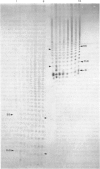
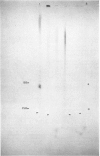
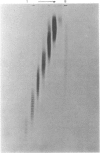
Abstract
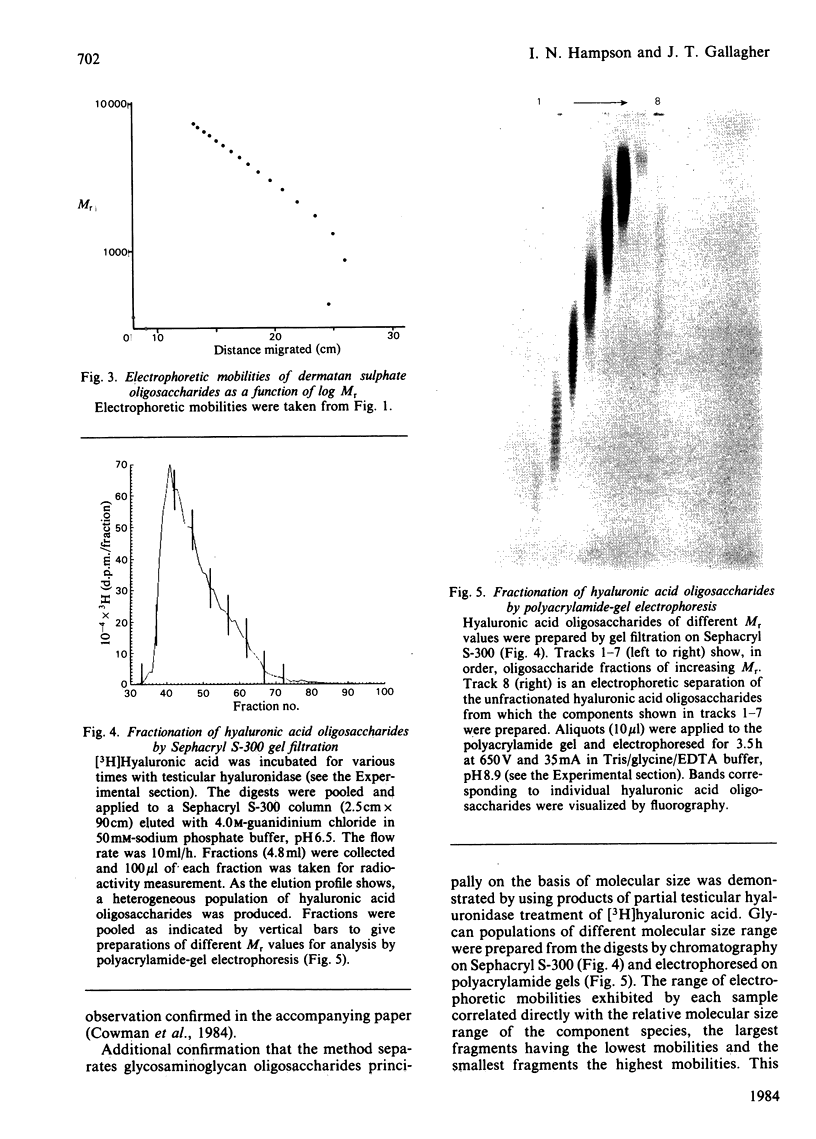
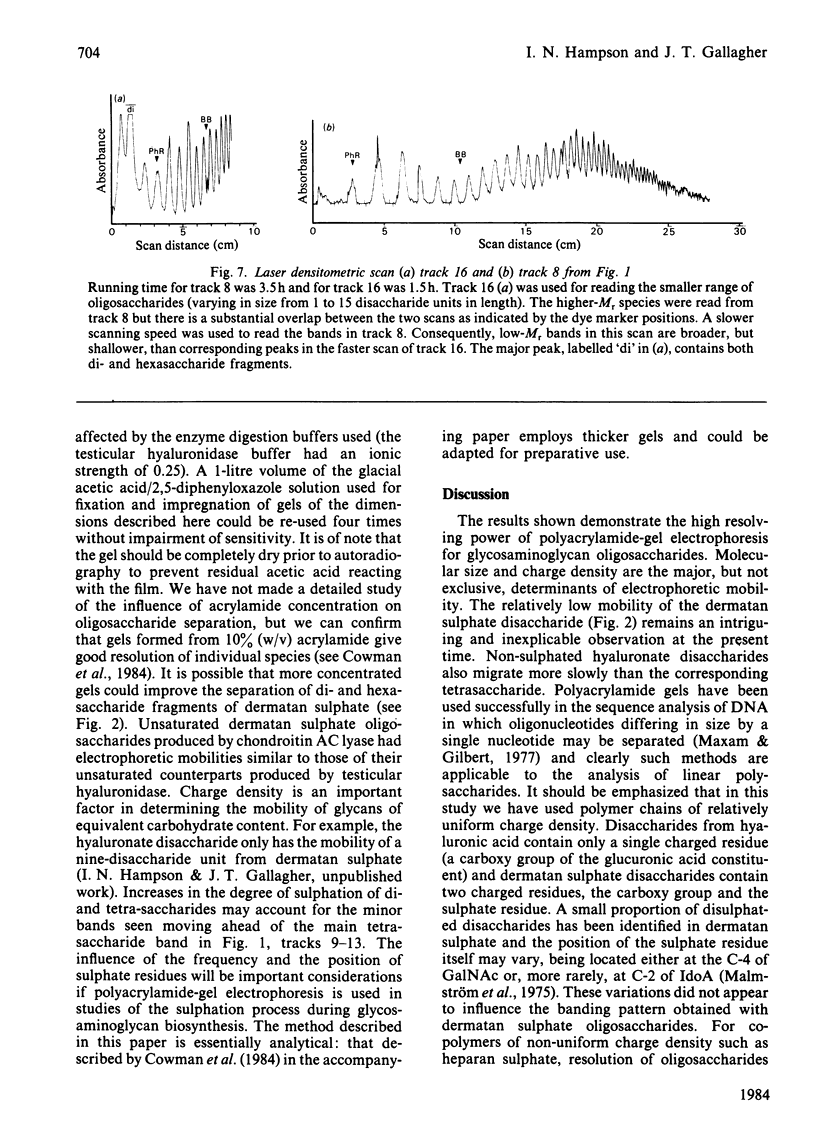
Glycosaminoglycan oligosaccharides generated by treatment of biosynthetically radiolabelled dermatan sulphate and hyaluronic acid with chondroitin AC lyase or testicular hyaluronidase may be resolved into a series of discrete bands by polyacrylamide-gel electrophoresis. Bands were identified by fixation in glacial acetic acid containing 20% (w/v) 2,5-diphenyloxazole followed by fluorography. The bands represented glycans which differed in size by one disaccharide unit. For the larger oligosaccharides (decasaccharides and above) of similar charge: mass ratio, there was a linear relationship between electrophoretic mobility and log Mr. However, the smaller species showed anomalous migration patterns. Consideration of the structures of the fragments produced by the different enzyme treatments suggests that copolymeric and homopolymeric oligosaccharides may be separated by polyacrylamide-gel electrophoresis. There are many potential applications of this technique, foremost amongst them being studies on the molecular size heterogeneity and patterns of enzyme-mediated depolymerization of native glycosaminoglycan chains and investigations into rates of polymer chain elongation and post-polymerization modification reactions so essential to glycosaminoglycan function.
