Abstract
Background
Neuronal ceroid lipofuscinosis (NCL) is a heterogeneous group of 13 rare, progressive neurodegenerative diseases of the brain and retina. CLN14 is a very rare subtype of NCL caused by pathogenic variants in the KCTD7 gene. Only four cases of this subtype have been reported in the literature.
Case presentation
A nine-month-old, previously healthy male who was firstborn to first-cousin parents presented with progressive psychomotor regression, dysmorphic facial features, myoclonus, and vision loss. Neurological examination showed generalized hypotonia and brisk reflexes. He continued to deteriorate until age 18 months, when he developed his first generalized tonic-clonic seizure. An ophthalmological examination showed a hypopigmented fundus and slight temporal disc pallor. Brain MRI showed mild generalized brain atrophy and white matter disease. EEG revealed a severely abnormal trace marked by generalized, high amplitude, sharply contoured, polymorphic delta slowing intermixed with theta slowing and some alpha activity, with disorganized and scattered spikes and sharp waves. The patient continued to have uncontrolled seizures and further psychomotor regression until he died of status epilepticus and pneumonia at the age of 44 months. WES identified a novel homozygous variant c.413T > C, p.(Leu138Pro) in the KCTD7 gene, causing an amino acid transition from leucine to proline at position 138. Both parents were carriers of the same variant.
Conclusions
We present the fifth known case of CLN14 in the literature and report the clinical course and a novel underlying likely causative variant in the KCTD7 gene. The improving accessibility and affordability of genetic testing will likely uncover more NCL cases and further expand the disease’s genotypic and phenotypic spectrum.
Keywords: Infantile neuronal Ceroid Lipofuscinosis; CLN14; Neurodegenerative diseases; Genetic diseases, Inborn; Epilepsy
Background
Neuronal ceroid lipofuscinosis (NCL) is a heterogeneous group of progressive neurodegenerative diseases of the brain and, often, the retina that is marked by a pathological intracellular accumulation of autofluorescent lipopigments (ceroid lipofuscin or similar materials) in neurons and other tissues [1]. The NCLs are genetically mediated and are typically autosomal recessive [2]. Although common among lysosomal storage diseases, NCLs are generally rare. Estimates vary between countries and geographic locations. Incidence is estimated to range between 1 and 8 per 100,000 people, and the prevalence between 1 and 4 per million [2, 3]. Due to the rarity of the condition and diagnostic obstacles, incidence figures rely mainly on literature from Western countries [2, 3].
Many studies and case series have demonstrated the condition’s clinical, pathological, and genetic heterogeneity [4–6]. This inherent heterogeneity and the discovery of an increasing list of causative genetic pathogenic variants have led to the old classification based on clinical phenotypes (age of onset and order of appearance of clinical features) being deemphasized in favor of a gene-based classification [7]. Presently, the NCLs include 13 confirmed forms that result from a genetic deficiency in genes CLN1 to CLN14 [2, 8].
Clinically, all NCL forms share the progressive course but exhibit varying durations of illness. Early-onset NCLs are generally more severe and carry a poorer prognosis [2]. Patients who present in early or late infancy may appear normal at birth but subsequently develop rapid intellectual, visual, and motor deterioration, with intractable seizures, myoclonus, and early death. Severe brain atrophy in those patients led to the term “walnut kernel brain” [2, 3, 9]. On the other hand, juvenile-onset patients present with progressive visual loss but may exhibit less marked seizures and brain atrophy. Meanwhile, patients who first manifest the disease during adulthood present with dementia and seizures, but visual abnormalities are usually absent [2, 3]. Patients with a later onset overlap phenotypically with progressive myoclonic epilepsy (PME). The pathologic hallmarks of NCL are the autofluorescent pigment and the ultrastructural intracytoplasmic deposits observed on electron microscopy (EM) of the brain and other tissues [2, 10]. The different clinical forms exhibit significant overlapping of electron microscopy findings. Thus, diagnosis increasingly rests on molecular genetic testing and, in some subtypes, may be supported by enzyme activity assays [11].
