Abstract
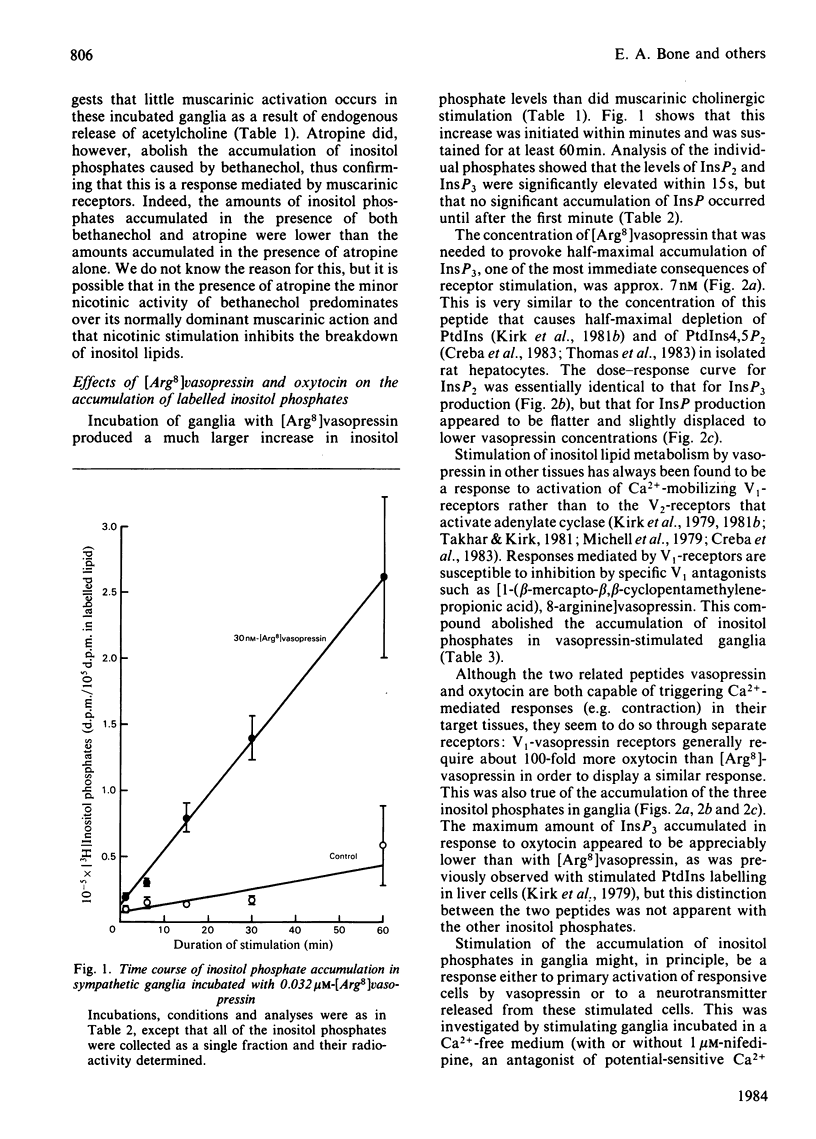
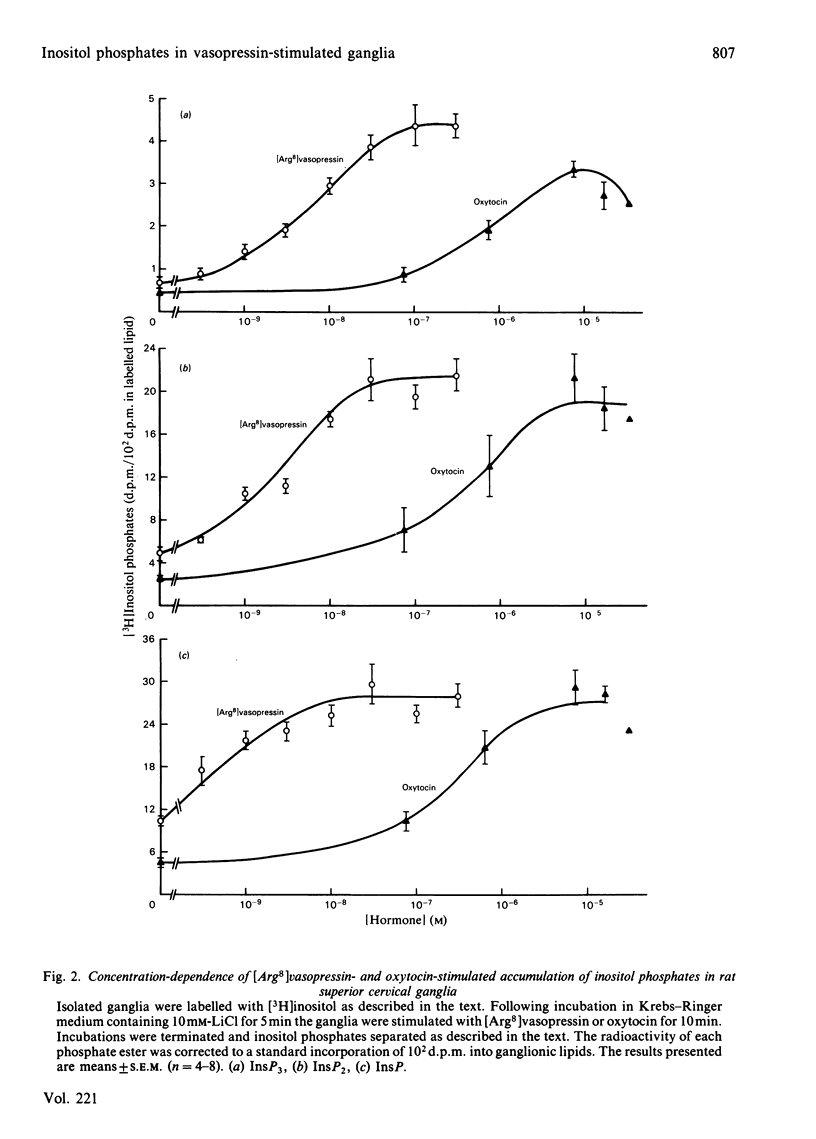
An accumulation of 3H-labelled inositol phosphates is observed when prelabelled rat superior cervical sympathetic ganglia are exposed to [8-arginine]vasopressin or to muscarinic cholinergic stimuli. The response to vasopressin is much greater than the response to cholinergic stimuli. The response to vasopressin is blocked by a V1-vasopressin antagonist, and oxytocin is a much less potent agonist than vasopressin. Vasopressin causes no increase in the cyclic AMP content of ganglia. These ganglia therefore appear to have functional V1-vasopressin receptors that are capable of activating inositol lipid breakdown, but no V2-receptors coupled to adenylate cyclase. The first [3H]inositol-labelled products to accumulate in stimulated ganglia are inositol trisphosphate and inositol bisphosphate, suggesting that the initiating reaction in stimulated inositol lipid metabolism is a phosphodiesterase-catalysed hydrolysis of phosphatidylinositol 4,5-bisphosphate (and possibly also phosphatidylinositol 4-phosphate). This response to exogenous vasopressin occurs in ganglia incubated in media of reduced Ca2+ concentration. The physiological functions of the V1-vasopressin receptors of these ganglia remain unknown.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar R. A., Abdel-Latif A. A. Requirement for calcium ions in acetylcholine-stimulated phosphodiesteratic cleavage of phosphatidyl-myo-inositol 4,5-bisphosphate in rabbit iris smooth muscle. Biochem J. 1980 Dec 15;192(3):783–791. doi: 10.1042/bj1920783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P. Receptor-stimulated inositol phospholipid metabolism in the central nervous system. Cell Calcium. 1982 Oct;3(4-5):413–428. doi: 10.1016/0143-4160(82)90027-6. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Kebabian J. W. Role of cyclic AMP in synaptic transmission in the mammalian peripheral nervous system. Fed Proc. 1974 Apr;33(4):1059–1067. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Effects of acetylcholine on phospholipides in the pancreas. J Biol Chem. 1954 Aug;209(2):549–558. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E., SHELP W. D. The effects of acetylcholine on the turnover of phosphatidic acid and phosphoinositide in sympathetic ganglia, and in various parts of the central nervous system in vitro. J Gen Physiol. 1960 Nov;44:217–226. doi: 10.1085/jgp.44.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hanley M. R., Benton H. P., Lightman S. L., Todd K., Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. A vasopressin-like peptide in the mammalian sympathetic nervous system. Nature. 1984 May 17;309(5965):258–261. doi: 10.1038/309258a0. [DOI] [PubMed] [Google Scholar]
