Abstract
Background
It is not clear which fixation of total knee arthroplasty obtains the best clinical, functional and radiographic results in people with osteoarthritis and other non‐traumatic diseases, such as rheumatoid arthritis.
Objectives
To assess the benefits and harms of cemented, cementless and hybrid knee prostheses fixation techniques in participants with primary osteoarthritis (osteoarthritis following trauma was not included) and other non‐traumatic diseases, such as rheumatoid arthritis.
Search methods
We searched CENTRAL (2011, issue 10), MEDLINE via PubMed, EMBASE, Current Controlled Trials, LILACS, The Cumulative Index to Nursing and Allied Health Literature, SPORTDiscus, Health Technology Assessment Database and the Database of Abstracts of Reviews of Effectiveness, all from implementation to October 2011, along with handsearches of high‐yield journals and reference lists of articles. No language restrictions were applied.
Selection criteria
Randomized controlled trials (RCTs) evaluating cemented, cementless and hybrid fixation. Participants included patients that were 18 years or older with osteoarthritis and other non‐traumatic diseases who were undergoing primary total knee arthroplasty.
Data collection and analysis
Three authors independently selected the eligible trials, assessed the trial quality, risk of bias and extracted data. Researchers were contacted to obtain missing information.
Main results
Five RCTs and 297 participants were included in this review. Using meta‐analysis on roentgen stereophotogrammetric analysis (RSA) we observed that cemented fixation of the tibial components demonstrated smaller displacement in relation to cementless fixation (with and without hydroxyapatite) after a follow‐up of two years (maximum total point‐motion, N = 167, two RCTs, mean difference (MD) = 0.52 mm, 95% confidence interval (CI) 0.31 to 0.74). However, the risk of future aseptic loosening with uncemented fixation was approximately half that of cemented fixation according to the arthroplasty instability classification (moderate quality as assessed by GRADE) inferred from RSA (N = 216, three RCTs, risk ratio (RR) = 0.47, 95% CI 0.24 to 0.92) with a 16% absolute risk difference between groups. The number needed to treat for an additional beneficial outcome (NNTB) to prevent future aseptic loosening was 7 (95% CI 5 to 44). There was a low risk of bias for RSA among the studies included. It was not possible to perform meta‐analysis on patient‐important outcomes, such as the survival rate of the implant (any change of a component), patient global assessments, functional measures, pain, health‐related quality of life measures and adverse events. Almost all included studies recorded functional measures of Knee Society and Hospital for Special Surgery knee scores, but the authors of each study found no significant difference between the groups.
Authors' conclusions
There was a smaller displacement of the cemented tibial component in relation to the cementless fixation in studies with osteoarthritis and rheumatoid arthritis participants who underwent primary total knee prosthesis with a follow‐up of two years; however, the cemented fixation presented a greater risk of future aseptic loosening than cementless fixation.
Keywords: Humans; Knee Prosthesis; Arthritis, Rheumatoid; Arthritis, Rheumatoid/surgery; Arthroplasty, Replacement, Knee; Arthroplasty, Replacement, Knee/methods; Biocompatible Materials; Biocompatible Materials/therapeutic use; Bone Cements; Bone Cements/therapeutic use; Durapatite; Durapatite/therapeutic use; Osteoarthritis, Knee; Osteoarthritis, Knee/surgery; Prosthesis Failure; Prosthesis Failure/etiology; Radiostereometric Analysis; Radiostereometric Analysis/methods; Randomized Controlled Trials as Topic; Time Factors; Treatment Outcome
Plain language summary
Fixation options of total knee replacement for osteoarthritis and other non‐traumatic diseases
This summary of a Cochrane review presents previous research about the effects of cemented, cementless or hybrid fixation of total knee replacement (arthroplasty) for osteoarthritis and other non‐traumatic diseases.
Through three high quality trials and 216 participants, we observed that:
‐ The risk of future aseptic loosening with uncemented fixation is approximately half that of cemented fixation in people with knee osteoarthritis and other non‐traumatic diseases.
‐ Sixteen fewer people out of 100 had a future prediction of arthroplasty instability with uncemented fixation (16% fewer, ranging from 27% fewer to 5% fewer).
‐ Thirteen people out of 100 had a future prediction of arthroplasty instability with uncemented fixation.
‐ Twenty‐nine people out of 100 had a future prediction of arthroplasty instability with cemented fixation.
These conclusions were based on an arthroplasty instability classification inferred from radiographic measures.
We have no available evidence provided by this review regarding the survival rate of the implant (any change of a component), patient global assessments, functional measures, pain and health‐related quality of life measures.
We often do not have precise information about adverse events and complications. This is particularly true for rare but serious adverse events. Possible adverse events may include deep vein thrombosis and rare complications may include infections.
What is osteoarthritis and other non‐traumatic diseases of the knee and what types of knee implant fixation methods are available?
Osteoarthritis, also known as degenerative joint disease, has a variety of causes. Osteoarthritis can be classified as either primary or secondary, depending on whether there is an identifiable underlying cause. Most cases of the disease have no known cause and are referred to as primary osteoarthritis. Primary osteoarthritis is mostly related to aging. The causes of secondary osteoarthritis include rheumatoid arthritis, a disease in which the immune system attacks the joints (post‐traumatic causes were not included in this review).
In some patients, damage and pain in the knee from arthritis may be severe enough for surgery. In these patients, the damaged joint surfaces can be replaced by an artificial joint or knee implant. In total knee replacement surgery, the ends of the long bones of the leg (femur and tibia) are usually replaced with metal ends, and an insert is placed between them. The femoral and tibial components can be fixed to the bone with or without cement. Cementation of the tibial component while leaving the femoral component cementless is a hybrid technique.
It is not clear which fixation obtains the best clinical, functional and radiographic results in people with osteoarthritis and other non‐traumatic diseases, such as rheumatoid arthritis. The use of cement in total knee arthroplasty fixation is considered by many authors to be the gold standard but remains a controversial issue.
Summary of findings
Summary of findings for the main comparison. Uncemented fixation with and without hydroxyapatite compared to cemented fixation for osteoarthritis and other non‐traumatic diseases.
| Uncemented fixation with and without hydroxyapatite compared to cemented fixation for osteoarthritis and other non‐traumatic diseases | ||||||
| Patient or population: Patients with osteoarthritis and other non‐traumatic diseases Settings: Osteoarthritis (non‐post‐traumatic patients) Intervention: Uncemented with and without hydroxyapatite Comparison: Cemented | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cemented | Uncemented with and without hydroxyapatite | |||||
|
Arthroplasty instability ‐ inferred from roentgen stereophotogrammetric analysis (Available data analysis) Follow‐up: 1 to 2 years |
287 per 1000 | 135 per 1000 (69 to 264) | RR 0.47 (0.24 to 0.92) | 216 (3 studies) | ⊕⊕⊕ moderate1 | Arthroplasty instability was considered an event. This outcome predicts knee arthroplasty revision rates because of aseptic loosening (provides indirect evidence). Absolute risk difference = 16% (95% CI ‐0.27 to ‐0.05) Relative percent change = 53% (95% CI 8 to 76%) NNTH = 7 (95% CI 5 to 44) |
| Survival rate of the implant (any change of a component) | See comment | See comment | 0 (0) | See comment | Survival rate of the implant is a long term outcome not available in included studies (2 to 5 year follow‐up). | |
| Global Assessment (Patient) | See comment | See comment | 27 (1) | See comment | Toksvig‐Larsen 1988 reported that all of the patients were satisfied with the results at a 2 year follow‐up. We found no mention of degrees of satisfaction. | |
| Functional measures with validated instruments | See comment | See comment | 240 (4) | See comment | Knee Society and HSS knee scores were recorded. The SD of the mean of the results was not reported. The authors found no significant difference between the groups. | |
| Pain | See comment | See comment | 0 (0) | See comment | Not reported. | |
| Health‐related quality of life measures | See comment | See comment | 0 (0) | See comment | Not reported. | |
| Total adverse event | See comment | See comment | 0 (0) | See comment | No evidence available2 | |
| *The basis for the assumed risk (e.g. the median control group risk among studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Roentgen stereophotogrammetric analysis predicts the arthroplasty instability. We downgraded for indirectness of evidence.
2The type of fixation of the femoral component was different from the tibial component in 3 out of 4 studies. It was not possible to correlate some adverse events to the cementation of the arthroplasty from the study description even when the type of fixation of the femoral and tibial components was the same (Nilsson 1999).
Background
Description of the condition
After many years of suffering from the knee joint pain due to disabling diseases, such as rheumatoid arthritis (RA) and osteoarthritis (OA), the only remaining treatment for many patients is surgery and replacement of the joint surfaces with a total knee arthroplasty (TKA). Approximately 20,000 patients undergo TKA in the UK with an annual hospital cost of £70 million showing the economic impact of this disease (McCormack 2009).
The major reason for prosthesis failure is aseptic loosening of the implant (Huiskes 1998). Aseptic loosening of the prosthesis can be related to the type of fixation of the implants, since the quality of fixation of the prosthesis and the load transfer characteristics between the implant and bone are important factors that affect the durability of the arthroplasty (Whiteside 2006). Continued developments are aimed at improving the results of TKA.
Description of the intervention
Prosthesis systems can be crudely divided into two groups: cemented and cementless systems. Additionally, two hybrid techniques are currently used: 1) cementation of the tibial component while leaving the femoral component cementless, and 2) partial cementation of the tibial component (i.e. cementing the base plate and leaving the tibial stem cementless). While some studies have reported outcomes of fully cemented or fully cementless total knee arthroplasties, some studies have simply reported the outcomes of tibial or femoral components from fully cemented, fully cementless, or hybrid systems. Early implants for TKA were fixed to the bone with cement. To improve the survival of the arthroplasty, the cementless implant was developed to proportionate biologic fixation by osseous ingrowth in the implant with a potentially more durable bond of the prosthesis to the bone than the cemented implant (Fehring 2006).