CLN14 is one of the diseases caused by biallelic loss of function of the KCTD7 gene [12]. KCTD7 is widely expressed in the brain and is believed to help regulate neuronal potassium conductance [13]. The spectrum of conditions caused by biallelic loss of KCTD7 function is rare overall [13]. Pathogenic KCTD7 variants usually cause a rare genetic form of PME (PME3), which manifests with early onset drug-resistant epilepsy, frequent myoclonic seizures, and neuro-regression. However, very few cases presented inclusions on skin biopsy and a more severe and rapidly progressing phenotype of NCL (CLN14) [13]. This subtype of NCL was first described in 2012 and is much rarer than the other NCLs. We conducted a literature search, which identified only four previously reported cases of CLN14.
The present report is the first case report from the Gaza Strip documenting any case with NCL and the fifth case in the literature with the CLN14 subtype due to a novel pathogenic variant in the KCTD7 gene. Documentation of this case’s clinical and genetic findings contributes to the increasing understanding of the NCLs and the awareness of the KCTD7-related phenotypic spectrum.
Case presentation
The patient was an initially healthy male infant born from an uneventful pregnancy and a normal vaginal delivery. He was his parents’ firstborn child. The parents were first cousins, but family history was unremarkable on both sides.
At nine months, the parents noticed that the patient could not sit unsupported. Three months later, around his first birthday, they began to notice sudden abnormal jerks of the patient’s extremities, trunk, and eyes, which a pediatrician diagnosed as myoclonus. The patient also displayed signs of psychomotor regression, marked by a gradual decline in previously acquired motor skills, such as sitting up and crawling, and cognitive skills, such as verbal communication and social engagement. He was also becoming less interactive with caregivers. Dysmorphic facial features were apparent, including low-set ears, up-slanted palpebral fissures, epicanthal folds, a depressed nasal bridge, a short nasal columella, and a fair complexion. There were no abnormal cutaneous lesions. Neurological examination revealed generalized hypotonia and brisk reflexes. Heart and chest examinations were unremarkable, and there was no evidence of organomegaly in the abdomen. Initial laboratory tests were within the normal range and included a complete hemogram, renal and liver functions, serum electrolytes, thyroid functions, and plasma lactate levels. A non-contrast brain CT scan and a brain MRI were ordered, and they returned as normal. An electroencephalogram (EEG) was reported as showing generalized slowing with spikes and slow waves, with no sensitivity to photic stimulation.
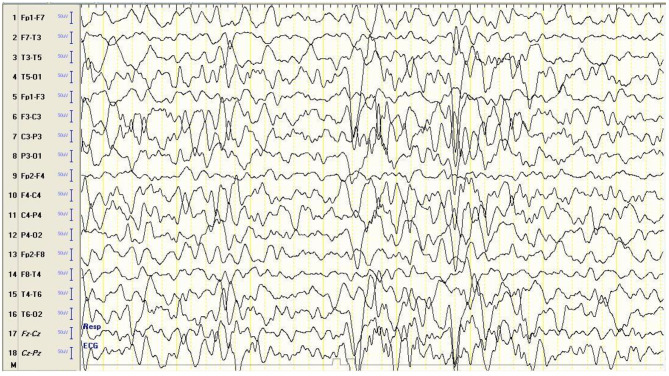
Around the age of 18 months, the patient experienced his first episode of a generalized tonic-clonic convulsion (GTC). Serial neurological examinations were also concerning for progressive generalized hypotonia and worsening motor and cognitive developmental regression. He could not maintain his balance while sitting and became increasingly unable to recognize familiar faces. Social interactions were becoming more sluggish. He did not talk or babble but displayed a distinctive, frequent laugh. The patient did not fixate his gaze on objects placed before him, track a moving object, or appreciate a torchlight. An ophthalmological examination uncovered a hypopigmented fundus and slight temporal disc pallor. A follow-up brain MRI indicated evidence of mild generalized brain atrophy and white matter disease. Follow-up EEG revealed a severely abnormal trace marked by generalized, high amplitude, sharply contoured, polymorphic delta slowing intermixed with theta slowing and some alpha activity, with disorganized and scattered spikes and sharp waves, indicating a generalized cerebral disturbance and increased seizure tendency (Fig. 1). However, photic stimulation was not performed. GTCs became more frequent as time progressed and did not respond to concomitant treatment with phenobarbital, levetiracetam, sodium valproate, and clonazepam. The clinical presentation suggested NCL as a possible diagnosis among other differential conditions, such as inborn errors of metabolism, leukodystrophy, and mitochondrial cytopathies. Hence, Whole Exome Sequencing (WES) was requested, including copy number variation (CNV) and mitochondrial genome.
