Abstract
Background
Diverse bacterial group behaviors are controlled by quorum sensing, a regulatory network of bacterial gene expression based on cell density, and involving communication through chemical signal molecules called autoinducers. Multidisciplinary research in toxigenic Vibrio cholerae the etiologic agent of cholera, appear to suggest group behavior in the ecology, epidemiology, pathogenesis and transmission of the pathogen. This review summarizes latest advances and known aspects of quorum regulated environmental survival form of V. cholerae, and their role in cholera outbreaks, as well as the significance of this knowledge in tracking the pathogen for prevention of cholera.
Main body
Pathogenic V. cholerae naturally exists in aquatic reservoirs, and infects humans, often leading to epidemic outbreaks of cholera. Effective detection and monitoring of the pathogen in surface waters have been a research focus in preventing cholera outbreaks. However, in the aquatic reservoirs, V. cholerae persists mostly in a quiescent state referred to as viable but non-culturable (VBNC), or conditionally viable environmental cells (CVEC), which fail to grow in routine bacteriological culture. The presence of CVEC can, however, be observed by fluorescent antibody based microscopy, and they appear as clumps of cells embedded in an exopolysaccharide matrix. Current studies suggest that CVEC found in water are derived from in-vivo formed biofilms excreted by cholera patients. The transition to CVEC occurs when dilution of autoinducers in water blocks quorum-mediated regulatory responses that would normally disperse the cellular aggregates. Consequently, CVEC are resuscitated to actively growing cells if autoinducers are replenished, either in the laboratory, or naturally by other environmental bacteria or the intestinal microbiota when CVEC are ingested by humans or aquatic animals.
Conclusion
Quorum sensing plays a crucial role in the environmental persistence of toxigenic V. cholerae in a latent state, and their periodic emergence to cause cholera outbreaks. Furthermore, the autoinducer driven resuscitation of these cells may be a basis for improving the detection of V. cholerae in water samples, and monitoring V. cholerae in their aquatic reservoirs in cholera endemic areas.
Keywords: Cholera, Vibrio cholerae, Biofilms, Quorum sensing, Autoinducers, Conditionally viable environmental cells (CVEC)
Background
Epidemics of cholera due to infection with toxigenic Vibrio cholerae O1 continue to be a significant public health problem in many developing countries of Asia, Africa and Latin America [1, 2]. V. cholerae as a species encompasses more than 200 serogroups which exist as a part of the normal bacterial flora of the aquatic ecosystems, although some strains are pathogenic and infect humans. V. cholerae belonging primarily to the O1 or O139 serogroups can cause cholera epidemics, and generally carry a set of virulence genes which encode the factors required for pathogenesis in humans [1, 3]. The serogroup O1 has two major serotypes, Ogawa and Inaba as well as a rarely reported serotype Hikojima. These serotypes have been further classified into two biotypes, classical and El Tor based on several biochemical properties [1]. The major virulence genes carried by the epidemic strains include a prophage encoded enterotoxin, cholera toxin (CT), and a pathogenicity island encoding a pilus colonization factor, toxin coregulated pilus (TCP). Besides, these strains are also known to carry genes for a number of putative accessory virulence factors such as mannose sensitive hemagglutinin (MSHA) pilus, hemolysins, and the RTX toxin [1]. V. cholerae O1 has caused all historically known pandemics of cholera, whereas V. cholerae O139 emerged in late 1992 as a new highly pathogenic strain causing explosive cholera epidemics, and continued to be associated with epidemics during a few subsequent years [3, 4]. However, V. cholerae O139 serogroup has not been reported to cause any cholera epidemic since the last reported O139 outbreak in Bangladesh in 2002 [5]. The virulence associated gene clusters in V. cholerae appear to have a structure that is capable of being propagated horizontally [6], and the genes for the major enterotoxin cholera toxin (CT) is transferred through the filamentous phage CTXφ [7]. The ecosystem comprising of diverse bacterial strains, CTXφ and other phages and genetic elements, and the intestinal environment of the host population may all contribute to the emergence and enrichment of new pathogenic V. cholerae strains through horizontal acquisition of virulence genes by precursor strains with lower virulence potential [1, 8, 9].
When a pathogenic V. cholerae strain becomes endemic in a region, it can persist in aquatic reservoirs, and cholera outbreaks may appear to occur periodically. For example, in the regions of the Ganges Delta of Bangladesh and India, seasonal epidemics are known to occur almost every year. Complex interactions of a multitude of genetic, ecological, and socioeconomic factors may drive the periodicity of the cholera epidemics. In these areas, viable toxigenic V. cholerae O1 can be readily isolated from surface water during cholera epidemics. However, during inter-epidemic periods, it becomes very challenging to culture these bacteria from water using conventional methods, because of their persistence in a quiescent state often referred to as viable but non-culturable (VBNC) or conditionally viable environmental cells (CVEC) [10, 11]. It has been suggested that CVEC are derived from bacterial biofilms formed in vivo during cholera pathogenesis, and the conversion to CVEC occurs when stools of cholera patients contaminate surface water [11, 12].