How the intervention might work
Roentgenographic (X‐ray) and histological evaluation of the bone‐cement interface in loosened arthroplasties indicate a pathologic response to foreign materials. Therefore, bone cement has been implicated to be involved in the pathogenesis of implant failure (Harris 1976; Mirra 1976). Cement has not been confirmed to be the initiator of osteolytic problems, and osteolysis has been reported with uncemented arthroplasties (Maloney 1996). Aseptic loosening is mainly related to wear particles of the prosthetic components, but it remains doubtful whether the use of cement affects the durability of the prosthesis (Khaw 2002). The fixation of the prosthesis without cement has the advantage of having a shorter surgical time and ease of arthroplasty revision as the cement is not interlocked with the bone (Fehring 2006). Because cement fixation is not present to help protect against excessive point loading, alignment is more crucial in cementless knee replacements (Brassard 2006).
On the other hand, the cemented fixation of the total knee replacement is considered to be the gold standard by many orthopedic surgeons (Fehring 2006). Cementation potentially creates a barrier against wear debris preventing osteolysis and loosening (McCaskie 1998). Additionally, cement may help to better distribute stresses and strains to the surrounding bone, thereby adding longevity to the implant.
The survival rate of the implant is one of the most important outcomes to be analyzed, and aseptic loosening is the major cause of arthroplasty failure. Most randomized controlled trials comparing the cemented total knee arthroplasty with uncemented total knee arthroplasty have a follow‐up of up to five years, which may be insufficient to show a difference in survival rate. Roentgen stereophotogrammetric analysis (RSA), a radiological method that can predict the risk of future aseptic loosening (predictive power of approximately 85%) through the analysis of small movements of the implant, has been used in studies with a follow‐up of two years (Kärrholm 2006; Ryd 1995).
In addition to the ability to maintain mechanical contact to the implantation site, various attributes, such as knee function, knee pain, susceptibility to certain complications, ease of re‐operation and cost consumption, are also of interest.
Why it is important to do this review
Gandhi 2009 conducted meta‐analyses to verify clinical function and survival of fully cemented and fully uncemented prostheses in total knee replacement. These meta‐analyses included randomized and quasi‐randomized controlled trials and observational studies. Gandhi 2009 excluded studies with hybrid fixation of the arthroplasty and did not exclude the studies with post‐traumatic participants. The survival rate of the implant free of aseptic loosening was based on studies with a minimum follow‐up of two years, and in a subgroup analysis of data only from RCTs showed no differences between the groups for odds of aseptic loosening (Odds ratio (OR) 1.9, 95% CI 0.55 to 6.40; P = 0.314).
Our systematic review included only randomized controlled studies. Studies with hybrid fixation were included and post‐traumatic studies were excluded. In addition to clinical function and survival of implants studied by Gandhi 2009, our systematic review included RSA to provide an idea of aseptic loosening even with a short follow‐up of two years. Other outcomes were foreseen in our systematic review like health‐related quality of life measures and total adverse events (Types of outcome measures).
A number of RCTs have been performed with conflicting results. The use of cement in TKA fixation is considered by many authors to be the gold standard but remains a controversial issue. There are other issues that can potentially affect the results of a total knee arthroplasty but remain inconclusive (mobile bearing versus fixed bearing, and posterior cruciate ligament sacrifice versus posterior cruciate ligament retention) and are analyzed in other systematic reviews (Wilco 2001; Wilco 2005).
Objectives
To assess the effects (benefits and harms) of cemented, cementless and hybrid knee prostheses fixation techniques in participants with primary osteoarthritis (osteoarthritis following trauma was not included) and other non‐traumatic diseases.
Methods
Criteria for considering studies for this review
Types of studies
We included reports of randomized controlled trials. In the case of multiple publications of a given data set, we included the first published article in our analysis. Exceptions to this rule were made if a more recent publication corroborated the results of a longer follow‐up.
Types of participants
Patients aged 18 years or older who were undergoing implantation of a primary knee arthroplasty were eligible. We considered all of the diagnostic indications for the implantation of a TKA, especially osteoarthritis and rheumatoid arthritis. Participants that received a TKA following trauma or revision arthroplasties were excluded.
Types of interventions
The intervention of interest was the primary implantation of a cemented, cementless or hybrid total knee prosthesis.
‐ Cemented fixation of the arthroplasty components versus cementless fixation of the arthroplasty components (with or without hydroxyapatite).
‐ Cemented fixation of the arthroplasty components versus hybrid fixation (cementless fixation with or without hydroxyapatite of one arthroplasty component and cemented fixation of the other component).
‐ Cementless fixation of the arthroplasty components (with or without hydroxyapatite) versus hybrid fixation (cementless fixation with or without hydroxyapatite of one arthroplasty component and cemented fixation of the other component).
Types of outcome measures
The major outcomes considered were the survival rate of the implant, roentgen stereophotogrammetric analysis (absolute values of rotation in three orthogonal axes (transverse, longitudinal and sagittal), maximum total point‐motion (MTPM), subsidence, lift‐off, induced displacement and arthroplasty instability (indirect outcome)), functional measures with validated instruments (e.g. OXFORD, IKDC), knee pain (e.g. VAS), range of motion (with validated methods), general functional abilities (e.g. WOMAC), health‐related quality of life measures (e.g. SF36), global assessment (patient), total adverse event (e.g. deep vein thrombosis).
Minor outcomes considered included length of surgery (in minutes), operative blood loss (in milliliters), red blood cell count, hemoglobin level, hematocrit, postoperative blood transfusion (in units), rate of lateral release, days to mobilization, length of hospital stay (days), discharge destination, walking aids at discharge, extensor mechanism function, gait analysis results and costs.
The outcomes were evaluated with validated instruments, and although all of the scales were reported in the review, the pooling of instruments that have not been validated in the next update of the review will be examined in a sensitivity analysis.
Search methods for identification of studies
The following databases were searched to identify randomized controlled trials from inception until 31 October 2011:
the Cochrane Central Register of Controlled Trials (CENTRAL in The Cochrane Library 2011, issue 10);
Current Controlled Trials (www.controlled‐trials.com);
MEDLINE via PubMed;
EMBASE;
LILACS (Latin American and Caribbean Health Science Literature available at http://bases.bvs.br);
Cumulative Index to Nursing and Allied Health Literature (CINAHL);
SPORTDiscus;
Health Technology Assessment Database (HTA);
Database of Abstracts of Reviews of Effectiveness (DARE).
The "optimal" sensitivity search strategies designed to identify clinical trials were used, as described by Dickersin et al (Dickersin 1994) and Castro et al (Castro 1999).
Complete strategies are described for CENTRAL (Appendix 1), MEDLINE via PubMed (Appendix 2), EMBASE via OVID (Appendix 3) and LILACS (Appendix 4).
In addition to these electronic search strategies, we performed handsearches of high‐yield journals (The Journal of Bone and Joint Surgery ‐ American Volume (1980 to October 2011), The Journal of Bone and Joint Surgery ‐ British Volume (1980 to October 2011), The Journal of Arthroplasty (1986 to October 2011) and Clinical Orthopaedics and Related Research (1980 to October 2011)), and additionally, we surveyed the reference lists of relevant articles. Researchers were contacted personally to retrieve information that was not presented in the original articles. No language restrictions were applied.
Data collection and analysis
Selection of studies
Two authors (GYN and OAL) independently selected trials for inclusion, extracted data, assessed trial quality and analyzed results. When there were disagreements, a third author (MSP) was consulted, and in situations where a consensus was not reached, the data were not included in the review until the author of the trial resolved the question.
Data extraction and management
The review authors screened the titles and abstracts of publications obtained by the search strategy. If a study fulfilled the inclusion criteria, the data concerning methodological issues, characteristics of participants, interventions and outcome measures were independently extracted using a standard extraction form. A one year follow‐up was considered a short‐term follow‐up and five years or more was considered a long‐term follow‐up.
Assessment of risk of bias in included studies
The risk of bias in included studies was assessed by two authors (GYN and OAL) according to the Cochrane Handbook (Higgins 2011). When there were disagreements a third author (MSP) was consulted.
The domains used in the risk of bias tool were:
‐ random sequence generation;
‐ allocation concealment;
‐ blinding of participants and personnel (surgeons);
‐ blinding of outcome assessment;
‐ incomplete outcome data;
‐ selective reporting.
Measures of treatment effect
For dichotomous data, risk ratios (RR) and 95% confidence intervals (CI) were estimated according to the intention‐to‐treat principles. For pooled data, the random‐effects model was used. For all statistically significant results, the number needed to treat for an additional beneficial outcome (NNTB) and/or the number needed to treat for an additional harmful outcome (NNTH) were also calculated using the Cates calculator for dichotomous data (Cates 2004).
Continuous outcomes were analyzed with the mean and standard deviation of endpoint measures. For the meta‐analysis of continuous outcomes, mean differences (MDs) between groups were estimated, and when different scales were used, standardized mean differences (SMDs) were estimated. A random‐effects model was used for all of the analyses in this review.
Data on continuous outcomes are frequently skewed with the mean being different from the center of the distribution. The statistics for meta‐analysis are thought to be able to cope with some skew but were actually formulated for parametric data. To avoid this potential pitfall, the following standards were applied to all of the data before inclusion: (1) standard deviations and means were reported or obtained from authors, and (2) for data with finite limits, such as endpoint scale data, the standard deviation (SD), when multiplied by two, had to be less than the mean. Otherwise, the mean would be unlikely to be an appropriate measure of the center of the distribution. In such circumstances, skewness must be handled through transforming the data, if possible, or through a sensitivity analysis (Higgins 2011). For skewed data, the analysis suggested by Altman (Altman 1995; Altman 1996) must be used. However, we found no skewed data.