Fig. 1.
EEG at 18 months showing a severely abnormal trace. The EEG shows generalized, high-amplitude, sharply contoured, polymorphic delta slowing intermixed with theta slowing and some alpha activity, with disorganized and scattered spikes and sharp waves (Montage: longitudinal bipolar, High cut: 35 Hz, Low cut: 1 Hz, Sensitivity: 7 µV/mm)
At 24 months, the patient continued to experience recurrent GTCs despite intensive treatment with anti-seizure medications. A follow-up neurological examination showed progression in hypotonia, exaggerated reflexes, and action myoclonus. Using Illumina TruSeq Exome Enrichment protocol for WES, we identified a novel homozygous variant c.413T > C p.(leu138pro) in the KCTD7 gene. This missense mutation caused an amino acid transition from leucine to proline at position 138. Both parents were heterozygous carriers of the same variant. The ensuing clinical course was marked by several admissions due to status epilepticus and pneumonia until the patient died in August 2023 at the age of forty-four months.
Discussion and conclusions
In the present report, we document the first genetically confirmed case of NCL in the Gaza Strip in a patient who presented with an infantile-onset, progressive disease that culminated in a premature death. Genetic testing revealed a novel c.413T > C, p.(Leu138Pro) variant in the KCTD7 gene, leading to the diagnosis of CLN14, which is extremely rare. This variant is most likely causative of the disease due to its prediction as probably damaging by different in silico tools. Due to its novelty, it was classified as a variant of unknown significance (VUS), but further testing could change the classification to likely pathogenic.
Presently, 13 genetic forms of NCL have been identified and assigned a “CLNx” number, and all are autosomal recessive except for CLN4. The NCLs are marked by progressive psychomotor decline, epilepsy, vision impairment, and premature death [14]. Besides suggestive history and neurological and ophthalmological examination, NCL diagnosis may be aided by pathologic studies that reveal the presence of variable ultrastructures, including granular, curvilinear, lamellar, or fingerprint cytosomes [10]. However, advances in genetic testing facilitate achieving an accurate and early diagnosis, which is necessary for treatment decision-making, anticipatory guidance, family planning, and avoiding prolonged and costly diagnostic odysseys. Genetic analysis can also facilitate differentiating the condition from other neurodegenerative disorders with overlapping presentations and is less invasive. Therefore, molecular diagnosis is the current gold standard diagnostic tool for NCL [2, 3, 11]. Another benefit from the increased accessibility and affordability of genetic testing is that it will facilitate the discovery and documentation of more NCL cases, especially from lower-income regions such as the Gaza Strip, which could change the current understanding of the condition’s prevalence, genotypic basis, and phenotypic spectrum [3]. It is important to note that as the spectrum of diseases associated with NCLs is uncovered further, it has become apparent that causative genes and specific pathogenic variants do not always predict phenotype. Also, although general phenotypic patterns, including age at onset, guide the diagnostic approach, age at onset does not necessarily predict genotype.