In cholera endemic regions, the dormant form of pathogenic V. cholerae O1 that exists in aquatic reservoirs is known to periodically revive and amplify prior to occurrence of seasonal epidemics [13]. The mechanism triggering this revival was however, unknown until resuscitation of CVEC in water was demonstrated in the laboratory by exposure to exogenous autoinducers (AIs) [14], which are bacterial signal molecules involved in quorum sensing [15]. Recently, natural resuscitation of CVEC has been proposed to occur in aquatic niches through exposure to AIs produced by V. cholerae non-O1 non-O139, or by the gut microbiota when CVEC are ingested by humans or other animals [16, 17]. The primary purpose of this review is to discuss and summarize the advances in our knowledge of the persistence of toxigenic V. cholerae O1 in a latent form in their reservoirs when there are no apparent cholera cases around, and the mechanism of their resuscitation prior to initiation of an outbreak of cholera.
Persistence of pathogenic V. cholerae in the environment
V. cholerae as a species belongs to a group of organisms which normally reside in the aquatic ecosystems [18], even though nonpathogenic strains are more commonly found in the environment, than the strains which are capable of causing cholera epidemics. This observation is explained by the fact that there are more than 200 different serogroups of V. cholerae, of which only two serogroups namely V. cholerae O1 and O139 carry the full array or virulence genes needed to cause epidemic disease in humans. However, some of the putative virulence genes or their homologues are also dispersed among non-epidemic environmental strains of V. cholerae belonging to diverse serogroups with varying degree of virulence potential, suggesting that the virulence associated factors or their homologues may have alternative function in the aquatic environment [19–21].
Like numerous other bacteria in the aquatic environment, V. cholerae are known to form surface associated bacterial communities known as biofilms. Formation of biofilms on chitin surfaces of crustaceans is enhanced by the TCP pili [19]. Chitin associated V. cholerae have been reported to use chitinase, an enzyme secreted by the bacteria to decompose chitin and obtain nutrients [22]. Furthermore, it has been found that V. cholerae biofilms formed on chitin surfaces are resistant to grazing by protozoa [23]. These observations suggested that while TCP functions as a major intestinal colonization factor during pathogenesis of V. cholerae, it may also promote persistence of V. cholerae O1 in the aquatic environment where TCP facilitates adhesion of the bacteria to other aquatic organisms. Similarly, the Type III secretion system (T3SS) carried by some V. cholerae strains may also have important functions in the environment [24], since V. cholerae come in contact with eukaryotic cells in the marine environment where the bacteria can associate with plankton, copepods, shrimp and insects. Furthermore, V. cholerae in their aquatic reservoirs, have to survive predation by unicellular eukaryotes, and it has been suggested that the type VI secretion system (T6SS) in V. cholerae is involved in the process [25]. The virulence associated secretion (VAS) genes of the T6SS in V. cholerae are essential for their contact-dependent cytotoxicity toward Dictyostelium discoideum, an aquatic amoebae species [25]. Presumably, because of the alternative roles of virulence factors in the environment, particular strains of pathogenic V. cholerae can persist for a long time in their aquatic reservoirs even in the absence of any known incidence of cholera due to that strain, but can potentially reemerge and cause sporadic disease in humans. Likewise, although the O139 serogroup of V. cholerae seems to have disappeared, it is not possible to ascertain whether strains of this epidemic serogroup still persist in any environmental niche. Notably, the classical biotype of V. cholerae O1 which was replaced by the El Tor biotype in 1973, reemerged in Bangladesh in 1982 and coexisted with the El Tor biotype until 1992 [26, 27].
In view of ample evidence from environmental and laboratory studies, the survival and persistence of pathogenic V. cholerae and possible alternative roles of virulence associated factors in the aquatic environment is now widely accepted. Recent studies are also beginning to provide insights of the metabolic state in which toxigenic V. cholerae O1 persist in the aquatic ecosystems, and suggest possible mechanisms which regulate their periodic emergence causing outbreaks of cholera [16, 17]. Originally, various survival states and conditions such as specific association of V. cholerae with aquatic plants or animals, and/or the existence in a dormant but viable form was proposed [10, 28]. It was suggested that in response to stress under unfavorable environmental conditions V. cholerae are converted to a metabolically less active VBNC state, in which the bacteria fail to grow in culture by conventional techniques, but remain potentially viable and infectious [10]. There were considerable debates on the precise nature and metabolic state of VBNC, since their resuscitation beyond reasonable uncertainty could not be demonstrated. Understandably, the ecological and public health significance of any dormant state depends on whether these cells are re-convertible to normal infectious bacteria. Subsequently, as described below a somewhat similar survival state referred to as conditionally viable environmental cells (CVEC) of V. cholerae that respond to quorum sensing autoinducers, and resuscitate to actively growing cells has been described [11, 12, 14]. However, for clarity both the terms VBNC and CVEC have been used interchangeably in this review.
Conditionally Viable Environmental Cells (CVEC) of V. cholerae
As described above, pathogenic V. cholerae O1 can be readily isolated from nearby environmental water sources during a cholera outbreak, whereas isolation of the bacteria from water when there is almost no incidence of cholera is challenging. However, with the development of modified enrichment and culture methods, it has become evident that environmental reservoirs indeed contain considerably more viable pathogenic V. cholerae O1 cells even during the absence of reported cholera cases, than are detected by conventional culture [11, 14, 29]. Since these latent environmental V. cholerae cells could actually be grown in culture by the modified methods [14, 29], for clarity the terminology “conditionally viable environmental cells (CVEC)” has been introduced to describe these cells [11].