Unit of analysis issues
There was no unit of analysis error. The number of observations matched the number of participants knees randomized.
Dealing with missing data
We performed an analysis of data based on available data and intention‐to‐treat principles (Higgins 2011).
For dichotomous data, we assumed either poor outcomes or good outcomes for withdrawn participants. For arthroplasty instability classification, we assumed either that all missing participants were stable, or that all missing participants were unstable.
For continuous data, when participants were lost to follow‐up and the individual data of all participants were available, the last available observation outcome of each participant withdrawn from the study was imputed in the intention‐to‐treat analysis. The mean value of the first evaluation outcome of the randomized group was attributed to each randomized participant withdrawn from the study before the first evaluation.
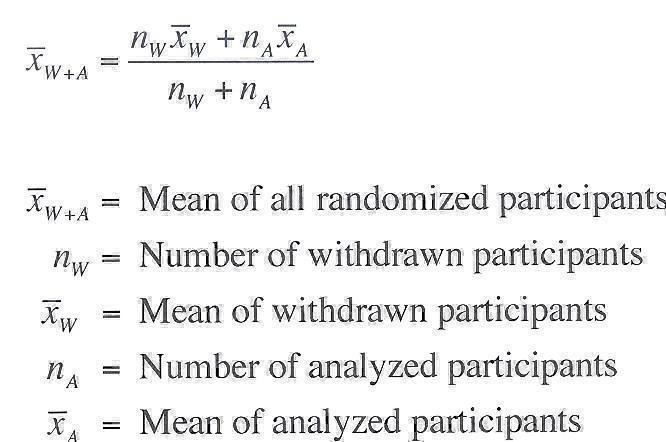
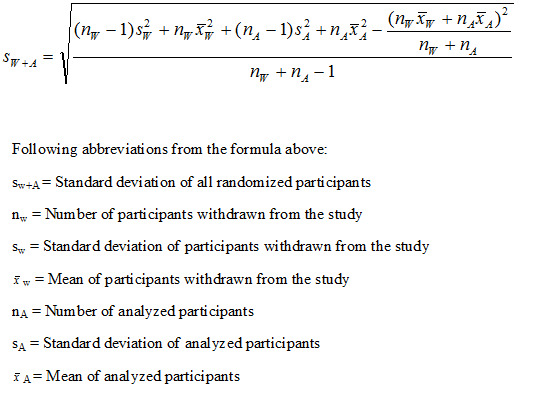
For continuous data, when participants were withdrawn from the study and the individual data of all participants were not available, the mean and standard deviation of all the randomized participants were calculated using the mean and standard deviation of the analyzed participant groups and the mean and standard deviation of the participants withdrawn from the study based on the known last evaluation outcome. The mean and standard deviation of all the randomized participants were calculated according to the formulas in Figure 1 and in Figure 2 (Morais 2012).
1.
2.
Assessment of heterogeneity
Heterogeneity was assessed using the Chi2 test in conjunction with the I2 statistic (a value greater than 50% was considered to demonstrate substantial heterogeneity). The significance for the Chi2 test was set at P < 0.10 due to the low power of this test (Higgins 2011). We also assessed the heterogeneity of estimate effects between the included studies by visual inspection of the forest plot analysis. When significant heterogeneity was present, we attempted to explain the differences based on the clinical characteristics of the included studies.
Assessment of reporting biases
When possible, we plotted data on a funnel graph in order to assess publication bias.
Data synthesis
Statistical analyses were conducted using Review Manager 5 (RevMan) software.
Continuous data were entered as means and standard deviations.
Dichotomous data were entered as events and number of participants.
The intention‐to‐treat analysis for dichotomous data assumed either that all of the missing participants experienced the event or that all of the missing participants did not experience the event.
In the absence of significant heterogeneity and given sufficient included trials, the results were combined for the meta‐analysis using the random‐effects model.
We used the grading system described in the GRADE handbook for grading the quality of evidence (Schünemann 2009) recommended by the Cochrane Handbook (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
It is possible that the treatment effects might differ not because of statistical heterogeneity, but rather clinical heterogeneity (e.g. cemented or cementless endoprosthesis may be more effective in osteoarthritis than in rheumatoid arthritis or vice versa). To evaluate this possibility, we carried out stratification for the most important clinical parameters (effect modifiers) that could influence the effect of the knee endoprosthesis technique on the selected clinical outcomes.
Sensitivity analysis
It was not possible to perform a sensitivity analysis.
Results
Description of studies
Results of the search
The database search resulted in 551 references. Of these, 533 studies were considered to be irrelevant based on the title, abstract and full text. After personal communication with at least one of the authors, six studies were withdrawn because they did not meet the systematic review inclusion criteria (Albrektsson 1992; Beaupré 2007; Ishii 2005a; Keblish 1993; Khaw 2002; Nilsson 2006). The studies by Önsten 1998 and Khaw 2002 were the same as the studies by Carlsson 2005 and Baker 2007 with a shorter follow‐up, respectively. Two studies (Nilsson 1992; Parker 2001) are awaiting classification because one author of each study was contacted, but there has not been a reply to our request concerning the etiology of osteoarthritis (i.e. post‐traumatic or not). Three studies (Demey 2010; Dunbar 2009; Nelissen 1998) are awaiting classification depending on participant characteristics (there was no feedback from authors to know if they included post‐traumatic osteoarthritis). For more information, see the table 'Characteristics of studies awaiting classification'. Five studies met the inclusion criteria. Full details of the included studies are given in the table 'Characteristics of included studies'. The studies by Carlsson 2005, Nilsson 1999 and Toksvig‐Larsen 1998 included 238 tibial components of participants that were evaluated in this systematic review. Uvehammer 2007 studied the femoral components. Ishii 2005b studied the perioperative blood loss in cementless and hybrid total knee arthroplasty. The study flow diagram is summarized in Figure 3.
3.
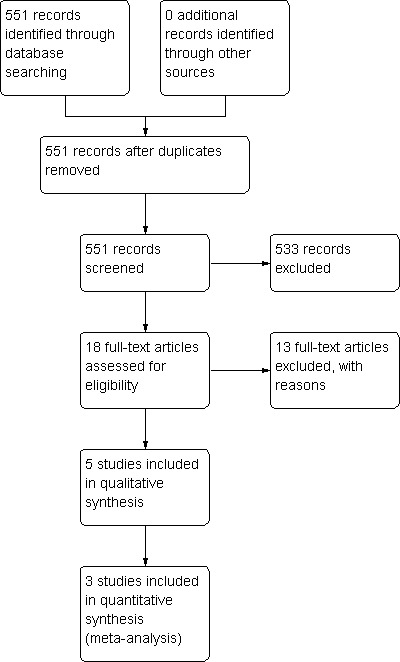
Study flow diagram.
Included studies
Toksvig‐Larsen 1998 analyzed the total knee arthroplasty tibial components randomized to cemented or uncemented without hydroxyapatite (HA) fixation. A follow‐up of two years was used for 27 OA participants (28 knees) that were included in this study. The following measures were evaluated: roentgen stereophotogrammetric analysis (RSA), induced displacement, maximum total point‐motion (MTPM) and subsidence, hip‐knee‐ankle‐angle (HKA), Hospital for Special Surgery knee score (HSS), extension, flexion and walking distance.
Nilsson 1999 analyzed the tibial components randomized to cemented or uncemented with hydroxyapatite fixation, and 43 OA participants (45 knees) and 13 RA (15 knees) were included in this study with a follow‐up of five years. The following measures were evaluated: RSA (absolute values of rotation in three orthogonal axes, MTPM, subsidence, and lift‐off), Knee Society knee score, range of motion and radiolucent line analysis at the interface of the tibial component as described by the Knee Society.
Carlsson 2005 analyzed the tibial components randomized to cemented, uncemented without hydroxyapatite, or uncemented with hydroxyapatite fixation, and 120 OA participants (150 knees) were included in this study with a follow‐up of five years. The following measures were evaluated: RSA (absolute values of rotation in three orthogonal axes, MTPM, subsidence, and lift‐off), the Knee Society clinical rating system and the method of Ewald (the radiographs were evaluated for any osteolytic lesions or any progressive implant bone or cement‐bone lucencies using the method of Ewald, in which the interface around the tibial component was analyzed in 10 zones on antero‐posterior and lateral radiographs).
Ishii 2005b randomized participants (54 OA and 3 RA participants) to the prosthesis fixed without cement or hybrid (cemented tibia and femur without cement) and analyzed total blood loss. Red blood cell count, hemoglobin level, and hematocrit were recorded preoperatively and one week, four weeks, and three months postoperatively. Torniquet time and operation time were recorded too.
Uvehammer 2007 analyzed the femoral components randomized to cemented or uncemented with hydroxyapatite fixation, and 50 OA participants (54 knees) were included in this study with a follow‐up of two years. The following measures were evaluated: RSA (absolute values of rotation in three orthogonal axes), HKA, and HSS.
Excluded studies
The following exclusion criteria were used for these eight studies (Albrektsson 1992; Baker 2007; Beaupré 2007; Ishii 2005a; Keblish 1993; Khaw 2002; Nilsson 2006; Önsten 1998): two studies evaluated post‐traumatic osteoarthritis participants (Albrektsson 1992; Nilsson 2006); in one study, the prosthesis was always cemented (Keblish 1993); the study by Önsten 1998 was the same as the study by Carlsson 2005 with a shorter follow‐up; two studies did not differentiate whether arthritis was post‐traumatic or not (Beaupré 2007; Khaw 2002); one study was not randomized (Ishii 2005a); and the study by Baker 2007 was the same as the study by Khaw 2002 with a longer follow‐up. For exclusion details, see the table 'Characteristics of excluded studies'.
