Abstract
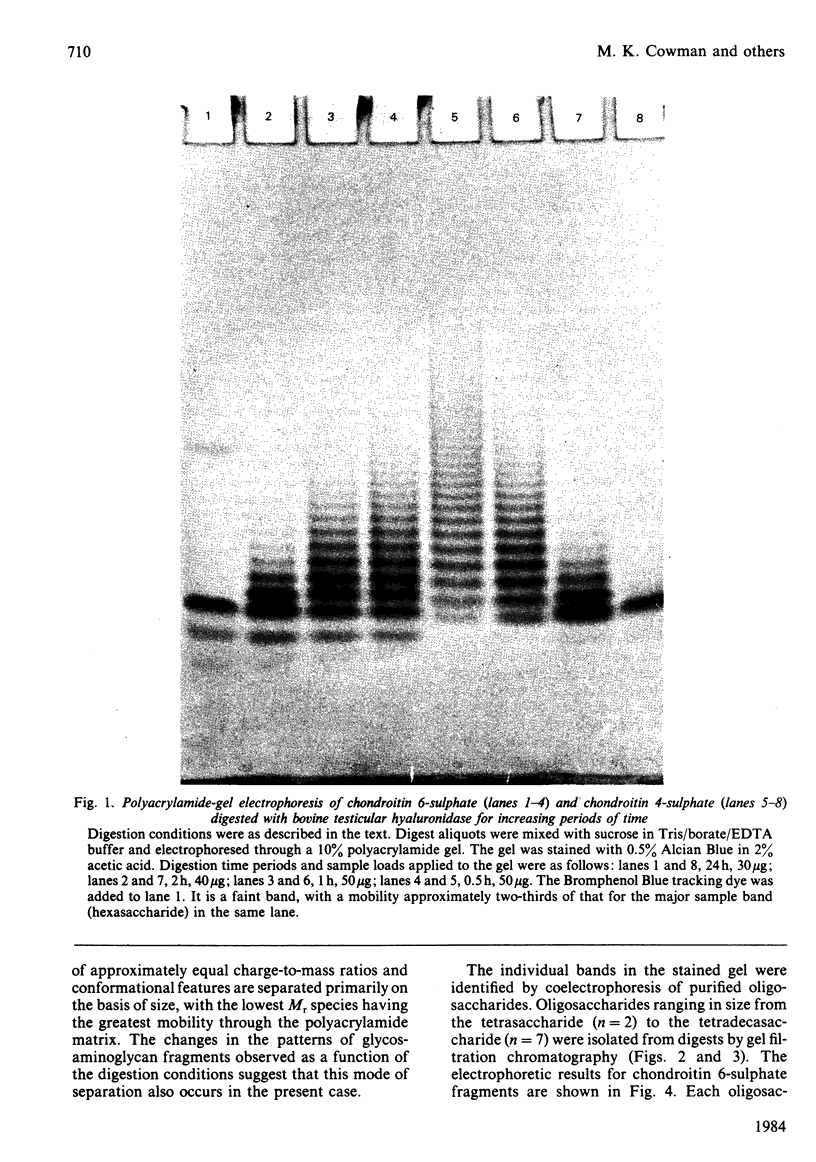
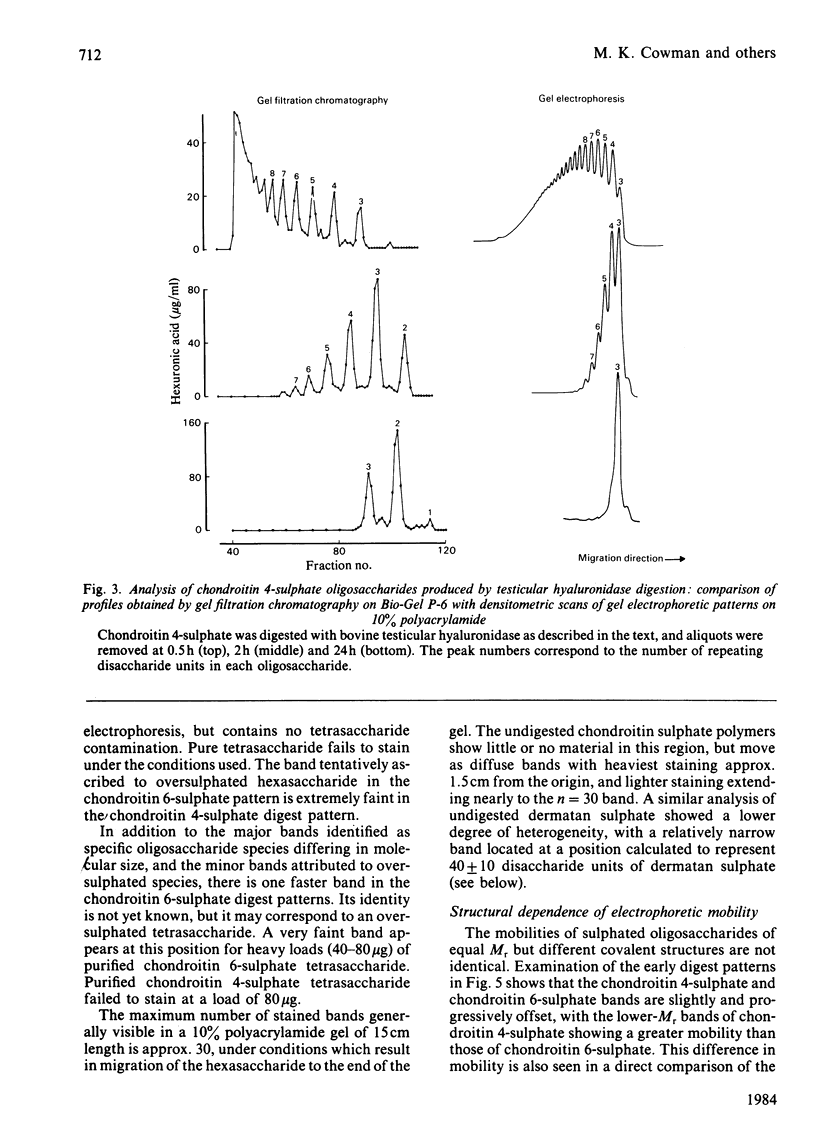
Oligosaccharide fragments of glycosaminoglycans may be separated for rapid analysis by electrophoresis through a 10% polyacrylamide matrix. An extensive ladder-like set of bands is observed for partial testicular hyaluronidase digests of chondroitin 4- or 6-sulphate, and for dermatan sulphate. Co-electrophoresis of purified oligosaccharides has established that the major bands of these patterns represent fragments differing in chain length by one disaccharide unit, with the smallest fragments having the greatest mobility. Additional minor bands, representing heterogeneity in the repeating unit structure, are also observed. There are slight differences in the mobilities of oligosaccharides derived from the three major types of sulphated glycosaminoglycans. Alcian Blue is employed for visualization of the digest fragments. Sample loads of 5-10 micrograms per band appear optimum. The smallest oligosaccharide which may be stained by this method is the hexasaccharide. After consideration of this effect, a good correlation is found to exist between densitometric scans of the gel-electrophoretic patterns and gel-filtration chromatographic profiles based on uronic acid concentration.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Ray D. A., Steck T. L. Electrophoretic determinations of hyaluronate produced by cells in culture. Biochim Biophys Acta. 1972 Mar 30;264(1):73–84. doi: 10.1016/0304-4165(72)90118-3. [DOI] [PubMed] [Google Scholar]
- Balazs E. A., Berntsen K. O., Karossa J., Swann D. A. An automated method for the determination of hexuronic acids. Anal Biochem. 1965 Sep;12(3):547–558. doi: 10.1016/0003-2697(65)90221-6. [DOI] [PubMed] [Google Scholar]
- Christner J. E., Brown M. L., Dziewiatkowski D. D. Interactions of cartilage proteoglycans with hyaluronate. Inhibition of the interaction by modified oligomers of hyaluronate. J Biol Chem. 1979 Jun 10;254(11):4624–4630. [PubMed] [Google Scholar]
- Cowman M. K., Balazs E. A., Bergmann C. W., Meyer K. Preparation and circular dichroism analysis of sodium hyaluronate oligosaccharides and chondroitin. Biochemistry. 1981 Mar 3;20(5):1379–1385. doi: 10.1021/bi00508a053. [DOI] [PubMed] [Google Scholar]