- Hawthorne J. N., Pickard M. R. Phospholipids in synaptic function. J Neurochem. 1979 Jan;32(1):5–14. doi: 10.1111/j.1471-4159.1979.tb04503.x. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Autoradiographic localization of the acetylcholine-stimulated synthesis of phosphatidylinositol in the superior cervical ganglion. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1369–1376. doi: 10.1073/pnas.53.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N., Brownfield M. S. Peptidergic transmitters in synaptic boutons of sympathetic ganglia. Nature. 1980 Nov 27;288(5789):380–382. doi: 10.1038/288380a0. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Michell R. H., Hems D. A. Phosphatidylinositol metabolism in rat hepatocytes stimulated by vasopressin. Biochem J. 1981 Jan 15;194(1):155–165. doi: 10.1042/bj1940155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Rodrigues L. M., Hems D. A. The influence of vasopressin and related peptides on glycogen phosphorylase activity and phosphatidylinositol metabolism in hepatocytes. Biochem J. 1979 Feb 15;178(2):493–496. doi: 10.1042/bj1780493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren R., Cass C. E., Paterson A. R. The kinetics of dissociation of the inhibitor of nucleoside transport, nitrobenzylthioinosine, from the high-affinity binding sites of cultured hamster cells. Biochem J. 1983 Nov 15;216(2):299–308. doi: 10.1042/bj2160299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W. Slow synaptic responses in autonomic ganglia and the pursuit of a peptidergic transmitter. J Exp Biol. 1980 Dec;89:257–286. doi: 10.1242/jeb.89.1.257. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., KLINGMAN J. D., LEICHT W. S. EFFECTS OF TEMPERATURE, CALCIUM AND ACTIVITY ON PHOSPHOLIPID METABOLISM IN A SYMPATHETIC GANGLION. J Neurochem. 1963 Aug;10:549–570. doi: 10.1111/j.1471-4159.1963.tb05053.x. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., LEICHT W. S. METABOLISM OF PHOSPHATIDYL INOSITOL AND OTHER LIPIDS IN ACTIVE NEURONES OF SYMPATHETIC GANGLIA AND OTHER PERIPHERAL NERVOUS TISSUES. THE SITE OF THE INOSITIDE EFFECT. J Neurochem. 1965 Jan;12:1–13. doi: 10.1111/j.1471-4159.1965.tb10245.x. [DOI] [PubMed] [Google Scholar]
- Lakshmanan J. Nerve growth factor induced turnover of phosphatidylinositol in rat superior cervical ganglia. Biochem Biophys Res Commun. 1978 Jun 14;82(3):767–775. doi: 10.1016/0006-291x(78)90848-3. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Brown W. E., Michell R. H. Muscarinic cholinergic stimulation of phosphatidylinositol turnover in isolated rat superior cervical sympathetic ganglia. J Neurochem. 1976 Mar;26(3):649–651. doi: 10.1111/j.1471-4159.1976.