Abstract
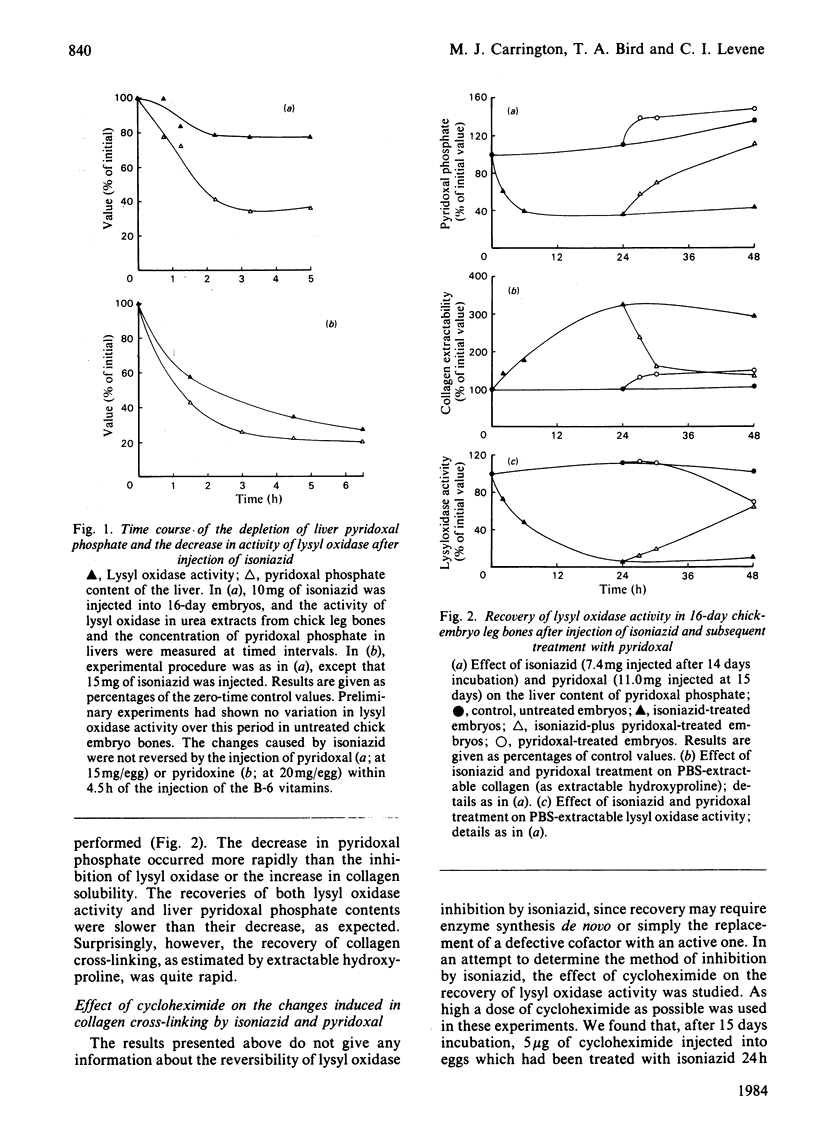
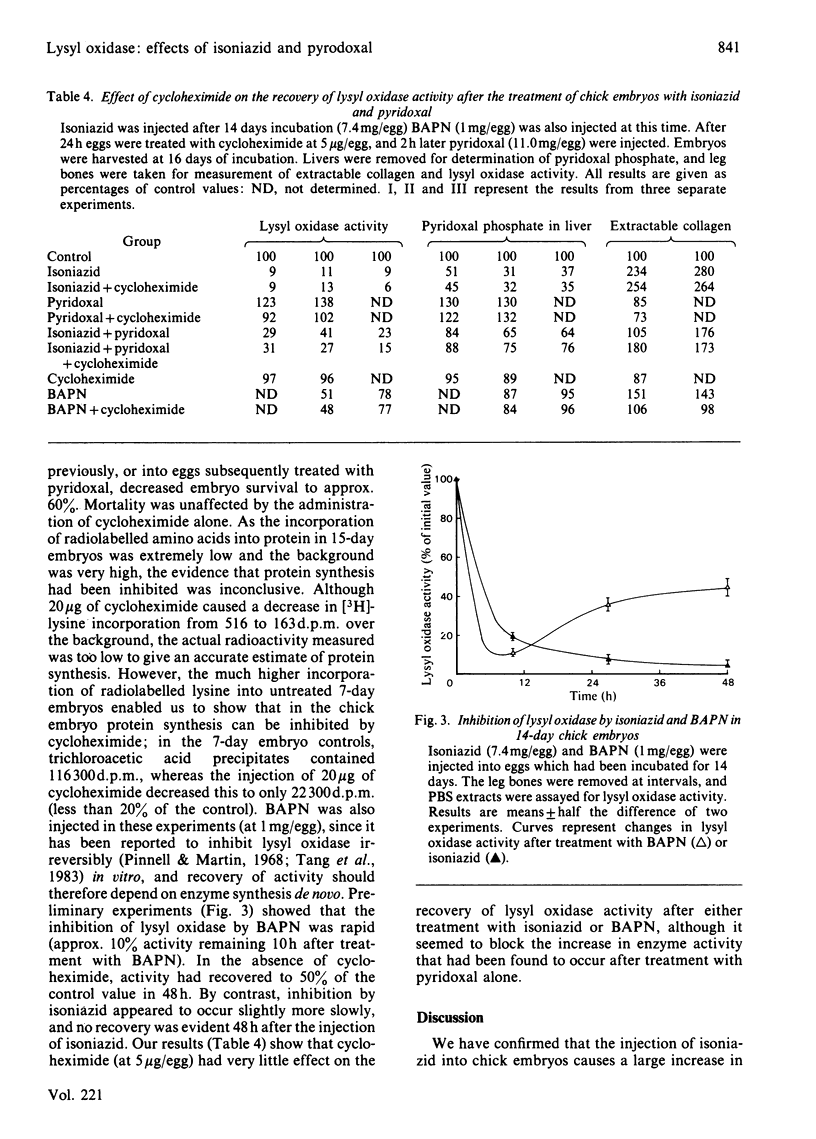
Isonicotinic acid hydrazide (isoniazid) causes a large increase in the salt-solubility of collagen when injected into chick embryos; this change is accompanied by the inactivation of lysyl oxidase (EC 1.4.3.13), the enzyme responsible for initiating cross-link formation in collagen and elastin. In addition, isoniazid markedly decreases the liver content of pyridoxal phosphate. The depletion of pyridoxal phosphate takes approx. 6 h, whereas the inhibition of lysyl oxidase and the increase in collagen solubility occur more slowly. A reversal of these effects of isoniazid can be produced by the subsequent injection of a stoichiometric amount of pyridoxal, supporting the role of pyridoxal as a cofactor for lysyl oxidase. Treatment of chick embryos with beta-aminopropionitrile, an irreversible inhibitor of lysyl oxidase, causes an inhibition of the enzyme, which begins to recover within 24 h but which is not affected by the administration of pyridoxal; with isoniazid inhibition, however, lysyl oxidase activity does not show any sign of recovery by 48 h. It is proposed that isoniazid may cause the inhibition of lysyl oxidase by competing for its obligatory cofactor, pyridoxal phosphate. The potential clinical implications in the therapeutic control of fibrosis are briefly discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. Fluorometric determination of pyridoxal phosphate in enzymes. Methods Enzymol. 1979;62:407–410. doi: 10.1016/0076-6879(79)62249-8. [DOI] [PubMed] [Google Scholar]
- Arem A. J., Misiorowski R. Lathyritic activity of isoniazid. J Med. 1976;7(3-4):239–248. [PubMed] [Google Scholar]
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Bird T. A., Levene C. I. Lysyl oxidase: evidence that pyridoxal phosphate is a cofactor. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1172–1180. doi: 10.1016/0006-291x(82)92124-6. [DOI] [PubMed] [Google Scholar]
- Fowler L. J., Peach C. M., Bailey A. J. In vitro studies on the enzymic biosynthesis of the collagen crosslinks. Biochem Biophys Res Commun. 1970 Oct 9;41(1):251–259. doi: 10.1016/0006-291x(70)90496-1. [DOI] [PubMed] [Google Scholar]
- Fuller G. C. Perspectives for the use of collagen synthesis inhibitors as antifibrotic agents. J Med Chem. 1981 Jun;24(6):651–658. doi: 10.1021/jm00138a001. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Gonnerman W. A., Savage J. E., O'Dell B. L. Connective tissue amine oxidase. II. Purification and partial characterization of lysyl oxidase from chick aorta. Biochim Biophys Acta. 1974 Apr 25;341(2):332–344. doi: 10.1016/0005-2744(74)90226-5. [DOI] [PubMed] [Google Scholar]