Risk of bias in included studies
We assessed the risk of bias of the trials included in this review (see table 'Characteristics of included studies') according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and generated risk of bias figures (Figure 4 and Figure 5).
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation concealment was considered adequate in four studies (Carlsson 2005; Nilsson 1999; Toksvig‐Larsen 1998; Uvehammer 2007). Ishii 2005b was the only study that did not describe the allocation concealment.
Blinding
Only Carlsson 2005 reported that participants were blinded for the intervention. In this type of study, the surgeon cannot be blinded to the fixation. The clinical measures were assessed by the surgeons in the studies by Toksvig‐Larsen 1998, Nilsson 1999 and Carlsson 2005. Uvehammer 2007 did not comment on whether the outcome assessors were blinded.
Incomplete outcome data
The withdrawal/drop‐out rate was described, acceptable (< 20%) and comparable in both groups in Carlsson 2005 and Ishii 2005b studies. The rate of drop‐out in the studies by Nilsson 1999 and Uvehammer 2007 was described and was comparable in both groups, but was not acceptable (missing outcome data were balanced in numbers across intervention groups with similar reasons for missing data across groups). In the study by Toksvig‐Larsen 1998, the withdrawal/drop‐out rate was described, was not acceptable and was not comparable between the groups.
Selective reporting
All of the included studies were considered to be free of selective reporting.
Other potential sources of bias
None known.
Effects of interventions
See: Table 1
Meta‐analysis
Carlsson 2005 provided us with the individual results of all of the participants (Carlsson 2005), which facilitated the intention‐to‐treat analysis. In the study by Nilsson 1999, we collected the mean and standard deviation of the analyzed participants group and the individual outcomes of the last evaluation of participants withdrawn from study. We have described the calculation of the mean and standard deviation of all of the randomized participants from the studies by Carlsson 2005 and Nilsson 1999 in the Data collection and analysis section (statistical analyses).
A meta‐analysis was performed on the measures presented for more than one study comparing the same follow‐up. An intention‐to‐treat analysis and the study available data analysis were performed. Comparable data included the following: absolute values around the three orthogonal axes (transverse, longitudinal and sagittal), maximum total point motion (MTPM) and arthroplasty instability (Ryd 1995).
We could not perform a meta‐analysis on clinical outcomes presented in the included studies (Knee Society knee score, Hospital for Special Surgery knee score, Range of Motion and Satisfaction) because the combination of tibial and femoral fixation was not comparable among the studies. For example, in the study by Nilsson 1999, if a patient was randomized to cemented tibial fixation, the femoral component should have been cemented as well. However, in the study by Carlsson 2005, if a patient was randomized to the cemented fixation, the femoral fixation was performed according to the preference of the surgeons.
It was not possible to include the study by Uvehammer 2007 in the meta‐analysis because it did not have comparable data.
Cemented fixation of the tibial arthroplasty component versus cementless fixation of the tibial component with hydroxyapatite
Major outcomes
1. Roentgen stereophotogrammetric analysis
1a. Absolute values of rotation in three orthogonal axes (transverse, longitudinal, and sagittal)
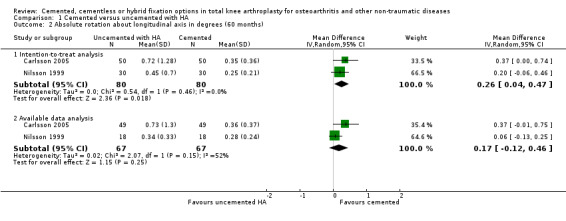
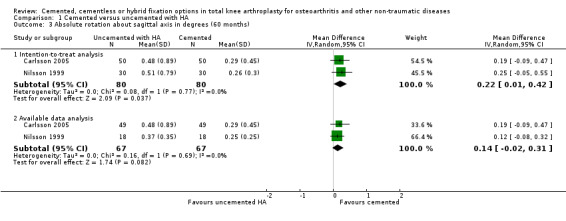
The absolute rotation around the longitudinal and sagittal axes (five year follow‐up) in an intention‐to‐treat analysis of the studies by Nilsson 1999 and Carlsson 2005 revealed a MD of 0.26 degrees (95% CI 0.04 to 0.47; P = 0.02) and 0.22 degrees (95% CI 0.01 to 0.42; P = 0.04), respectively, which favored the cemented fixation over the uncemented fixation with hydroxyapatite.
We found no differences among the groups in the transverse axis in available data analyses of the three orthogonal axes.
1b. Arthroplasty instability
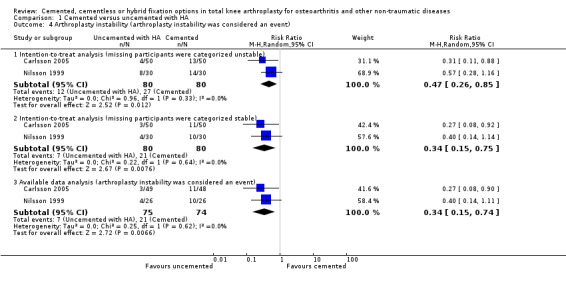
According to instability classification, uncemented fixation with hydroxyapatite was superior to cemented fixation according to available data (arthroplasty instability was considered an event), an intention‐to‐treat analysis (missing participants were categorized as unstable), and an intention‐to‐treat analysis (missing participants were categorized as stable), which revealed a RR of 0.34 (95% CI 0.15 to 0.74; P = 0.007), a RR of 0.47 (95% CI 0.26 to 0.85; P = 0.01), and a RR of 0.34 (95% CI 0.15 to 0.74; P = 0.008), respectively (Carlsson 2005; Nilsson 1999). A NNTH of 6 was found for available data, an intention‐to‐treat analysis (missing participants were categorized as unstable), and an intention‐to‐treat analysis (missing participants were categorized as stable). One additional person will likely incur arthroplasty instability for every six participants receiving cemented fixation rather than uncemented fixation with hydroxyapatite.
Cemented fixation of the tibial arthroplasty component versus cementless fixation of the tibial component with and without hydroxyapatite
Major outcomes
1. Roentgen stereophotogrammetric analysis
1a. Absolute values of rotation in three orthogonal axes (transverse, longitudinal, and sagittal)
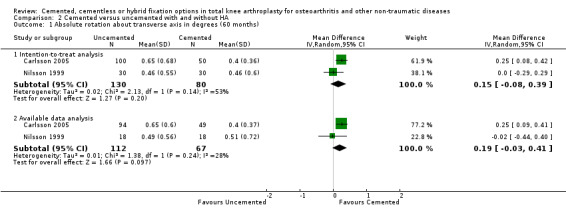
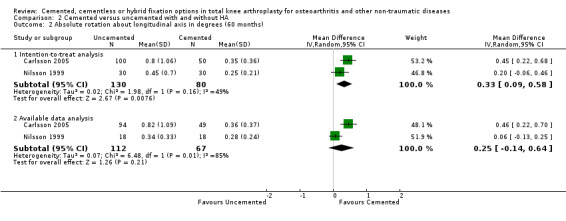
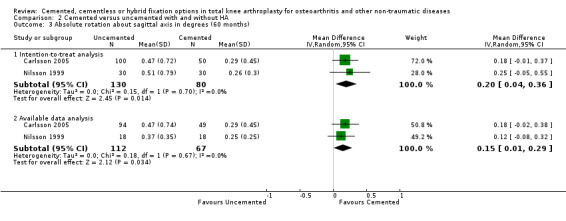
A meta‐analysis of Nilsson 1999 and Carlsson 2005 demonstrated that cemented fixation was superior to uncemented fixation with and without hydroxyapatite (uncemented types of fixation were grouped together). The MD was 0.33 degrees (95% CI 0.09 to 0.58; P = 0.008) for absolute rotation around the longitudinal axis (five year follow‐up) through an intention‐to‐treat analysis. For absolute rotation around the sagittal axis (five year follow‐up), the MD was 0.15 degrees (95% CI 0.01 to 0.29; P = 0.03) through available data analysis and 0.20 degrees (95% CI 0.04 to 0.36; P = 0.02) through an intention‐to‐treat analysis.
We found no differences among the groups in the transverse axis.
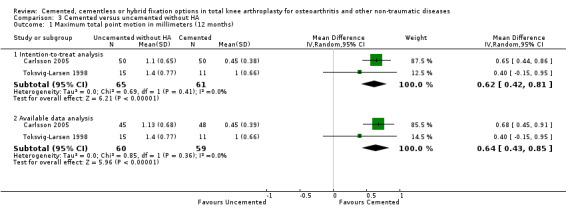
1b. Maximum total point‐motion (MTPM)
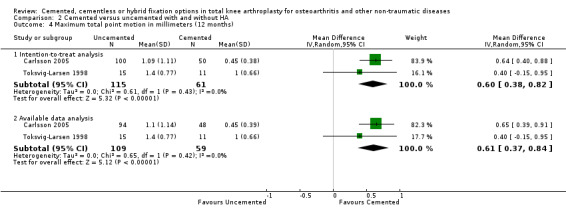
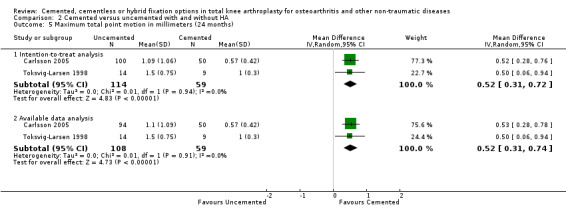
Regarding the 12‐month MTPM (Carlsson 2005; Toksvig‐Larsen 1998), cemented fixation was superior to uncemented fixation with or without hydroxyapatite according to the available data and an intention‐to‐treat analysis, revealing MTPMs of 0.61 mm (MD) (95% CI 0.37 to 0.84; P < 0.00001) and 0.60 mm (MD) (95% CI 0.38 to 0.82; P < 0.00001), respectively. The 24‐month MTPM data confirmed the superiority of cemented fixation to uncemented fixation with or without hydroxyapatite according to the available data and an intention‐to‐treat analysis, which revealed MTPMs of 0.52 mm (MD) (95% CI 0.31 to 0.74; P < 0.00001) and 0.52 mm (MD) (95% CI 0.31 to 0.72; P < 0.00001), respectively.
