Abstract
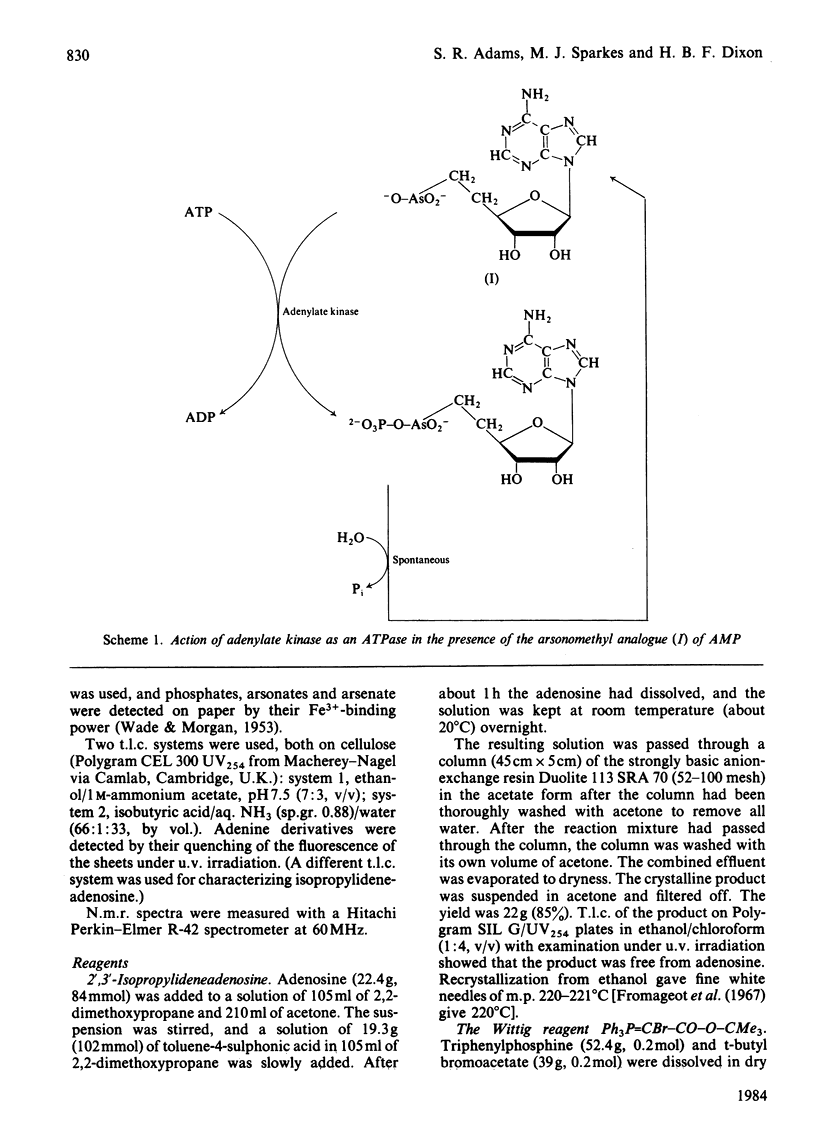
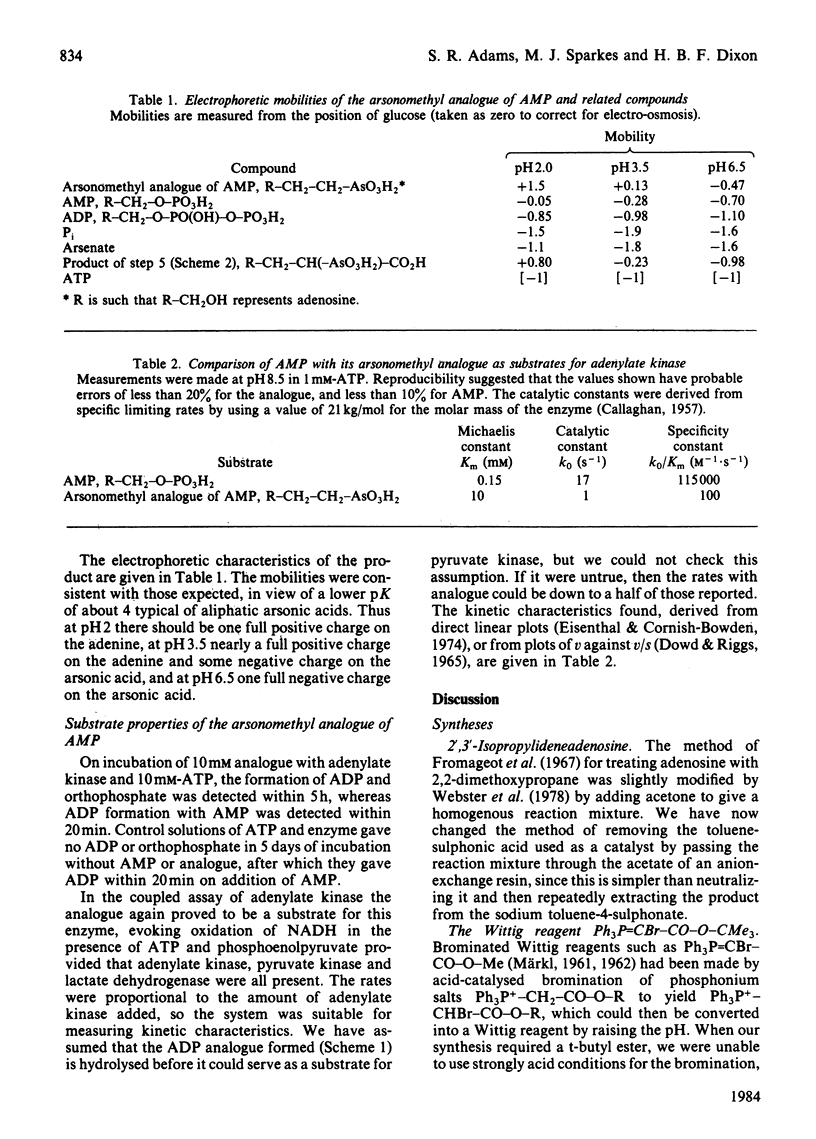
Adenosine was converted into the arsonomethyl analogue of AMP. The reactions used provide a general route for converting an alcohol, R-CH2-OH, into the arsonomethyl analogue, R-CH2-CH2-AsO3H2, of its phosphate, R-CH2-O-PO3H2. The analogue of AMP proves to be a substrate for rabbit adenylate kinase, which shows a limiting velocity with it of 1/17 that with AMP, a Michaelis constant raised 70-fold to about 10 mM, and hence a specificity constant lowered about 1200-fold. The product of transfer of a phospho group from ATP to the analogue is, like all anhydrides of arsonic acids, unstable to hydrolysis, and so breaks down to yield orthophosphate and regenerate the analogue. Hence adenylate kinase is converted into an ATPase by the presence of the analogue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. R., Sparkes M. J., Dixon H. B. The arsonomethyl analogue of 3-phosphoglycerate. Biochem J. 1983 Jul 1;213(1):211–215. doi: 10.1042/bj2130211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromageot H. P., Griffin B. E., Reese C. B., Sulston J. E. The synthesis of oligoribonucleotides. 3. Monoacylation of ribonucleosides and derivatives via orthoester exchange. Tetrahedron. 1967 May;23(5):2315–2331. doi: 10.1016/0040-4020(67)80068-1. [DOI] [PubMed] [Google Scholar]
- Gresser M. J. ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J Biol Chem. 1981 Jun 25;256(12):5981–5983. [PubMed] [Google Scholar]
- Jones G. H., Moffatt J. G. The synthesis of 6'-deoxyhomonucleoside-6'-phosphonic acids. J Am Chem Soc. 1968 Sep 11;90(19):5336–5338. doi: 10.1021/ja01021a086. [DOI] [PubMed] [Google Scholar]
