M. H., Kent T. A., Kennedy M. C., Beinert H., Münck E. Mössbauer and EPR studies of activated aconitase: development of a localized valence state at a subsite of the [4Fe-4S] cluster on binding of citrate. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4674–4678. doi: 10.1073/pnas.80.15.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron O., Sr, Kennedy M. C., Rauner R. A. Properties of pig heart aconitase. Biochem J. 1974 Dec;143(3):717–722. doi: 10.1042/bj1430717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron O., Waheed A., Glaid A. J., 3rd, Jaklitsch A. Iron and aconitase activity. Biochem J. 1974 Jun;139(3):709–714. doi: 10.1042/bj1390709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Czernuszewicz R. S., Spiro T. G., Ramsay R. R., Singer T. P. Resonance Raman studies of beef heart aconitase and a bacterial hydrogenase. J Biol Chem. 1983 Nov 10;258(21):12771–12774. [PubMed] [Google Scholar]
- Johnson M. K., Thomson A. J., Richards A. J., Peterson J., Robinson A. E., Ramsay R. R., Singer T. P. Characterization of the Fe-S cluster in aconitase using low temperature magnetic circular dichroism spectroscopy. J Biol Chem. 1984 Feb 25;259(4):2274–2282. [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Kent T. A., Dreyer J. L., Kennedy M. C., Huynh B. H., Emptage M. H., Beinert H., Münck E. Mössbauer studies of beef heart aconitase: evidence for facile interconversions of iron-sulfur clusters. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1096–1100. doi: 10.1073/pnas.79.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Ramsay R. R., Dreyer J. L., Schloss J. V., Jackson R. H., Coles C. J., Beinert H., Cleland W. W., Singer T. P. Relationship of the oxidation state of the iron-sulfur cluster of aconitase to activity and substrate binding. Biochemistry. 1981 Dec 22;20(26):7476–7482. doi: 10.1021/bi00529a023. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R. Evidence that the activation of aconitase involves a conformation change. Biochem J. 1982 Apr 1;203(1):327–330. doi: 10.1042/bj2030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem. 1967 Apr 25;242(8):1870–1879. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A mitochondrial iron protein with properties of a high-potential iron-sulfur protein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):556–563. doi: 10.1016/s0006-291x(74)80456-0. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. The soluble "high potential" type iron-sulfur protein from mitochondria is aconitase. J Biol Chem. 1978 Apr 25;253(8):2514–2517. [PubMed] [Google Scholar]
- Villafranca J. J., Mildvan A. S. The mechanism of aconitase action. II. Magnetic resonance studies of the complexes of enzyme, manganese(II), iron(II), and substrates. J Biol Chem. 1971 Sep 25;246(18):5791–5798. [PubMed] [Google Scholar]