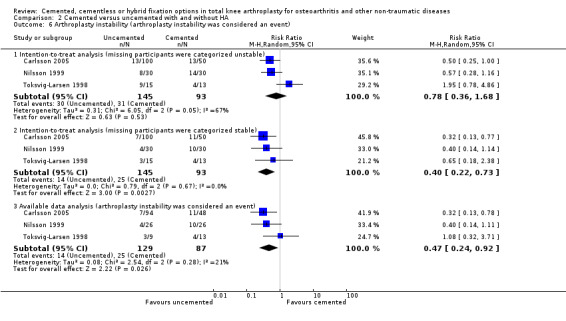
1c. Arthroplasty instability
Concerning arthroplasty instability (Ryd 1995), uncemented fixation with and without hydroxyapatite was found to be superior to cemented fixation through available data (arthroplasty instability was considered an event) and an intention‐to‐treat analysis (missing participants were categorized as stable), revealing an RR of 0.47 (95% CI 0.24 to 0.92; P = 0.03) and an RR of 0.40 (95% CI 0.22 to 0.73; P = 0.003), respectively. We found no difference with the same comparison using an intention‐to‐treat analysis when missing participants were categorized as unstable, which revealed an RR of 0.78 (95% CI 0.36 to 1.68; P = 0.53 (Carlsson 2005; Nilsson 1999; Toksvig‐Larsen 1998). Numbers needed to treat for an additional harmful outcome (NNTH) were 7 and 8 for available data analysis and an intention‐to‐treat analysis, respectively (missing participants were categorized as stable).
Cemented fixation of the tibial arthroplasty component versus cementless fixation of the tibial component without hydroxyapatite
Major outcomes
1. Roentgen stereophotogrammetric analysis
1a. Maximum total point‐motion (MTPM)
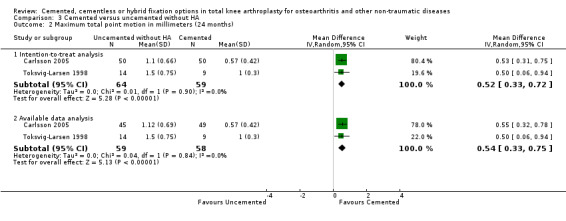
Regarding the 12‐month MTPM (Carlsson 2005; Toksvig‐Larsen 1998), cemented fixation was superior to uncemented fixation without hydroxyapatite according to available data and an intention‐to‐treat analysis, which reported MTPMs of 0.64 mm (MD) (95% CI 0.43 to 0.85; P < 0.00001) and 0.62 mm (MD) (95% CI 0.42 to 0.81; P < 0.00001), respectively. The 24‐month MTPM data confirmed the superiority of cemented fixation to uncemented fixation with or without hydroxyapatite according to the available data and an intention‐to‐treat analysis, which revealed MTPMs of 0.54 mm (MD) (95% CI 0.33 to 0.75; P < 0.00001) and 0.52 mm (MD) (95% CI 0.33 to 0.72; P < 0.00001), respectively.
1b. Arthroplasty instability
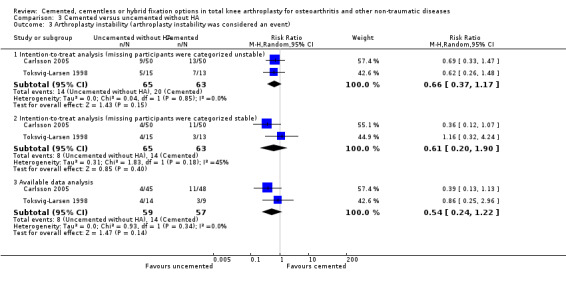
We found no differences regarding arthroplasty instability when uncemented fixation without hydroxyapatite was compared to cemented fixation using available data (arthroplasty instability was considered an event), an intention‐to‐treat analysis (missing participants were categorized as unstable), and another intention‐to‐treat analysis (missing participants were categorized as stable), which revealed RRs of 0.54 (95% CI 0.24 to 1.22; P = 0.14), 0.66 (95% CI 0.37 to 1.17; P = 0.15), and 0.61 (95% CI 0.20 to 1.90; P = 0.4), respectively (Carlsson 2005; Toksvig‐Larsen 1998).
Outcomes not analyzed by meta‐analysis
A meta‐analysis and an intention‐to‐treat analysis were performed on the available data when outcomes were observed in more than one study with the same follow‐up time. The tibial component was randomized in all of the studies except the study by Uvehammer 2007 (randomization of the femoral component). We could not perform a meta‐analysis on the following outcomes.
The Hospital for Special Surgery knee score (HSS) results were reported in the studies by Uvehammer 2007 and Toksvig‐Larsen 1998. This rating system generates a maximum score of 100 points. The resultant score was classified in the following ranges: > 85 was excellent, 70 to 84 was good, 60 to 69 was fair and < 60 was poor. According to Uvehammer 2007, at the two year follow‐up, the median HSS scores for the cemented (N = 27) and uncemented with hydroxyapatite (N = 21) femoral component groups were 92 (no SD; range 66 to 98) and 94 (no SD; range 70 to 98), respectively (P = 0.4) (the preoperative score was not reported). In the study by Toksvig‐Larsen 1998, the median HSS preoperative scores for the cemented (N = 9) and uncemented without hydroxyapatite (N = 14) groups were 59 (no SD; range 46 to 79) and 59 (no SD; range 42 to 87), respectively. The postoperative scores at two years for the cemented and uncemented groups were 93 (no SD; range 90 to 97) and 88 (no SD; range 74 to 98), respectively. No significant differences were detected between the groups.
The Knee Society clinical rating system results were reported by Carlsson 2005 and Nilsson 1999. The Knee Society clinical rating score consists of two scores: a knee score and a functional score, which range from 0 to 100 points, with 100 points being the best score. Fifty of the 100 points in the knee score reflect pain assessment. In the study by Carlsson 2005, knee osteoarthritis cases were stratified into two series. Series I comprised 90 patients submitted to unilateral total knee replacement, and Series II comprised 30 patients with bilateral, simultaneous knee replacements. The knees were randomized with respect to the fixation of the tibial component: cemented, uncemented with hydroxyapatite and uncemented without hydroxyapatite. The study did not individualize the preoperative and postoperative results to the type of tibial fixation but found no difference between the groups (five year follow‐up). In the study by Nilsson 1999, the median Knee Society knee scores for the cemented (N = 26) and uncemented with hydroxyapatite (N = 27) groups were 15 (no SD; range 0 to 59) and 11 (no SD; range 0 to 43), respectively. At the five year follow‐up, the median Knee Society knee scores for the cemented and uncemented with hydroxyapatite groups were 93 (no SD; range 69 to 99) and 93 (no SD; range 74 to 99), respectively. No significant difference was detected between the groups.
According to the study by Toksvig‐Larsen 1998, all of the patients (9 cemented and 14 uncemented without hydroxyapatite) were satisfied with the results at a two year follow‐up.
Total blood loss, red blood cell count, hemoglobin level, and hematocrit were reported by Ishii 2005b. Total blood loss did not differ between the two groups (cemented tibial component, 731 ± 288 mL; cementless tibial component, 731 ± 331 mL; P = 0.9117). The red blood cell count, hemoglobin level, and hematocrit returned to the preoperative levels within three months in both groups. There were no significant differences in tourniquet time (mean 65 ± 13 versus 60 ± 12 minutes in cementless and hybrid, respectively) or operation time (59 ± 12 and 55 ± 13 minutes, respectively) between the groups. No patient needed an additional transfusion. No significant difference was detected between the groups.
Complications
Complications are summarized in Table 2.
1. Complications.
| Complications | Toksvig‐Larsen 1998 | Nilsson 1999 | Carlsson 2005 | Uvehammer 2007 |
| Unrelated deaths (N and fixation) |
2 cemented | 3 cemented, 5 uncemented with hydroxyapatite | 1 cemented, 4 uncemented without hydroxyapatite, 1 uncemented with hydroxyapatite |
2 cemented, 1 uncemented with hydroxyapatite |
| Cardiac infarction (N and fixation) |
1 cemented | 1? (5‐day PO) |
‐ | ‐ |
| Revision because of loosening (N and fixation) |
‐ | 1 uncemented with hydroxyapatite | ‐ | ‐ |
| Revision because of infection (N and fixation) |
‐ | 2 uncemented with hydroxyapatite | 1 uncemented without hydroxyapatite | ‐ |
| Revision because of instability (N and fixation) |
‐ | ‐ | 1 cemented | ‐ |
| Cerebral hemorrhage (N and fixation) |
‐ | 1 ? | ‐ | ‐ |
| Cerebral infarction (N and fixation) |
‐ | 1 cemented (2‐year PO) | ‐ | ‐ |
| Periprosthetic fractures (N and fixation) |
‐ | 2 cemented, 1 uncemented with hydroxyapatite | ‐ | ‐ |
| Aseptic necrosis of the patella (N and fixation) |
‐ | 1? | ‐ | ‐ |
? = Type of fixation not described
PO = postoperative
N = number of participants
Two deaths not related to surgical intervention and one myocardial infarction before the first roentgen stereophotogrammetric analysis (RSA) occurred in the study by Toksvig‐Larsen 1998 (all of the participants underwent tibial component fixation with cement).