Initially, certain simple modifications incorporated in the methods for recovering V. cholerae from water samples began to provide a better understanding of the environmental prevalence of pathogenic V. cholerae. In one of these early methods termed Antibiotic Selection Technique (AST), after usual enrichment for vibrios, the culture was subjected to selection on an antibiotic (to which the target V. cholerae strain was known to be resistant) in order to suppress the growth of numerous other strains of mostly nonpathogenic V. cholerae normally found in water [29]. This modification significantly enhanced isolation of V. cholerae O1 from water samples that were found to be negative for the organism by conventional culture. In another novel method, it was demonstrated that when water samples which were apparently negative for V. cholerae in usual culture were inoculated in the intestines of adult rabbits, culturable V. cholerae O1 could be isolated from the intestinal fluids after several hours [30]. These findings suggested that VBNC or CVEC present in water samples could convert to actively growing culturable cells either on exposure to replenished nutrients or passage in the intestinal environment. Taken together, several studies involving passage in rabbit models, AST, fluctuation analysis, and fluorescent-antibody based microscopy established that CVEC are clumps of partially dormant cells, and these cells can be revived under appropriate in vitro and in vivo conditions [11, 14, 30].
Relationship between CVEC and biofilms
V. cholerae is known to form biofilms, and the metabolic pathway involved in this process has been described [31, 32]. Biofilms are surface associated communities of bacteria, in which the cells are embedded in an exopolysaccharide matrix and protected from adverse environmental conditions. The formation and dispersion of biofilms are regulated by quorum sensing through detection of autoinducers and involving an intricate signal transduction cascade, which are components of the quorum sensing system [31–33]. A number of studies have suggested that quorum sensing also controls expression of virulence genes in V. cholerae, and that the bacteria form biofilms under in-vivo conditions as well while colonizing the host intestine [11, 15, 34, 35]. Consequently, microscopic analysis of stool samples from cholera patients, revealed that the stools contain both free swimming planktonic cells as well as large clumps of V. cholerae cells resembling biofilms [11, 36]. In agreement with these observations, experiments in the rabbit model involving inoculation of pathogenic V. cholerae cells into the ileal loops of adult rabbits, showed the presence of both free swimming planktonic cells and biofilm-like clumped cells in the ileal loop fluids recovered after 16 h of inoculation [12]. It was further demonstrated that when cholera stools are diluted in filter sterilized environmental water samples, CVEC are formed from the clumps of V. cholerae cells excreted in the stools. Comparative genetic fingerprinting of V. cholerae isolates derived from naturally occurring CVEC, and isolates from cholera patients suggested that V. cholerae cells that enter into the CVEC state typically exist as clumps of cells that comprise a single clone closely related to isolates causing the most recent local cholera epidemic. These findings are consistent with the suggestion that in cholera endemic areas, contamination of water by stools of cholera patients may lead to the formation of CVEC, which are also the abundant environmental survival form of the pathogen [11, 12].
Evidence for association of quorum sensing with CVEC
Attempts have been made to understand the mechanisms involved in the transition of V. cholerae to the CVEC state. Formation of surface associated biofilms, or cellular aggregates in bacterial suspension, and colonization of the intestine have been found to be influenced by the V. cholerae quorum sensing system [31–34, 37]. Fluorescent antibody based microscopic examination of surface water samples revealed the presence of naturally occurring CVEC, and these cells appeared to be organized in aggregates embedded in an exopolysachharide material [11]. The genes responsible for production of Vibrio extracellular polysaccharides are controlled through the quorum sensing pathway that includes autoinducers (AIs) and their cognate receptors, as well as a signal transduction cascade [31–33].