- Cowman M. K., Bush C. A., Balazs E. A. Vacuum-ultraviolet circular dichroism of sodium hyaluronate oligosaccharides and polymer segments. Biopolymers. 1983 May;22(5):1319–1334. doi: 10.1002/bip.360220506. [DOI] [PubMed] [Google Scholar]
- Delaney S. R., Conrad H. E., Glaser J. H. A high-performance liquid chromatography approach for isolation and sequencing of chondroitin sulfate oligosaccharides. Anal Biochem. 1980 Oct;108(1):25–34. doi: 10.1016/0003-2697(80)90689-2. [DOI] [PubMed] [Google Scholar]
- Delaney S. R., Leger M., Conrad H. E. Quantitation of the sulfated disaccharides of heparin by high performance liquid chromatography. Anal Biochem. 1980 Jul 15;106(1):253–261. doi: 10.1016/0003-2697(80)90145-1. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Malmström A. Structure of pig skin dermatan sulfate. 1. Distribution of D-glucuronic acid residues. Eur J Biochem. 1971 Feb 1;18(3):422–430. doi: 10.1111/j.1432-1033.1971.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Rodén L. Structure of dermatan sulfate. I. Degradation by testicular hyaluronidase. J Biol Chem. 1967 Sep 25;242(18):4161–4169. [PubMed] [Google Scholar]
- Fransson L. A., Rodén L. Structure of dermatan sulfate. II. Characterization of products obtained by hyaluronidase digestion of dermatan sulfate. J Biol Chem. 1967 Sep 25;242(18):4170–4175. [PubMed] [Google Scholar]
- Fransson L. A. Structure of dermatan sulfate. 3. The hybrid structure of dermatan sulfate from umbilical cord. J Biol Chem. 1968 Apr 10;243(7):1504–1510. [PubMed] [Google Scholar]
- Fransson L. A. Structure of dermatan sulfate. IV. Glycopeptides from the carbohydrate-protein linkage region of pig skin dermatan sulfate. Biochim Biophys Acta. 1968 Mar 11;156(2):311–316. [PubMed] [Google Scholar]
- HOFFMAN P., MEYER K., LINKER A. Transglycosylation during the mixed digestion of hyaluronic acid and chondroitin sulfate by testicular hyaluronidase. J Biol Chem. 1956 Apr;219(2):653–663. [PubMed] [Google Scholar]
- Hampson I. N., Gallagher J. T. Separation of radiolabelled glycosaminoglycan oligosaccharides by polyacrylamide-gel electrophoresis. Biochem J. 1984 Aug 1;221(3):697–705. doi: 10.1042/bj2210697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hilborn J. C., Anastassiadis P. A. Estimation of the molecular weights of acidic mucopolysaccharides by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Jan;39(1):88–92. doi: 10.1016/0003-2697(71)90465-9. [DOI] [PubMed] [Google Scholar]