During the study by Nilsson 1999, eight deaths not related to surgical intervention occurred (three from the cemented group and five from the uncemented with hydroxyapatite group). Before RSA, three participants were excluded: one due to myocardial infarction on the third postoperative day, one due to hemorrhage on the fifth postoperative day, and one participant was not treated according to the protocol. From these participants, two belonged to the cemented group and one belonged to the uncemented with hydroxyapatite group. However, it was not possible to correlate these participants to the events from the study description. Three participants were revised (loosening of the tibial component with uncemented fixation with hydroxyapatite occurred to one participant in the seventh postoperative month, and two participants with rheumatoid arthritis from the uncemented with hydroxyapatite group were revised between the first and second year due to infection).
Three participants were submitted to osteosynthesis due to a supracondylar femoral fracture (one uncemented with hydroxyapatite and two cemented) and remained in the analysis. Patellar aseptic necrosis occurred in one participant treated nonoperatively that evolved painlessly in a five year follow‐up. A participant from the cemented group was excluded due to cerebral infarction after two years. In the study by Carlsson 2005, one participant from the uncemented without hydroxyapatite group was revised due to infection, another due to instability (cemented group), and six participants died due to causes not related to the intervention (one from the cemented group, four from the uncemented without hydroxyapatite group and one from the uncemented with hydroxyapatite group).
In the study by Uvehammer 2007, three unrelated deaths occurred (two from the cemented group and one from the uncemented with hydroxyapatite group).
Ishii 2005b reported no complications like persistent drainage, dehiscence or infection.
Heterogeneity
Except for rotation about the longitudinal axis, the data for the cemented fixation group versus the uncemented with and without hydroxyapatite groups comparison demonstrated no statistically significant heterogeneity using the Chi2 test in conjunction with I2 for all of the meta‐analyses, including rotation about the transversal and sagittal axes in the same studies. The heterogeneity was also assessed by visual inspection of the forest plots (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 3.1; Analysis 3.2; Analysis 3.3).
1.1. Analysis.
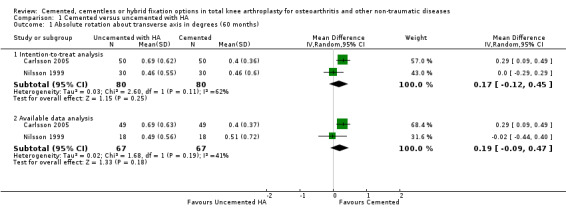
Comparison 1 Cemented versus uncemented with HA, Outcome 1 Absolute rotation about transverse axis in degrees (60 months).
1.2. Analysis.
Comparison 1 Cemented versus uncemented with HA, Outcome 2 Absolute rotation about longitudinal axis in degrees (60 months).
1.3. Analysis.
Comparison 1 Cemented versus uncemented with HA, Outcome 3 Absolute rotation about sagittal axis in degrees (60 months).
1.4. Analysis.
Comparison 1 Cemented versus uncemented with HA, Outcome 4 Arthroplasty instability (arthroplasty instability was considered an event).
2.1. Analysis.
Comparison 2 Cemented versus uncemented with and without HA, Outcome 1 Absolute rotation about transverse axis in degrees (60 months).
2.2. Analysis.
Comparison 2 Cemented versus uncemented with and without HA, Outcome 2 Absolute rotation about longitudinal axis in degrees (60 months).
2.3. Analysis.
Comparison 2 Cemented versus uncemented with and without HA, Outcome 3 Absolute rotation about sagittal axis in degrees (60 months).
2.4. Analysis.
Comparison 2 Cemented versus uncemented with and without HA, Outcome 4 Maximum total point motion in millimeters (12 months).
2.5. Analysis.
Comparison 2 Cemented versus uncemented with and without HA, Outcome 5 Maximum total point motion in millimeters (24 months).
2.6. Analysis.
Comparison 2 Cemented versus uncemented with and without HA, Outcome 6 Arthroplasty instability (arthroplasty instability was considered an event).
3.1. Analysis.
Comparison 3 Cemented versus uncemented without HA, Outcome 1 Maximum total point motion in millimeters (12 months).
3.2. Analysis.
Comparison 3 Cemented versus uncemented without HA, Outcome 2 Maximum total point motion in millimeters (24 months).
3.3. Analysis.
Comparison 3 Cemented versus uncemented without HA, Outcome 3 Arthroplasty instability (arthroplasty instability was considered an event).
Subgroup analysis
Since Carlsson 2005 evaluated three types of tibial fixation (cemented, uncemented without hydroxyapatite and uncemented with hydroxyapatite), a comparison between the cemented group and the two types of uncemented fixation was possible (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6). While migration analysis through the MTPM demonstrated that the cemented fixation migrated less than the uncemented types of fixation in 24 months, the uncemented types of fixation appeared to be more stable.
All of the studies included in this review evaluated osteoarthritis participants. Nilsson 1999 included rheumatoid arthritis participants, and because there was no significant difference between these diagnoses, this author did not present separated results. Therefore, it was not possible to analyze subgroups according to the diagnosis. Ishii 2005b also included three rheumatoid arthritis participants but separated results were not provided.
Assessing publication bias
A funnel plot with three and four studies was not considered useful and, therefore, was not produced.
Grading of the evidence and summary of findings table
The meta‐analysis outcome was graded (Table 1) according to the GRADE approach (Schünemann 2009).
The following seven important outcomes were selected for the summary of findings table: arthroplasty instability, survival of the implant, global assessment (patient), functional measures with validated instruments, pain, health‐related quality of life, and total adverse events.
The quality of evidence was classified as moderate for arthroplasty instability because of the indirectness of the evidence.
We could not perform an analysis on the other patient‐important outcomes.
Discussion
Summary of main results
Four of the five included studies (Carlsson 2005; Nilsson 1999; Toksvig‐Larsen 1998; Uvehammer 2007) evaluated clinical and radiographic measures, with a particular focus on roentgen stereophotogrammetric analyses (RSA).
Roentgen stereophotogrammetric analysis allows the accurate three‐dimensional measurement of micromotion between an implant and the host bone using roentgen rays (Kärrholm 2006; Valstar 2005). Usually tantalum markers are fixed into the arthroplasty implant and into the bone where this implant is placed. After the surgery, control radiographs capture the distances between markers inside the implant and those in the bone. During follow‐up, new radiographs are performed to measure the distance between markers, and consequently between the implant and bone. The most commonly used variables are absolute rotation around the three axes, maximum total point‐motion (MTPM), lift‐off, and subsidence. The rotations are measured around the transverse (flexion/extension), longitudinal (internal/external rotation) and sagittal (varus/valgus) axes. The MTPM is the total three‐dimensional vector translation of the marker with the greatest motion.
Using high resolution analyses, small movements of the knee implants in relatively low numbers of participants measured by RSA after a short follow‐up can predict the risk of future aseptic loosening (Kärrholm 2006; Ryd 1995). Ryd 1995 showed that migration of the tibial component (MTPM) of more than 0.2 mm between one and two years predicted the risk for future aseptic loosening (predictive power of approximately 85%). Therefore, cases migrating at a higher rate were considered unstable, while those migrating at a lower rate were classified as stable.
For radiographic measures (using RSA), the risk of bias was considered low for most of the key domains among the studies. The blinding of outcome assessor was at high risk or unclear risk of bias in all of the studies, but there is no importance of blinding for RSA since this is an objective outcome. The quality of evidence was considered high for analyses of absolute values of rotation in the three orthogonal axes (transverse, longitudinal, and sagittal) but was considered moderate for arthroplasty instability because it was an indirect outcome. We considered there to be a high risk of attrition bias in the study by Toksvig‐Larsen 1998 because the withdrawal/drop‐out rate was described, was unacceptable and was not comparable between the groups.
Using the available means and standard deviations from the selected studies, we were able to execute the meta‐analysis and verify that tibial component fixation with cement presented a smaller displacement than fixation without cement (with or without hydroxyapatite) when evaluating MTPM through two years of follow‐up data (MTPM, N = 167, two RCTs, MD = 0.52 mm, 95% CI 0.31 to 0.74). Furthermore, the intention‐to‐treat analysis verified the superiority in this previously analyzed variable (24‐months MTPM, N = 173, two RCTs, MD = 0.52 mm, 95% CI 0.31 to 0.72).
These results were statistically significant; however, it is important to note that the MDs of the studied variables were less than one degree and less than one millimeter.
Carlsson 2005 study demonstrated the superiority of cemented fixation over uncemented fixation concerning displacement at five years and is in agreement with our systematic review. Toksvig‐Larsen 1998 showed that cemented tibial components had lesser subsidence than uncemented components at two years.
Contrary to the previously mentioned findings, cementless components demonstrated 4.7 times lower risk of future aseptic loosening compared to cemented fixation according to the arthroplasty instability classification inferred from RSA (N = 216, three RCTs, RR = 0.47, 95% CI 0.24 to 0.92). The quality of this evidence is moderate and was downgraded by one level because of indirectness of evidence. Probably uncemented total knee arthroplasties are more stable and durable than cemented arthroplasties, but this information must be confirmed through RCTs with longer follow‐up. Although aseptic loosening is mainly related to wear debris, the role of bone cement as initiator of osteolysis remains controversial (Harris 1976; Khaw 2002; Mirra 1976). On the other side, the cemented system can provide a more effective seal from wear particles improving arthroplasty durability (McCaskie 1998).
Although Nilsson 1999 observed no difference between the hydroxyapatite‐coated and cemented implants concerning displacement at five years, he noted distinct migration patterns. The uncemented components stabilized after an initial period of early and larger migration, whereas the cemented components did not show a tendency toward stabilization over time. This presumably reflected the different biological reactions at the implant‐bone interface. A small difference at the level of the tibial cut implied that the uncemented component initially rests on only a small area of bone and gradually migrates until stronger bone and an equilibrium are reached. According to Nilsson 1999, because hydroxyapatite is osteoconductive, even in partially unstable conditions, it can be expected to shorten this period. The continuous migration pattern of cemented components suggests the presence of long‐lasting bone remodeling at the interface when cement is used.