- Kamiya K., Cruse W. B., Kennard O. The arsonomethyl group as an analogue of phosphate. An X-ray investigation. Biochem J. 1983 Jul 1;213(1):217–223. doi: 10.1042/bj2130217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas R., Pestaña D., Diez-Masa J. C. Arsenic mononucleotides. Separation by high-performance liquid chromatography and identification with myokinase and adenylate deaminase. Biochemistry. 1984 Feb 28;23(5):955–960. doi: 10.1021/bi00300a024. [DOI] [PubMed] [Google Scholar]
- Lagunas R., Solis A. Arsenate induced activity of certain enzymes on their dephosphorylated subtrates. FEBS Lett. 1968 Jul;1(1):32–34. doi: 10.1016/0014-5793(68)80011-0. [DOI] [PubMed] [Google Scholar]
- Lagunas R. Sugar-arsenate esters: thermodynamics and biochemical behavior. Arch Biochem Biophys. 1980 Nov;205(1):67–75. doi: 10.1016/0003-9861(80)90084-3. [DOI] [PubMed] [Google Scholar]
- Long J. W., Ray W. J., Jr Kinetics and thermodynamics of the formation of glucose arsenate. Reaction of glucose arsenate with phosphoglucomutase. Biochemistry. 1973 Sep 25;12(20):3932–3937. doi: 10.1021/bi00744a023. [DOI] [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. Detection of phosphate esters on paper chromatograms. Nature. 1953 Mar 21;171(4351):529–530. doi: 10.1038/171529a0. [DOI] [PubMed] [Google Scholar]
- Webster D., Sparkes M. J., Dixon H. B. An arsenical analogue of adenosine diphosphate. Biochem J. 1978 Jan 1;169(1):239–244. doi: 10.1042/bj1690239. [DOI] [PMC free article] [PubMed] [Google Scholar]