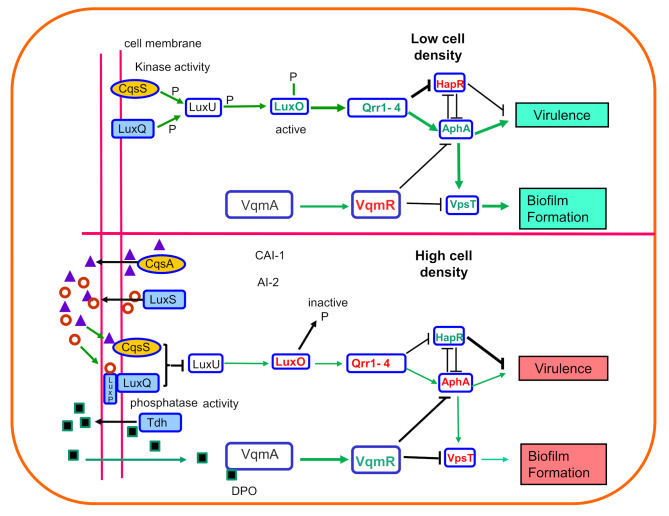
In the V. cholerae quorum sensing system (Fig. 1), AI signal molecules are sensed by their respective receptors and the signals from the sensory circuits converges to LuxO, the response regulator which negatively controls HapR, a transcriptional regulator that in turn controls several genes involved in the formation or dissolution of biofilms or in intestinal colonization. Considering the clumped organization of cells observed in CVEC, possible association of quorum sensing with CVEC formation has been further explored by creating and analyzing genetic mutants with altered quorum sensing ability. Subsequently, it has been shown that mutations in crucial genes required for quorum sensing and biofilm formation also impair CVEC formation by V. cholerae, when such mutants are added into environmental water samples following intestinal infections [12]. For example, in laboratory experiments, an isogenic strain carrying a mutation in the hapR gene produced more robust biofilms than the wild type strain, and formed CVEC when inoculated in water after passage in the rabbit intestine. On the other hand, a luxO mutant produced very little biofilm as compared to the wild type strain, and also failed to form CVEC under the above condition. Generally, mutations which enhance or block the formation of quorum regulated biofilms were found to similarly enhance or block the formation of CVEC. Mutants that produced more robust biofilms under in vitro conditions, also produced more compact and larger clumps of cells when inoculated in the intestine of adult rabbits. These mutants were more capable in forming CVEC when introduced into water samples after passage in the rabbit ileal loops [12]. The above studies concluded that CVEC are in fact derived from in vivo-formed bacterial biofilms, and are formed when aquatic reservoirs are contaminated by stools of cholera patients. It has been further proposed that while in the water, rapid dilution of AIs resulting in an inability to sense these signals by the clumped cells enhance the process of CVEC formation [12]. The biofilm associated cells enter into a quiescent metabolic state as dilution of AIs blocks quorum-mediated regulatory responses that would normally disperse the cellular aggregates excreted in the stools of cholera patients [11, 14]. In agreement with these observations, Wu et al. [38] demonstrated that a hapR mutant of V. cholerae O1 rapidly enters into a dormant VBNC state as compared to the wild type strain when incubated at 4oC in artificial sea water, whereas upregulation of hapR results in a prolonged culturable state.
Fig. 1.
Quorum sensing systems in Vibrio cholerae. Quorum sensing in V. cholerae involves at least three autoinducers (CAI-1, AI-2 and DPO) and their respective receptors CqsS, LuxP, and VqmA together with intricate signal transduction cascades. The autoinducers CAI-1 and AI-2 are produced by autoinducer synthase CqsA and LuxS respectively, whereas DPO is derived from threonine catabolism, and requires threonine dehydrogenase (Tdh). The CAI-1 and AI-2 are sensed by the membrane-bound receptors CqsS and LuxPQ which channel information into a common regulatory pathway. Conversely, DPO is detected by the VqmA receptor-transcription factor in the cytoplasm. VqmA in turn activates expression of vqmR gene that encodes the VqmR small RNA (sRNA) which regulates target mRNAs. At low autoinducer concentrations (low cell concentrations), CqsS and LuxPQ act as kinases to phosphorylate LuxU. Phosphorylated LuxU transfers the phosphate to LuxO, and LuxO-P induces the expression of the Qrr1–4 sRNAs. The Qrr sRNAs repress hapR and activate aphA, promoting virulence gene expression and biofilm formation. AphA also activates the transcription of vpsT. At high cell concentration (autoinducer concentrations), on binding of CAI-1 and AI-2 to CqsS and LuxPQ, respectively, the receptors act as phosphatases, which reduces LuxO-P levels and inhibits qrr1–4 expression. Under these conditions, aphA is repressed and hapR is activated. The VqmA-DPO complex induces the transcription of the VqmR sRNA. VqmR inhibits biofilm formation by repressing VpsT and virulence gene expression by inhibiting AphA. In summary, virulence and biofilm formation are upregulated at low cells density (shown in green). At high cell density, all three QS systems repress virulence and biofilm formation (shown in red). Active key factors are shown in green whereas repressed or inactive factors are shown in red font
Response of CVEC to exogenous autoinducers
In an attempt to verify the above assumption that CVEC are formed because the clumped cells fail to sense sufficient concentration of AIs which are vastly diluted in the water, it was explored whether supplementation of the enrichment culture medium for isolation of V. cholerae from water samples with exogenous AIs would reverse the process, and lead to the dispersion and/or resuscitation of CVEC. It was found that the quiescent form of pathogenic V. cholerae that exist in aquatic reservoirs can be converted to actively growing ‘culturable’ cells by exposure to either of two different AIs, namely cholera autoinducer-1 (CAI-1) and AI-2 produced exogenously [14, 16]. The observations that CVEC formation is enhanced in an AI depleted state (due to dilution in water) [12], and the ability of these clumped cells to respond to AIs and resuscitate into actively growing planktonic cells suggest that the pathways for formation and dispersion of biofilms of V. cholerae, and that of CVEC are linked and both involve quorum sensing. In another study with V. vulnificus, it was reported that autoinducer AI-2 could induce resuscitation of the VBNC form of V. vulnificus [39], and VBNC cells of a mutant unable to produce AI-2 were resuscitated when cultures were supplemented with exogenous AI-2. Furthermore, a quorum sensing inhibitor cinnamaldehyde delayed the resuscitation, thus confirming the importance of quorum sensing in the resuscitation process [39].