Other issues that remain inconclusive in total knee arthroplasty (mobile bearing versus fixed bearing, and posterior cruciate ligament sacrifice versus posterior cruciate ligament retention) are analyzed in other systematic reviews (Wilco 2001; Wilco 2005). Wilco 2001 systematic review included two studies and found no superiority between mobile and fixed bearing for total knee arthroplasty with regard to range of motion or functional performance of the participants. Wilco 2005 systematic review included studies with variable methodological quality and concluded that there was no solid base to decide whether or not to retain or sacrifice the posterior cruciate ligament in total knee replacement.
Overall completeness and applicability of evidence
This systematic review has external validity for patients with osteoarthritis of the knee and other non‐traumatic diseases, for example, rheumatoid arthritis. The exclusion criteria used in included studies did not affect the external validity of the meta‐analyses performed (Characteristics of included studies). It is important to remember that: 1) The results of this systematic review do not apply to patients with post‐traumatic osteoarthritis, and 2) The most common type of knee osteoarthritis is primary osteoarthritis (no known cause and is not a result of trauma). The included studies were insufficient to address all of the objectives of the review. We could not perform a meta‐analysis on clinical and functional outcomes presented in the included studies because the combination of tibial and femoral fixation was not comparable among the studies.
Primary studies have reported some complications or adverse outcomes, and we could not investigate whether there was any possible correlation with the type of fixation.
Nilsson 1999 was the only study to report revision due to loosening (one participant with uncemented fixation with hydroxyapatite). Any significant difference between the types of fixation could be presented in studies with follow‐ups longer than five years.
Some complications were occasionally influenced by the type of fixation of both components, but the studies were designed to evaluate tibial or femoral components, and it was not possible to compare studies analyzing the total knee prosthesis with both components (femoral and tibial) fixed with cement, cementless or hybrid. Nilsson 1999 and Carlsson 2005 analyzed the same follow‐up period, but we could not perform a meta‐analysis on the adverse outcomes because the femoral component was cemented or was not consistent with the preference of the surgeon in the study of Carlsson. As there were not two comparable groups between the studies by Toksvig‐Larsen 1998 (uncemented femur/cemented tibia or uncemented femur/uncemented tibia) and Uvehammer 2007 (uncemented femur/cemented tibia or cemented femur/uncemented tibia), we could not perform a meta‐analysis on these studies.
Other comparisons were not possible because the means and standard deviations of other outcomes were not available.
The results of the review suggest that cementless total knee arthroplasty is more durable than a cemented one, but this supposition must be confirmed through RCTs with longer follow‐up. We must bear in mind that cementless total knee arthroplasty is not available in all countries because it has a higher cost.
Future research must focus on major outcomes, such as health‐related quality of life measures.
Quality of the evidence
Through available data, uncemented fixation with and without hydroxyapatite was found to be superior to cemented fixation according to arthroplasty instability classification inferred from RSA (N = 216, three RCTs, RR = 0.47, 95% CI 0.24 to 0.92). The quality of this evidence is moderate and was downgraded one level because this is an indirect outcome that predicts arthroplasty instability. We found no key methodological limitations of the studies and their results were consistent. Arthroplasty instability is an important outcome for the surgeon and is something the patient can understand. Taking into account the methodological quality and power of included studies, the results of the review suggest higher durability of cementless total knee arthroplasty.
Potential biases in the review process
The strengths of this systematic review are: there were no language restrictions in search strategy, two authors independently selected studies and a third author was consulted when there were disagreements, and contact was made with all of the authors of primary studies to obtain missing data.
Agreements and disagreements with other studies or reviews
Gandhi 2009 conducted meta‐analyses to verify clinical function and survival of fully cemented and fully uncemented prostheses in total knee replacement. In addition to these types of fixation, our systematic review also studied hybrid fixation. Gandhi 2009 included studies with participants with knee osteoarthritis and rheumatoid arthritis, without exclusion of studies with post‐traumatic arthritis as in our systematic review. This increases the external validity of Gandhi 2009, but increases the heterogeneity of their patients. Gandhi 2009 included randomized and quasi‐randomized controlled trials and observational studies, while our systematic review aimed to include only randomized controlled trials. The inclusion of unpublished abstracts increased the power of Gandhi 2009 meta‐analyses. Gandhi 2009 assessed only the survival of the implant free of aseptic loosening at a minimum follow‐up of two years and joint function measured by the Knee Society Score. In our systematic review the number of outcomes is broader (Types of outcome measures), including RSA that can predict aseptic loosening.
The search strategy of Gandhi 2009 resulted in five RCTs (Khaw 2002; Gicquel 2000; McCaskie 1998; Nilsson 1992; Nilsson 1993) and 10 observational studies while our search strategy resulted in five RCTs (three could be used for meta‐analyses). The study of Gandhi 2009 should have included the Nilsson 1999 study since the authors observed the clinical function of fully cemented and fully uncemented total knee arthroplasties. We did not include Nilsson 1993 and McCaskie 1998 studies because the authors performed a quasi‐randomized method of allocation. We did not include Khaw 2002; Gicquel 2000 and Nilsson 1992 studies because there was no differentiation between post‐traumatic and non post‐traumatic osteoarthritis.
According to Gandhi 2009, the combined odds ratio for aseptic loosening for the uncemented group was 4.2 (95% CI 2.7 to 6.5; P < 0.0001). However, subgroup analysis of data only from RCTs showed no differences between the groups for odds of aseptic loosening (OR 1.9, 95% CI 0.55 to 6.40; P = 0.314). Gandhi 2009 found no difference between the groups for the Knee Society Knee Score (MD 0.005, 95% CI ‐0.26 to 0.26; P = 0.972).
The concept of survival of the implant free of aseptic loosening was based on a minimum follow‐up of two years in Gandhi 2009 study. This follow‐up can be considered short to evaluate implant survival and can explain why Gandhi 2009 found no difference when he made a subgroup analysis with only the randomized controlled trials.
There are follow‐up limitations in current primary studies, in which case RSA can provide a better idea of risk of future aseptic loosening as in our systematic review, even with a relative low number of participants after a short follow‐up of two years (Kärrholm 2006; Ryd 1995).
Authors' conclusions
Implications for practice.
We observed that cemented fixation of the tibial component demonstrated a smaller displacement in relation to cementless fixation in a two to five year follow‐up.
The cemented tibial components demonstrated an increased risk of subsequent loosening compared to the cementless components using RSA which has a predictive power of approximately 85%.
Through the included studies, we found no indication that either cemented or uncemented total knee arthroplasty results in better functional performance of the participants.
Implications for research.
A specific problem related to comparing prostheses is that the differences are small, and consequently, the effect on participant performance for a given parameter can be difficult to detect and can only be detected with large sample sizes with longer follow‐ups.
Although RSA in two to five year follow‐ups suggested the most stable fixation for total knee prosthesis, to assure the superiority of one type of fixation in relation to another, we must base the results on the following: 1) revision rate, 2) adverse events, and 3) functional status and health‐related quality of life.
Some studies failed to present validated instruments, blinding of outcomes, means, standard deviations or an intention‐to‐treat analysis. In future studies, we suggest that these methodological aspects must be considered to attempt to solve the question of total knee prosthesis fixation.
History
Protocol first published: Issue 4, 2006 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 11 July 2011 | New search has been performed | updated search |
| 6 January 2010 | Amended | CMSG ID: C115‐R |
Acknowledgements
We would like to acknowledge Regis Bruni Andriolo, Jose Fausto de Morais, Elizabeth Ghogomu and Lara J. Maxwell for methodological and statistical support and to Mario Lenza (surgical editor) for his comments.