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Terenius L., Hökfelt T., Martling C. R., Tatemoto K., Mutt V., Polak J., Bloom S., Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982 Dec;116(4):477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J. Studies of receptor-stimulated inositol lipid metabolism should focus upon measurements of inositol lipid breakdown. Biochem J. 1981 Jul 15;198(1):247–248. doi: 10.1042/bj1980247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Mikoshiba K., Tsukada Y. Effect of potassium ions on glucose and phospholipid metabolism in the rat's cervical sympathetic ganglia with and without axotomy. Brain Res. 1973 Jun 29;56:259–269. doi: 10.1016/0006-8993(73)90340-5. [DOI] [PubMed] [Google Scholar]
- Pickard M. R., Hawthorne J. N., Hayashi E., Yamada S. Effects of surugatoxin and other nicotinic and muscarinic antagonists on phosphatidylinositol metabolism in active sympathetic ganglia. Biochem Pharmacol. 1977 Mar 1;26(5):448–450. doi: 10.1016/0006-2952(77)90208-8. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Burgess G. M., Halenda S. P., McKinney J. S., Rubin R. P. Effects of secretagogues on [32P]phosphatidylinositol 4,5-bisphosphate metabolism in the exocrine pancreas. Biochem J. 1983 May 15;212(2):483–488. doi: 10.1042/bj2120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Recent hypotheses regarding the phosphatidylinositol effect. Life Sci. 1981 Sep 21;29(12):1183–1194. doi: 10.1016/0024-3205(81)90221-6. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Terenius L., Elfvin L. G., Lundberg J. M., Brandt J., Elde R. P., Goldstein M. Enkephalin immunoreactive nerve fibres and cell bodies in sympathetic ganglia of the guinea-pig and rat. Neuroscience. 1979;4(2):249–270. doi: 10.1016/0306-4522(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Takhar A. P., Kirk C. J. Stimulation of inorganic-phosphate incorporation into phosphatidylinositol in rat thoracic aorta mediated through V1-vasopressin receptors. Biochem J. 1981 Jan 15;194(1):167–172. doi: 10.1042/bj1940167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. P., Marks J. S., Coll K. E., Williamson J. R. Quantitation and early kinetics of inositol lipid changes induced by vasopressin in isolated and cultured hepatocytes. J Biol Chem. 1983 May 10;258(9):5716–5725. [PubMed] [Google Scholar]
- Wali F. A. Effects of oxytocin and vasopressin on ganglionic transmission at the rabbit superior cervical ganglion. Pharmacol Res Commun. 1984 Jan;16(1):55–62. [PubMed] [Google Scholar]
- Weiss S. J., McKinney J. S., Putney J. W., Jr Receptor-mediated net breakdown of phosphatidylinositol 4,5-bisphosphate in parotid acinar cells. Biochem J. 1982 Sep 15;206(3):555–560. doi: 10.1042/bj2060555. [DOI] [PMC free article] [PubMed] [Google Scholar]