Resuscitation of CVEC under natural conditions
The CAI-1 is produced by a narrow range of vibrio species [40], whereas AI-2 is produced by numerous bacterial species in the environment [41, 42]. Therefore, it is possible that fully viable cells of pathogenic V. cholerae can emerge from the CVEC state in the environment in response to AIs produced by the same or other bacterial species. It was explored whether possible genetic variants of V. cholerae which overproduce one or more AIs exist in nature, since hypothetically such variants could produce AIs at a sufficiently high concentration to enhance the resuscitation of CVEC in water. As a proof of concept, initially screening of a mutant library of a typical V. cholerae O1 strain C6706 [43] was conducted to identify possible mutants which could overproduce AIs, and indeed a derivative of the parent strain was identified which markedly overproduced AI-2 compared to the parent strain [16]. Furthermore, environmental surveillance confirmed the abundance of AI-2 hyper-producing variants of V. cholerae in natural waters [16, 17]. Remarkably, AI-2 produced in the culture supernatants of these variant strains were also found to enhance resuscitation of CVEC in environmental water samples in the laboratory. Notably, natural V. cholerae O1 isolates with different quorum sensing functions and constitutive expression of quorum sensing genes with a variety of abilities to remain culturable or enter into the dormant state have been described [38].
Role of non-cholera vibrios
V. cholerae belonging to the non-O1 non-O139 serogroups (non-cholera-vibrios) are abundant in aquatic ecosystems, and naturally occurring variant strains of V. cholerae non-O1 non-O139 which overproduce AI-2 have also been identified [17]. Similar to the V. cholerae O1 strains, the AI-2 produced in the culture supernatant of the non-O1 non-O139 variant strains has been found to resuscitate CVEC in water samples. Temporal variation in environmental prevalence of such AI-2 hyper-producing non-O1 non-O139 strains and their co-occurrence with V. cholerae O1 in water samples have been monitored [17]. Increased prevalence of V. cholerae O1 in surface was found to coincide with an increase in AI-2 overproducing V. cholerae non-O1 non-O139 strains. These observations suggest that V. cholerae non-O1 non-O139 variant strains overproducing AI-2 presumably contribute in resuscitation of the latent pathogen, prior to seasonal cholera epidemics.
Role of host intestinal environment
It is now recognized that the dormant environmental form of toxigenic V. cholerae O1 are potentially infectious, and ingestion of these forms can cause disease. In agreement with this assumption when water samples which were apparently negative for V. cholerae O1 in conventional culture, were introduced into rabbit intestine, actively growing toxigenic V. cholerae O1 could be recovered from the intestinal fluids [30]. The recent demonstration of the effect of exogenous AIs on the resuscitation of CVEC sheds light on how CVEC might be revived in the intestinal environment. It follows that since AI-2 is produced by numerous bacterial species, the resuscitation of CVEC in response to AIs could also occur inside the host through exposure to AIs produced by the normal intestinal microbiota. Therefore, CVEC could also be resuscitated into actively growing cells when ingested by humans or aquatic animals.
Example of one such aquatic host could be the vertebrate fish species Danio rerio, widely known as zebrafish. Both V. cholerae and zebrafish are abundant in natural waters in the Indian subcontinent where cholera is endemic, and the zebrafish can be rapidly colonized by V. cholerae [44]. Moreover, intestinal colonization of zebrafish by V. cholerae leads to bacterial multiplication, and development of diarrhea excreting V. cholerae [44, 45]. The zebrafish is likely to be a natural aquatic host for V. cholerae, as colonization of the zebrafish intestine occurs via a natural process when exposed to V. cholerae, without requiring any manipulation. Given that the zebrafish is known to have a core gut microbiota [46], it seems possible that the intestinal microbiota might contribute to the resuscitation of CVEC when ingested by zebrafish. There may be a natural association between zebrafish and V. cholerae in certain settings overlapping regions of cholera endemicity, and the habitat of the zebrafish [47].
CVEC resuscitation and seasonal cholera outbreaks
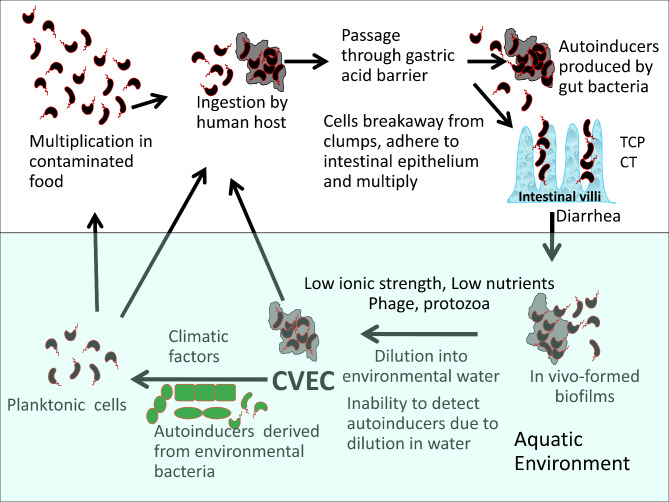
An important aspect of cholera epidemiology in endemic areas is the periodic occurrence of epidemics and persistence of the pathogen in the aquatic reservoirs predominantly in a ‘nonculturable’ or CVEC state during the inter-epidemic period. The model described in Fig. 2, seeks to explain likely natural mechanism of CVEC resuscitation and its association with the occurrence of cholera outbreaks. Prior to the epidemic, environmental parameters (e.g., temperature, salinity, presence of aquatic hosts, etc.) may cause blooms of diverse bacterial species that produce AIs, particularly AI-2 which is known to be produced and sensed by numerous bacterial species including non-cholera vibrios which are abundant in natural waters [17, 48, 49]. In response to the AIs, nearby CVEC form of V. cholerae may resuscitate to the actively growing state. Moreover, the presence of AI-2 overproducing variants of environmental bacteria [17] might enhance this process, and lead to an index case of cholera (Fig. 2).