Full text
PDF


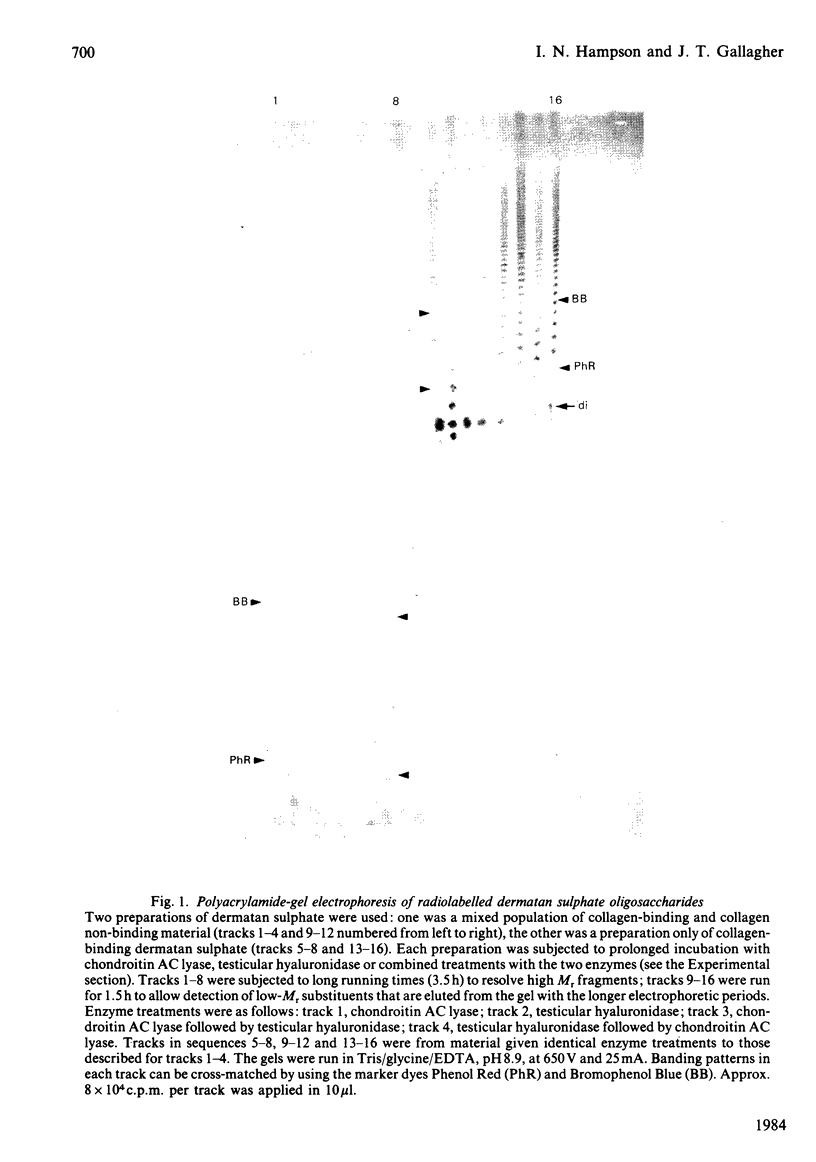



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowman M. K., Slahetka M. F., Hittner D. M., Kim J., Forino M., Gadelrab G. Polyacrylamide-gel electrophoresis and Alcian Blue staining of sulphated glycosaminoglycan oligosaccharides. Biochem J. 1984 Aug 1;221(3):707–716. doi: 10.1042/bj2210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Havsmark B., Sheehan J. K. Self-association of heparan sulfate. Demonstration of binding by affinity chromatography of free chains on heparan sulfate-substituted agarose gels. J Biol Chem. 1981 Dec 25;256(24):13039–13043. [PubMed] [Google Scholar]
- Gallagher J. T., Gasiunas N., Schor S. L. Specific association of iduronic acid-rich dermatan sulphate with the extracellular matrix of human skin fibroblasts cultured on collagen gels. Biochem J. 1983 Oct 1;215(1):107–116. doi: 10.1042/bj2150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Spooncer E., Dexter T. M. Role of the cellular matrix in haemopoiesis. I. Synthesis of glycosaminoglycans by mouse bone marrow cell cultures. J Cell Sci. 1983 Sep;63:155–171. doi: 10.1242/jcs.63.1.155. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Garvin J. H., Jr, Chipman D. M. Mechanism of action of bovine testicular hyaluronidase. Mapping of the active site. J Biol Chem. 1975 Sep 25;250(18):7473–7480. [PubMed] [Google Scholar]