The KCTD7 gene codes for the potassium channel tetramerization domain-containing protein 7, which is widely expressed throughout the brain and is believed to play an important role as a modulator of neuron survival and excitability [12, 15]. Among its modulating roles, KCTD7 modulates proteostasis by interacting with cullin-3, a component of cullin-RING E3 ubiquitin ligase complexes. This explains how its mutations may cause disease through the undesired accumulation of undegraded materials [15]. The different roles believed to be played by KCTD7 are reflected in the phenotypic spectrum caused by KCTD7 gene mutations, where PME is presented on one end and CLN14 on the other [11]. A recent publication illustrated the phenotypic spectrum of molecularly confirmed KCTD7 pathogenic variants, including rare cases with opsoclonus myoclonus ataxia syndrome and myoclonus dystonia [13]. CLN14 is much rarer compared to PME and is more severe. Over 40 different KCTD7 gene pathogenic variants have been identified in relation to PME [16]. On the other hand, the limited number of previous case reports of CLN14 have reported two different homozygous and one compound heterozygous KCTD7 pathogenic variants (Table 1). WES testing in our patient revealed a novel homozygous variant (c.413T > C), which caused an amino acid change from leucine to proline at position 138 p.(leu138pro).
Table 1.
Clinical findings, investigations, and outcomes of the present case and four previously reported cases of CLN14
| Sex | Age at onset | Presenting symptoms and signs | Ophthalmoscopic findings | Brain MRI findings | EEG findings | KCTD7 gene pathogenic variant | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Case 1 (12) | Male | 9 months | Neurocognitive regression; microcephaly multifocal action myoclonus; spasticity; vision loss | Vision loss, normal retinal exam | Cerebellar cortical atrophy, thinning of the corpus callosum, loss of subcortical white matter (12 years) | Not Reported |
Homozygous: c.550 C > T (p.Arg184Cys) |
Death at 13 years |
| Case 2 (12) | Female | 8 months | Neurocognitive regression; microcephaly; action myoclonus; vision loss | vision loss, mild optic atrophy | Moderate generalized brain atrophy more pronounced in the frontal lobes and cerebellum (13 years) | Not Reported |
Homozygous: c.550 C > T (p.Arg184Cys) |
Death at 17 years |
| Case 3 (25) | Female | 13 months | Action myoclonus; developmental delay | Not Reported | Predominantly anterior white matter atrophy (15 months) | Poorly organized and markedly slowed background activity, multifocal epileptiform discharges mainly involving the central regions, no photosensitivity |
Homozygous: [172G > A] p.[(Gly58Arg)] |
Wheelchair bound; loss of language, drug- resistant seizures |
| Case 4 (6) | Male | 21 months | Cognitive and motor deterioration, ataxia, epileptic paroxysms |
Not Reported (Vision was preserved) |
Cerebellar subatrophy, moderate expansion of the lateral ventricles (4 years) | Outbreaks of epileptiform activity |
Compound heterozygous: c.190 A > G (p.Thr64Ala) and c.337T > C (p.Ser113Pro) |
Not Reported |
| Present case | Male | 9 months | Neurocognitive regression, myoclonus, vision loss | Vision loss, hypopigmented fundi, slight temporal disc pallor | Mild generalized brain atrophy, white matter disease (2 years) |
Generalized slowing but no photosensitivity in the first EEG; Follow-up EEG showed generalized, high amplitude, sharply contoured, polymorphic delta slowing intermixed with theta slowing and some alpha activity, with disorganized and scattered spikes and sharp waves, photic stimulation not performed |
Homozygous: c.413T > C, p.(Leu138Pro) |
Death at 44 months |
Clinically, PME and CLN14 are distinguished by the earlier onset, more rapid progression, accompanying visual loss, progressive brain atrophy, and early death seen in CLN14. The early onset often follows an initial period of normal development. Intractable myoclonic seizures usually appear before the age of two, accompanied by the hallmark findings of psychomotor regression and loss of vision, eventually progressing to death. These features, which manifested in our patient, and the results of WES sequencing, permitted the diagnosis of CLN14 [3, 11]. In practice, a genetic diagnosis suffices to guide management and inform genetic counseling, making further examination rarely necessary [3, 11].