Fig. 2.
Role of CVEC and diverse environmental and host factors in cholera outbreaks. Diagrammatic representation of the formation and resuscitation of CVEC, and their involvement with environmental and host factors in the occurrence of periodic cholera outbreaks. Stools of cholera patients contain V. cholerae as a heterogenous mixture of active planktonic cells, and biofilm-like clumped cells. Upon introduction of cholera stools into environmental water which vastly dilutes extracellular autoinduecrs (AIs), a temporary loss of quorum sensing occurs. As a result, quorum-mediated regulatory responses that would normally disperse biofilm-associated cells are blocked and the cells exist embedded in thick exopolysaccharides in a dormant form referred to as CVEC. Presumably, the CVEC form of V. cholerae survives phage predation, and persists in water during the inter-epidemic time. The CVEC can provide a large dose of the pathogen when ingested by a potential victim, and may be resuscitated in the intestine in response to AIs produced by the gastrointestinal microbiota. On the other hand, CVEC may be resuscitated in the environment when climatic factors lead to multiplication of diverse environmental bacteria many of which produce AIs. The resulting planktonic cells seed the environment for rapid spread and amplification of the strain, together with selective enrichment of the pathogen in the human gastrointestinal environment. The gradual build up of the pathogenic clone in the environmental water and consumption of contaminated food or drinks may causes an index case of cholera and may eventually initiate an epidemic
Resuscitation of CVEC might also occur in the human gut through the influence of AIs produced by the human gastrointestinal microbiota, when CVEC in water are ingested by a potential victim. Epidemics may be preceded by a gradual enrichment of the pathogenic V. cholerae through passage in humans who consume surface water. The role of the human host in selective amplification of pathogenic V. cholerae O1 from the majority of environmental non-pathogenic V. cholerae has been proposed to be a crucial step that leads the onset of an epidemic [30]. Studies have also suggested that passage in the human intestine is linked with the generation of a hyperinfectious form of the pathogen [50], and that cholera transmission dynamics involve multiple host and environmental factors [51]. Because of the hyperinfectivity, cholera spreads rapidly through water contaminated with the stools of cholera patients. Moreover, it has been shown that stools of cholera patients contain large clumps of V. cholerae cells, and so ingestion of a clump of cells facilitates the delivery of a high dose of the pathogen to a potential victim. Once the epidemic subsides due to predation of the causative bacteria by phages [51–53] or other interventions, the remnant clumps of epidemic V. cholerae cells presumably continue to persists in the water as aggregates of latent cells in the CVEC state. This model of cholera epidemiology appears to unify several observations involving both environmental and host factors in the initiation of periodic epidemics and also explains the limited clonality apparent in the strains causing the cholera epidemics.
Conclusions and future directions
The findings from numerous studies discussed here can explain the mode of environmental persistence of pathogenic V. cholerae in which CVEC originate from aggregates of V. cholerae cells excreted in the stools of cholera patients, upon introduction into environmental water [11, 12]. During an epidemic both free-swimming planktonic form and clumped CVEC form of V. cholerae are found in water. On the other hand, during the inter-epidemic periods the pathogen predominantly exist in aquatic reservoirs in the CVEC state, and therefore, the prevalence of the pathogen in water can not be accurately estimated using conventional culture methods when there are no reported cholera cases in the area. Several studies could further explain aspects of reappearance of the pathogen in an actively growing state leading to cholera outbreaks in endemic regions such as the Ganges Delta [16, 17]. The seasonal epidemics may be caused by pathogenic strains resuscitated from the CVEC state caused by exogenous AIs produced by diverse environmental bacteria or by the intestinal microbiota, if CVEC are ingested by animals or humans. Asymptomatic infections of humans may account for the pre-epidemic buildup of epidemic strains which then initiate the index case of cholera.
Cholera is known to spread through water contaminated with V. cholerae excreted in the stools of cholera patients. Therefore, increased survival of the pathogen in water is likely to favor epidemic or pandemic spread of the pathogen, as well as its persistence in aquatic reservoirs after the epidemic, as a potential threat of future cholera outbreaks. Monitoring of the presence and concentration of the pathogen in surface water is essential to track the pathogen and control the initiation and spread of an epidemic in a community. Enrichment techniques incorporating exogenous AIs CAI-1 or AI-2 have been shown to remarkably improve the assays for detecting V. cholerae in environmental water samples [14, 16, 17]. Therefore, use of modified techniques using exogenous AIs (either chemically synthesized or produced by cloning relevant genes) may be recommended for more accurate detection of pathogenic V. cholerae in water. Since quorum sensing systems are present in numerous bacterial species, the applicability of the improved enrichment culture might also be useful for detection of other waterborne bacterial pathogens beyond V. cholerae as such. Further studies could be designed and conducted to test this possibility. Lastly, despite significant advances in understanding the survival state in which pathogenic V. cholerae persist in aquatic reservoirs during inter-epidemic periods, and their resuscitation prior to epidemics, there is scope for further investigations to more completely appreciate the role of diverse natural factors and the precise genetic regulation that lead to the formation and reactivation of the CVEC state of pathogenic V. cholerae.