- Horner A. A. Electrophoresis of acidic mucopolysaccharides in agarose gel. Can J Biochem. 1967 Jul;45(7):1009–1013. doi: 10.1139/o67-116. [DOI] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Iverius P. H. Distribution of sulphate and iduronic acid residues in heparin and heparan sulphate. Biochem J. 1974 Jan;137(1):33–43. doi: 10.1042/bj1370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C., Solursh M. Biosynthesis of proteoglycans and their assembly into aggregates in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1979 Apr 25;254(8):2600–2609. [PubMed] [Google Scholar]
- MATHEWS M. B., ROSEMAN S., DORFMAN A. Determination of the chondroitinase activity of bovine testicular preparations. J Biol Chem. 1951 Jan;188(1):327–334. [PubMed] [Google Scholar]
- MEYER K., RAPPORT M. M. The hydrolysis of chondroitin sulfate by testicular hyaluronidase. Arch Biochem. 1950 Jul;27(2):287–293. [PubMed] [Google Scholar]
- Malmström A., Fransson L. A. Structure of pig skin dermatan sulfate. 2. Demonstration of sulfated iduronic acid residues. Eur J Biochem. 1971 Feb 1;18(3):431–435. doi: 10.1111/j.1432-1033.1971.tb01260.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Quintarelli G., Scott J. E., Dellovo M. C. The chemical and histochemical properties of Alcian Blue. II. Dye binding of tissue polyanions. Histochemie. 1964 Jul 17;4(2):86–98. doi: 10.1007/BF00306150. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Quintarelli G., Dellovo M. C. The chemical and histochemical properties of Alcian Blue. I. The mechanism of Alcian Blue staining. Histochemie. 1964 Jul 17;4(2):73–85. doi: 10.1007/BF00306149. [DOI] [PubMed] [Google Scholar]
- Seno N., Anno K., Yaegashi Y., Okuyama T. Microheterogeneity of chondroitin sulfates from various cartilages. Connect Tissue Res. 1975;3(1):87–96. doi: 10.3109/03008207509152345. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- WEISSMANN B., MEYER K., SAMPSON P., LINKER A. Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J Biol Chem. 1954 May;208(1):417–429. [PubMed] [Google Scholar]
- WEISSMANN B. The transglycosylative action of testicular hyaluronidase. J Biol Chem. 1955 Oct;216(2):783–794. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]