- Hiyama K., Okada S. Crystallization and some properties of chondroitinase from Arthrobacter aurescens. J Biol Chem. 1975 Mar 10;250(5):1824–1828. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Malström A., Carlstedt I., Aberg L., Fransson L. A. The copolymeric structure of dermatan sulphate produced by cultured human fibroblasts. Different distribution of iduronic acid and glucuronic acid-containing units in soluble and cell-associated glycans. Biochem J. 1975 Dec;151(3):477–489. doi: 10.1042/bj1510477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Characterization of the synthase. Biochem J. 1983 Apr 1;211(1):181–189. doi: 10.1042/bj2110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Booth G. M. Improved methods for the fluorographic detection of weak beta-emitting radioisotopes in Agarose and acrylamide gel electrophoresis media. J Biochem Biophys Methods. 1981 Jun;4(5-6):339–346. doi: 10.1016/0165-022x(81)90074-9. [DOI] [PubMed] [Google Scholar]
- Riesenfeld J., Hök M., Lindahl U. Biosynthesis of heparin. Concerted action of early polymer-modification reactions. J Biol Chem. 1982 Jan 10;257(1):421–425. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- WEISSMANN B. The transglycosylative action of testicular hyaluronidase. J Biol Chem. 1955 Oct;216(2):783–794. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]