Acknowledgements
Not applicable.
Author contributions
S.N.F. wrote initial draft of the manuscript text and prepared Figure-1; S.Y. provided academic feedback and contributed to scientific editing; S.M.F. provided overall guidance and concept of the review, and proposed the model in Figure-2. All authors reviewed the manuscript.
Funding
Research by Shah M. Faruque is funded in part by the Wellcome Trust through a senior investigator award no. WT-105927/Z/14/Z.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the manuscript for publication. The manuscript does not contain any individual person’s data in any form.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Cholera Annual Report 2022. Weekly Epidemiological Record 38. 2023; 98:431 – 52.
- 3.Ramamurthy T, Garg S, Sharma R, Bhattacharya SK, Nair GB, Shimada T, Takeda T, Karasawa T, Kurazano H, Pal A, Takeda Y. Emergence of a novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;341:703–4. [DOI] [PubMed] [Google Scholar]
- 4.Faruque SM, Sack DA, Colwell RR, Sack RB, Nair GB. Emergence and evolution of Vibrio cholerae O139. Proc Natl Acad Sci USA. 2003;100:1304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faruque SM, Chowdhury N, Kamruzzama M, Ahmad QS, Faruque AS, Salam MA, Ramamurthy T, Nair GB, Weintraub A, Sack DA. Reemergence of epidemic Vibrio cholerae O139, Bangladesh. Emerg Infect Dis. 2003;9:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faruque SM, Mekalanos JJ. Pathogenicity islands and phages in Vibrio cholerae evolution. Trends Microbiol. 2003;11:505–10. [DOI] [PubMed] [Google Scholar]
- 7.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous bacteriophage encoding cholera toxin. Science. 1996;272:1910–14. [DOI] [PubMed] [Google Scholar]
- 8.Hassan F, Kamruzzaman M, Mekalanos J, Faruque SM. Satellite phage TLCφ enables toxigenic conversion by CTX phage through dif site alteration. Nature. 2010;467:982–5. 10.1038/nature09469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faruque SM, Asadulghani, Saha MN, Alim ARMA, Albert MJ, Islam KMN, Mekalanos JJ. Analysis of clinical and environmental strains of nontoxigenic Vibrio cholerae for susceptibility to CTXF: molecular basis for the origination of new strains with epidemic potential. Infect Immun. 1998;66:5819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colwell RR, Huq A. Vibrios in the environment: viable but non culturable Vibrio cholerae. In: Wachsmuth IK, Blake PA, Dlsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington DC, ASM Press. 1994; pp. 117–133.
- 11.Faruque SM, Biswas K, Udden SM, Ahmad QS, Sack DA, Nair GB, Mekalanos JJ. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci USA. 2006;103:6350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamruzzaman M, Udden SMN, Cameron DE, Calderwood SB, Nair GB, Mekalanos JJ, Faruque SM. Quorum-regulated biofilms enhance the development of conditionally viable, environmental Vibrio cholerae. Proc Natl Acad Sci USA. 2010;107:1588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver JD. The public health significance of viable but nonculturable bacteria. In: Colwell RR, Grimes DJ, editors. Nonculturable microorganisms in the Environment. Washington DC: ASM; 2000. p.277 – 99. [Google Scholar]
- 14.Bari SM, Roky MK, Mohiuddin M, Kamruzzaman M, Mekalanos JJ, Faruque SM. Quorum-sensing autoinducers resuscitate dormant Vibrio cholerae in environmental water samples. Proc Natl Aca Sci USA. 2013;110:9926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–6. [DOI] [PubMed] [Google Scholar]
- 16.Naser IB, Hoque MM, Faruque SN, Kamruzzaman M, Yamasaki S, Faruque SM. Vibrio cholerae strains with inactivated cqsS gene overproduce autoinducer-2 which enhances resuscitation of dormant environmental V. cholerae. PLoS ONE. 2019;14:e0223226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naser IB, Shishir TA, Faruque SN, Hoque MM, Hasan A, Faruque SM. Environmental prevalence of toxigenic Vibrio cholerae O1 in Bangladesh coincides with V. Cholerae non-O1 non-O139 genetic variants which overproduce AI-2. PLoS ONE. 2021;16:e0254068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colwell RR. Infectious disease and environment: cholera as a paradigm for waterborne disease. Int Microbiol. 2004;7:285–9. [PubMed] [Google Scholar]
- 19.Reguera G, Kolter R. Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J Bacteriol. 2005;187:3551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiavelli DA, Marsh JW, Taylor RK. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl Environ Microbiol. 2001;67:3220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watnick PI, Fullner KJ, Kolter R. A role for the mannose sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J Bacteriol. 1999;181:3606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahar S, Sultana M, Naser MN, Nair GB, Watanabe H, Ohnishi M, Yamamoto S, Endtz H, Cravioto A, Sack RB, Hasan NA, Sadique A, Huq A, Colwell RR, Alam M. Role of shrimp chitin in the ecology of toxigenic Vibrio cholerae and cholera transmission. Front Microbiol. 2012;2:260. 10.3389/fmicb.2011.