Ophthalmoscopic findings can occur even before the onset of vision loss in some NCL patients. Possible early changes include defective macular light reflex and optic disc pallor followed by attenuation of vessels, pigmentary retinal changes, degeneration of the macula, and optic atrophy [17]. In our patient, ophthalmoscopic evaluation was performed after visual symptoms became prominent, revealing a hypopigmented fundus and a slight temporal optic disc pallor. Interestingly, one case of NCL14 was reported to have preserved vision, which illustrates the condition’s likely phenotypic heterogeneity [6]. Imaging alone is nonspecific and is insufficient for establishing an NCL diagnosis. Still, it may provide important clues that suggest a neurodegenerative condition is present, especially when cerebral and cerebellar atrophy are observed, which has been reported across different NCLs and in all previous cases with CLN14 (Table 1) [18]. In our case, the follow-up brain MRI revealed generalized brain atrophy.
EEG findings are also generally nonspecific. It has been suggested that photosensitivity to low-frequency photic stimulation (1 to 5 Hz) may be a hallmark of CLN6, but this has not been studied extensively across other NCLs [19]. In the present case, EEG showed severely abnormal traces with marked generalized slowing, high amplitude spikes, and sharp waves, a pattern suggestive of a generalized cerebral disturbance and increased seizure tendency. Photosensitivity was absent from the initial EEG but was not performed in the follow-up recording six months later. EEG findings were reported in two of the four previous CLN14 cases, showing significant abnormalities. Only one previous case had reported the outcome of photic stimulation, with no evidence of photosensitivity. In terms of outcomes, three of the cases had their outcome reported. Of those, two had died, and the third suffered from severe symptoms and disability and perhaps died after the case report was published. Overall, our case suffered from a more severe and more rapidly progressive presentation compared to the other CLN14 cases, with onset in infancy, rapid clinical progression, severe EEG findings, and earlier death. Table 1 summarizes the main clinical findings, investigations, and the outcome of the present case and the four previously reported cases of CLN14 in the literature.
Metz et al., in a 2018 study, examined samples from several patients with PME and confirmed biallelic KCTD7 mutations [20]. Ultrastructural analysis identified the accumulation of lipid droplets and the presence of abnormal phagolysosomes containing undegraded materials and lipofuscin granules in the examined cells. Nonetheless, other features considered to be characteristic of NCL were lacking [20]. This raises the possibility that an ultrastructural spectrum mirrors the phenotypic spectrum of PME-CLN14. Keeping this in mind, and although the role of biopsy and ultrastructural examination has faded in favor of genetic testing, the increasing discovery of cases with earlier onset, more severe, or rapidly progressive PME and a confirmed biallelic KCTD7 mutation creates a blurry zone where distinguishing PME from CLN14 is not easy [13]. Additionally, the rate of clinical deterioration is often insufficient to distinguish PME from NCL reliably. In severe cases, the former condition might present with rapid deterioration, while the latter might occasionally deteriorate in a steady course. This is probably true of the PME-CLN14 spectrum as well. For instance, the age of death in four reported cases ranges widely from 44 months to 17 years (Table 1). These facts present us with a set of cases where biopsy, despite its cost, invasiveness, and technical limitations, may still be needed to fully delineate the KCTD7 clinical spectrum and outline the nosology of CLN14. A precise nosological diagnosis will become essential when a disease-modifying treatment directed at the lysosomal storage mechanism in CLN14 is introduced.
The physiological roles and pathways of the KCTD7 complex have not yet been fully elucidated. Thus, there is no definitive treatment for KCTD7 mutation–induced disorders [21]. Seizures are usually challenging to control and require polytherapy. Stem cell transplants and gene therapy have been tried with different NCLs, but none have shown long-term benefits. Several pharmacological agents (e.g., flupirtine) have similarly demonstrated no or mediocre therapeutic roles in other NCL forms [22, 23]. Replacement with the recombinant enzyme Cerliponase Alfa showed promise in slowing the progression of CLN2, marking the first therapy for an NCL disease that targets the disease etiology [24].