- Kagan H. M., Hewitt N. A., Salcedo L. L., Franzblau C. Catalytic activity of aortic lysyl oxidase in an insoluble enzyme-substrate complex. Biochim Biophys Acta. 1974 Sep 13;365(1):223–234. doi: 10.1016/0005-2795(74)90267-0. [DOI] [PubMed] [Google Scholar]
- LEVENE C. I. Structural requirements for lathyrogenic agents. J Exp Med. 1961 Sep 1;114:295–310. doi: 10.1084/jem.114.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENE C. I. The effect of lathyrogenic compounds on the glycogen content of the chick embryo liver. Br J Exp Pathol. 1962 Dec;43:596–599. [PMC free article] [PubMed] [Google Scholar]
- LEVENE C. I. The lathyrogenic effect of isonicotinic acid hydrazide (INAH) on the chick embryo and its reversal by pyridoxal. J Exp Med. 1961 Apr 1;113:795–810. doi: 10.1084/jem.113.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene C. I., Bye I., Saffiotti U. The effect of beta-aminopropionitrile on silicotic pulmonary fibrosis in the rat. Br J Exp Pathol. 1968 Apr;49(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- Moorhead L. C. Inhibition of collagen cross-linking: a new approach to ocular scarring. Curr Eye Res. 1981;1(2):77–83. doi: 10.3109/02713688109001730. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Fraser D. R., Levene C. I. The effect of pyridoxine deficiency on lysyl oxidase activity in the chick. Exp Mol Pathol. 1978 Jun;28(3):301–308. doi: 10.1016/0014-4800(78)90004-7. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Levene C. I. Evidence for the role of vitamin C-6 as a cofactor of lysyl oxidase. Biochem J. 1977 Nov 1;167(2):463–467. doi: 10.1042/bj1670463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A. S., Siegel R. C., Martin G. R. Stability and purification of lysyl oxidase. Arch Biochem Biophys. 1974 May;162(1):231–237. doi: 10.1016/0003-9861(74)90123-4. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Martin G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968 Oct;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C. Lysyl oxidase. Int Rev Connect Tissue Res. 1979;8:73–118. doi: 10.1016/b978-0-12-363708-6.50009-6. [DOI] [PubMed] [Google Scholar]
- Siegel R. C., Pinnell S. R., Martin G. R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970 Nov 10;9(23):4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- Tang S. S., Trackman P. C., Kagan H. M. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem. 1983 Apr 10;258(7):4331–4338. [PubMed] [Google Scholar]