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S, Tay QXM, Kjelleberg S, Rice SA, McDougald D. Quorum sensing-regulated chitin metabolism provides grazing resistance to Vibrio cholerae biofilms. ISME J. 2015;9:1812–20. 10.1038/ismej.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dziejman M, Serruto D, Tam VC, Sturtevant D, Diraphat P, Faruque SM, Rahman MH, Heidelberg JF, Decker J, Li L, Montgomery KT, Grills G, Kucherlapati R, Mekalanos JJ. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci USA. 2005;102:3465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci USA. 2006;103:1528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan MU, Samadi AR, Huq MI, Greenough WB. Reappearance of classical Vibrio cholerae in Bangladesh. Advances in Research on Cholera and Related Diarrheas. Tokyo: KTK Scientific; 1986. pp. 3–12. [Google Scholar]
- 27.Faruque SM, Alim ARMA, Rahman MM, Siddique AK, Sack RB, Albert MJ. Clonal relationships among classical Vibrio cholerae O1 strains isolated between 1961 and 1992 in Bangladesh. J Clin Microbiol. 1993;31:2513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colwell RR, Spira WM. (1992). The ecology of Vibrio cholerae. In Cholera, Barua D., and Greenough III WB. editors, Plenum Medical Book Co., New York, USA. pp. 107–127.
- 29.Faruque SM, Islam MJ, Ahmad QS, Biswas K, Faruque AS, Nair GB, Sack RB, Sack DA, Mekalanos JJ. An improved technique for isolation of environmental Vibrio cholerae with epidemic potential: monitoring the emergence of a multiple-antibiotic-resistant epidemic strain in Bangladesh. J Infect Dis. 2006;193:1029–36. [DOI] [PubMed] [Google Scholar]
- 30.Faruque SM, Chowdhury N, Kamruzzaman M, Dziejman M, Rahman MH, Sack DA, Nair GB, Mekalanos JJ. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc Natl Acad Sci USA. 2004;101:2123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50:101–4. [DOI] [PubMed] [Google Scholar]
- 32.Hammer BK, Bassler BL. Inaugural article: Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc Natl Acad Sci USA. 2007;104:11145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5:647–6. [DOI] [PubMed] [Google Scholar]
- 35.Silva AJ, Benitez JA. Vibrio cholerae biofilms and cholera pathogenesis. PLoS Negl Trop Dis. 2016;10:e0004330. 10.1371/journal.pntd.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson EJ, Chowdhury A, Harris JB, Begum YA, Chowdhury F, Khan AI, LaRocque RC, Bishop AL, Ryan ET, Camilli A, Qadri F, Calderwood SB. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. PNAS. 2007;104:19091–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jemielita M, Wingreen NS, Bassler BL. Quorum sensing controls Vibrio Cholere multicellular aggregate formation eLife 2018; 7:e42057. 10.7554/eLife.42057 [DOI] [PMC free article] [PubMed]
- 38.Wu B, Liang W, Yan M, Li J, Zhao H, Cui L, Zhu F, Zhu J, Kan B. Quorum sensing regulation confronts the development of a viable but non-culturable state in Vibrio cholerae. Environ Microbiol. 2020;22:4314–22. 10.1111/1462-2920.15026. [DOI] [PubMed] [Google Scholar]
- 39.Ayrapetyan M, Williams TC, Oliver JD. Interspecific quorum sensing mediates the resuscitation of viable but Nonculturable Vibrios. Appl Environ Microbiol. 2014;80:2478–83. 10.1128/AEM.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters CM, Bassler BL. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 2006;20:2754–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xavier KB, Bassler BL. LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol. 2003;6:191–7. [DOI] [PubMed] [Google Scholar]
- 43.Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc Natl Acad Sci USA. 2008;105:8736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol. 2014;80:1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nag D, Mitchell K, Breen P, Withey JH. Quantifying Vibrio cholerae colonization and diarrhea in the adult zebrafish model. J Vis Exp. 2018;137:57767. 10.3791/57767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011;5:1595–608. 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 48.Pereira CS, Thompson JA, Xavier KB. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev. 2013;37:156–81. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Li S, Liu X, Wang Z, Jiang M, Wang R, Xie L, Liu Q, Xie X, Shang D, Li M, Wei Z, Wang Y, Fan C, Luo ZQ, Shen X. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat Commun. 2020;11:5371. 10.1038/s41467-020-19243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417:642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson E, Harris J, Glenn Morris J, et al. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7:693–702. 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faruque SM, Naser IB, Islam MJ, Faruque AS, Ghosh AN, Nair GB, Sack DA, Mekalanos JJ. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci U S A. 2005;102:1702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faruque SM, Islam MJ, Ahmad QS, Faruque AS, Sack DA, Nair GB, Mekalanos JJ. Self-limiting nature of seasonal cholera epidemics: role of host-mediated amplification of phage. Proc Natl Acad Sci USA. 2005;102:6119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.