In conclusion, CLN14 is a very rare condition and a relatively new addition to the NCL group of diseases. Different mutations in the KCTD7 gene can cause this disease. The reporting of new cases will likely expand the genotypic and phenotypic presentations of CLN14 and the current understanding of the KCTD7-related phenotypic spectrum. We reported a novel homozygous variant in the KCTD7 gene (c.413T > C), which is most likely causative for our patient’s phenotype. Further clinical assessment and genetic testing of relatives are required to provide phenotypic specificity evidence and show co-segregation.
Acknowledgements
Dr. Ahmed M Yassin, MD, PhD, from the Department of Clinical Neurophysiology and Epilepsy at King Abdullah University Hospital, Jordan. Dr. Abeer J Qannan, MD, PhD, from the Genomics and Precision Medicine department at Hamad Bin Khalifa University, Qatar.
Abbreviations
- NCL
Neuronal ceroid lipofuscinosis
- PME
Progressive myoclonic epilepsy
- EM
Electron microscopy
- EEG
Electroencephalogram
- GTC
Generalized tonic-clonic convulsion
- WES
Whole Exome Sequencing
- CNV
Copy number variation
Author contributions
SZ, GM, YA, and BA contributed to the literature review. SZ, GM, and YA collected the data. SZ and BA analyzed the data. MA and BA contributed to brain MRI and EEG interpretation. SZ and BA prepared the figures and table. SZ and BA led the writing of the study manuscript. BA responded to reviewer comments. All authors contributed to the manuscript revision and have approved the final version.
Funding
The study was not funded.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Institutional Review Board (IRB) at the Islamic University of Gaza. The patient’s parents provided written consent, and confidentiality was maintained throughout the manuscript.
Consent for publication
The parents provided written consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haltia M, Goebel HH. The neuronal ceroid-lipofuscinoses: a historical introduction. Biochim Biophys Acta. 2013;1832(11):1795–800. [DOI] [PubMed] [Google Scholar]
- 2.Kaminiow K, Kozak S, Paprocka J. Recent insight into the genetic basis, clinical features, and diagnostic methods for neuronal ceroid lipofuscinosis. Int J Mol Sci. 2022;23(10). [DOI] [PMC free article] [PubMed]
- 3.Simonati A, Williams RE. Neuronal ceroid lipofuscinosis: the Multifaceted Approach to the Clinical issues, an overview. Front Neurol. 2022;13:811686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dozieres-Puyravel B, Nasser H, Elmaleh-Berges M, Lopez Hernandez E, Gelot A, Ilea A, et al. Paediatric-onset neuronal ceroid lipofuscinosis: first symptoms and presentation at diagnosis. Dev Med Child Neurol. 2020;62(4):528–30. [DOI] [PubMed] [Google Scholar]
- 5.Kamate M, Prashanth GP, Hattiholi V. Clinico-investigative profile of infantile and late-infantile neuronal ceroid lipofuscinoses. Neurol India. 2012;60(3):316–20. [DOI] [PubMed] [Google Scholar]
- 6.Kozina AA, Okuneva EG, Baryshnikova NV, Kondakova OB, Nikolaeva EA, Fedoniuk ID, et al. Neuronal ceroid lipofuscinosis in the Russian population: two novel mutations and the prevalence of heterozygous carriers. Mol Genet Genomic Med. 2020;8(7):e1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology. 2012;79(2):183–91. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg JB, Chen A, Kaminsky SM, Crystal RG, Sondhi D. Advances in the treatment of neuronal ceroid lipofuscinosis. Expert Opin Orphan Drugs. 2019;7(11):473–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goebel HH, Schochet SS, Jaynes M, Bruck W, Kohlschutter A, Hentati F. Progress in neuropathology of the neuronal ceroid lipofuscinoses. Mol Genet Metab. 1999;66(4):367–72. [DOI] [PubMed] [Google Scholar]
- 10.Anderson GW, Goebel HH, Simonati A. Human pathology in NCL. Biochimica et Biophysica Acta (BBA) -. Mol Basis Disease. 2013;1832(11):1807–26. [DOI] [PubMed]
- 11.Gardner E, Mole SE. The genetic basis of phenotypic heterogeneity in the neuronal ceroid lipofuscinoses. Front Neurol. 2021;12:754045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staropoli JF, Karaa A, Lim ET, Kirby A, Elbalalesy N, Romansky SG, et al. A homozygous mutation in KCTD7 links neuronal ceroid lipofuscinosis to the ubiquitin-proteasome system. Am J Hum Genet. 2012;91(1):202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoganathan S, Whitney R, Thomas M, Danda S, Chettali AM, Prasad AN, et al. -related progressive myoclonic epilepsy: report of 42 cases and review of literature. Epilepsia. 2024;65(3):709–24. [DOI] [PubMed] [Google Scholar]
- 14.Schulz A, Kohlschütter A, Mink J, Simonati A, Williams R. NCL diseases - clinical perspectives. Biochim Biophys Acta. 2013;1832(11):1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang JH, Alevy J, Akhanov V, Seo R, Massey CA, Jiang D et al. Kctd7 deficiency induces myoclonic seizures associated with Purkinje cell death and microvascular defects. Dis Model Mech. 2022;15(9). [DOI] [PMC free article] [PubMed]
- 16.Teng X, Aouacheria A, Lionnard L, Metz KA, Soane L, Kamiya A, et al. KCTD: a new gene family involved in neurodevelopmental and neuropsychiatric disorders. CNS Neurosci Ther. 2019;25(7):887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouseph MM, Kleinman ME, Wang QJ. Vision loss in juvenile neuronal ceroid lipofuscinosis (CLN3 disease). Ann N Y Acad Sci. 2016;1371(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamate M, Reddy N, Detroja M, Hattiholi V. Neuronal ceroid lipofuscinoses in children. Ann Indian Acad Neurol. 2021;24(2):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkovic SF, Oliver KL, Canafoglia L, Krieger P, Damiano JA, Hildebrand MS, et al. Kufs disease due to mutation of CLN6: clinical, pathological and molecular genetic features. Brain. 2019;142(1):59–69. [DOI] [PubMed] [Google Scholar]
- 20.Metz KA, Teng X, Coppens I, Lamb HM, Wagner BE, Rosenfeld JA, et al. KCTD7 deficiency defines a distinct neurodegenerative disorder with a conserved autophagy-lysosome defect. Ann Neurol. 2018;84(5):766–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Cao X, Liu P, Zeng W, Peng R, Shi Q, et al. KCTD7 mutations impair the trafficking of lysosomal enzymes through CLN5 accumulation to cause neuronal ceroid lipofuscinoses. Sci Adv. 2022;8(31):eabm5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makoukji J, Saadeh F, Mansour KA, El-Sitt S, Al Ali J, Kinarivala N, et al. Flupirtine derivatives as potential treatment for the neuronal ceroid lipofuscinoses. Ann Clin Transl Neurol. 2018;5(9):1089–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cialone J, Augustine EF, Newhouse N, Adams H, Vierhile A, Marshall FJ, et al. Parent-reported benefits of flupirtine in juvenile neuronal ceroid lipofuscinosis (Batten disease; CLN3) are not supported by quantitative data. J Inherit Metab Dis. 2011;34(5):1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis G, Morrill AM, Conway-Allen SL, Kim B. Review of Cerliponase Alfa: recombinant human enzyme replacement therapy for late-infantile neuronal ceroid lipofuscinosis type 2. J Child Neurol. 2020;35(5):348–53. [DOI] [PubMed] [Google Scholar]
- 25.Mastrangelo M, Sartori S, Simonati A, Brinciotti M, Moro F, Nosadini M, et al. Progressive myoclonus epilepsy and ceroidolipofuscinosis 14: the multifaceted phenotypic spectrum of KCTD7-related disorders. Eur J Med Genet. 2019;62(12):103591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.