Appendices
Appendix 1. Search strategy for CENTRAL
| CENTRAL |
| #1 arthritis[mesh] #2 arthrit*:ti,ab #3 osteoarthr*:ti,ab #4 gonarthrosis:ti,ab #5 arthralgia:ti,ab #6 felty* next syndrome:ti,ab #7 caplan* next syndrome:ti,ab #8 sjogren*next syndrome:ti,ab #9 sicca next syndrome:ti,ab #10 still* next disease:ti,ab #11 bechterew* next disease:ti,ab #12 rheuma*:ti,ab #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 #14 knee[mesh] #15 knee joint[mesh] #16 knee*:ti,ab #17 #14 OR #15 OR #16 #18 #13 AND #17 #19 knee osteoarthritis[mesh] #20 #18 OR #19 #21 knee arthroplasty[mesh] #22 knee prosthesis[mesh] #23 knee*[tw] AND (arthroplast* OR implant* OR replace* OR prosthe* OR endoprosthe*):ti,ab #24 #21 OR #22 OR #23 #25 "Prostheses and Implants"[mesh] #26 #17 AND #25 #27 #24 OR #26 #28 cementation[mesh] #29 "Bone cements"[mesh] #30 "Durapatite"[mesh] #31 cement* OR uncement* OR Hydroxyapatite OR Durapatite OR Ingrowth OR hybrid OR porous* OR coat* OR press‐fit:ti,ab #32 #28 OR #29 OR #30 OR #31 #33 #20 AND #27 AND #32 |
Appendix 2. Search strategy for MEDLINE (via Pubmed)
| MEDLINE (via PubMed) |
| #1 arthritis[mesh] #2 arthrit*[tw] #3 osteoarthr*[tw] #4 gonarthrosis[tw] #5 arthralgia[tw] #6 felty* syndrome[tw] #7 caplan* syndrome[tw] #8 sjogren* syndrome[tw] #9 sicca syndrome[tw] #10 still* disease[tw] #11 bechterew* disease[tw] #12 rheuma*[tw] #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 #14 knee[mesh] #15 knee joint[mesh] #16 knee*[tw] #17 #14 OR #15 OR #16 #18 #13 AND #17 #19 knee osteoarthritis[mesh] #20 #18 OR #19 #21 knee arthroplasty[mesh] #22 knee prosthesis[mesh] #23 knee*[tw] AND (arthroplast* OR implant* OR replace* OR prosthe* OR endoprosthe*)[tw] #24 #21 OR #22 OR #23 #25 "Prostheses and Implants"[mesh] #26 #17 AND #25 #27 #24 OR #26 #28 cementation[mesh] #29 "Bone cements"[mesh] #30 "Durapatite"[mesh] #31 cement* OR uncement* OR Hydroxyapatite OR Durapatite OR Ingrowth OR hybrid OR porous* OR coat* OR press‐fit[tw] #32 #28 OR #29 OR #30 OR #31 #33 #20 AND #27 AND #32 #34 randomized controlled trial[pt] #35 controlled clinical trial[pt] #36 randomized controlled trials[mh] #37 random allocation[mh] #38 double‐blind method[mh] #39 single‐blind method[mh] #40 clinical trial[pt] OR clinical trials[mh] #41 ("clinical trial"[tw] #42 (singl*[tw] OR doubl*[tw] #43 trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) #44 ("latin square"[tw]) #45 placebos[mh] #46 placebo*[tw] #47 random*[tw] #48 research design[mh:noexp] #49 comparative study[mh] #50 evaluation studies[mh] #51 follow‐up studies[mh] #52 prospective studies[mh] #53 cross‐over studies[mh] #54 control*[tw] #55 prospectiv*[tw] #56 volunteer*[tw] NOT (animal[mh] NOT human[mh]) #57 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #55 OR #56 #58 #33 AND #57 |
Appendix 3. Search strategy for EMBASE (via OVID)
| EMBASE (via OVID) |
| 1. exp Arthritis/ 2. arthrit$.tw. 3. osteoarthr$.tw. 4. gonarthrosis.tw. 5. arthralgia.tw. 6. (felty$ adj2 syndrome).tw. 7. (caplan$ adj2 syndrome).tw. 8. (sjogren$ adj2 syndrome).tw. 9. (sicca adj2 syndrome).tw. 10. still$ disease.tw. 11. bechterew$ disease.tw. 12. rheuma$.tw. 13. or/1‐12 14. exp knee/ 15. exp knee joint/ 16. knee$.tw. 17. or/14‐16 18. 13 and 17 19. exp Knee Osteoarthritis/ 20. exp Knee Arthritis/ 21. or/18‐20 22. exp Knee Arthroplasty/ 23. exp Knee Prosthesis/ 24. (knee$ adj2 (arthroplast$ or implant$ or replace$ or prosthe$ or endoprosthe$)).tw. 25. exp Prosthesis/ 26. exp IMPLANTATION/ 27. 25 or 26 28. 17 and 27 29. or/22‐24,28 30. exp CEMENTATION/ 31. exp HYDROXYAPATITE/ 32. (cement$ or uncement$ or Hydroxyapatite or Durapatite or Ingrowth or hybrid or porous$ or coat$ or press‐fit).tw. 33. or/30‐32 34. and/21,29,33 35. random$.ti,ab. 36. factorial$.ti,ab. 37. (crossover$ or cross over$ or cross‐over$).ti,ab. 38. placebo$.ti,ab. 39. (doubl$ adj blind$).ti,ab. 40. (singl$ adj blind$).ti,ab. 41. assign$.ti,ab. 42. allocat$.ti,ab. 43. volunteer$.ti,ab. 44. crossover procedure.sh. 45. double blind procedure.sh. 46. randomized controlled trial.sh. 47. single blind procedure.sh. 48. or/35‐47 49. exp animal/ or nonhuman/ or exp animal experiment/ 50. exp human/ 51. 49 and 50 52. 49 not 51 53. 48 not 52 54. 34 and 53 |
Appendix 4. Search strategy for LILACS
| LILACS |
| (((arthritis OR arthrit* OR osteoarthr* OR gonarthrosis OR arthralgia OR felty* syndrome OR caplan* syndrome OR sjogren* syndrome OR sicca syndrome OR still* disease OR bechterew* disease OR rheuma*) AND (knee OR knee joint OR knee*)) OR knee osteoarthritis) AND ((knee arthroplasty OR knee prosthesis OR (knee* AND (arthroplast* OR implant* OR replace* OR prosthe* OR endoprosthe*)) OR (prostheses and implants AND (knee OR knee joint OR knee*))) AND (cementation OR bone cements OR durapatite OR (cement* OR uncement* OR hydroxyapatite OR durapatite OR ingrowth OR hybrid OR porous* OR coat* OR press‐fit)) AND ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) |
Data and analyses
Comparison 1. Cemented versus uncemented with HA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Absolute rotation about transverse axis in degrees (60 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Intention‐to‐treat analysis | 2 | 160 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.12, 0.45] |
| 1.2 Available data analysis | 2 | 134 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.09, 0.47] |
| 2 Absolute rotation about longitudinal axis in degrees (60 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Intention‐to‐treat analysis | 2 | 160 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.04, 0.47] |
| 2.2 Available data analysis | 2 | 134 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.12, 0.46] |
| 3 Absolute rotation about sagittal axis in degrees (60 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Intention‐to‐treat analysis | 2 | 160 | Mean Difference (IV, Random, 95% CI) | 0.22 [0.01, 0.42] |
| 3.2 Available data analysis | 2 | 134 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.02, 0.31] |
| 4 Arthroplasty instability (arthroplasty instability was considered an event) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Intention‐to‐treat analysis (missing participants were categorized unstable) | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.85] |
| 4.2 Intention‐to‐treat analysis (missing participants were categorized stable) | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.75] |
| 4.3 Available data analysis (arthroplasty instability was considered an event) | 2 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.74] |
Comparison 2. Cemented versus uncemented with and without HA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Absolute rotation about transverse axis in degrees (60 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Intention‐to‐treat analysis | 2 | 210 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.08, 0.39] |
| 1.2 Available data analysis | 2 | 179 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.03, 0.41] |
| 2 Absolute rotation about longitudinal axis in degrees (60 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Intention‐to‐treat analysis | 2 | 210 | Mean Difference (IV, Random, 95% CI) | 0.33 [0.09, 0.58] |
| 2.2 Available data analysis | 2 | 179 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.14, 0.64] |
| 3 Absolute rotation about sagittal axis in degrees (60 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Intention‐to‐treat analysis | 2 | 210 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.04, 0.36] |
| 3.2 Available data analysis | 2 | 179 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.01, 0.29] |
| 4 Maximum total point motion in millimeters (12 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Intention‐to‐treat analysis | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.38, 0.82] |
| 4.2 Available data analysis | 2 | 168 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.37, 0.84] |
| 5 Maximum total point motion in millimeters (24 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Intention‐to‐treat analysis | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.31, 0.72] |
| 5.2 Available data analysis | 2 | 167 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.31, 0.74] |
| 6 Arthroplasty instability (arthroplasty instability was considered an event) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Intention‐to‐treat analysis (missing participants were categorized unstable) | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.36, 1.68] |
| 6.2 Intention‐to‐treat analysis (missing participants were categorized stable) | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.73] |
| 6.3 Available data analysis (arthroplasty instability was considered an event) | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.24, 0.92] |
Comparison 3. Cemented versus uncemented without HA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum total point motion in millimeters (12 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Intention‐to‐treat analysis | 2 | 126 | Mean Difference (IV, Random, 95% CI) | 0.62 [0.42, 0.81] |
| 1.2 Available data analysis | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 0.64 [0.43, 0.85] |
| 2 Maximum total point motion in millimeters (24 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Intention‐to‐treat analysis | 2 | 123 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.33, 0.72] |
| 2.2 Available data analysis | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.54 [0.33, 0.75] |
| 3 Arthroplasty instability (arthroplasty instability was considered an event) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Intention‐to‐treat analysis (missing participants were categorized unstable) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.37, 1.17] |
| 3.2 Intention‐to‐treat analysis (missing participants were categorized stable) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.20, 1.90] |
| 3.3 Available data analysis | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carlsson 2005.
| Methods | RCT, computer generated randomization. The primary outcome measure in this study was RSA, which is a radiographic "measuring tool", and was not influenced by the assessor. For the secondary outcome measure, i.e. the clinical data, the assessor was not blinded. | |
| Participants | Inclusion: Osteoarthrosis: 120 participants (150 knees). Exclusion: Revision, history of joint sepsis, recent systemic corticosteroids, primary or secondary carcinoma in the last five years, metabolic bone disease, such as Paget’s disease, psychosocial disorders limiting rehabilitation, previous intraarticular knee fracture, over 20° valgus or varus deformity, extension loss over 30°, unsuitable for cruciate‐retaining arthroplasty, unsuitable for cementless fixation of the tibial component, need for augmentation wedges or bone grafts, previous proximal tibial osteotomy, and greater than 80 years old. Average age: 73 years. Average weight: 79 kg. Seven participants lost. | |
| Interventions | Randomized tibial component fixation: Cemented (only under the tibial plateau), uncemented porous, or uncemented porous hydroxyapatite. According to the preference of the surgeons, the femoral component was cemented or not, although according to the protocol, the patella should not be resurfaced, and a surgeon chose to implant a patellar component in 2 participants. The Press‐Fit Condylar (PFC) modular, posterior‐cruciate‐retaining prosthesis with a posterior lipped polyethylene insert (Johnson and Johnson Orthopaedics) was used in all cases. Vacuum‐mixed, gentamicin‐loaded cement (Palacos cum Gentamicin; Schering‐Plough, Kenilworth, NJ) was used. |
|
| Outcomes | RSA (absolute values of rotation around the three orthogonal axes, MTPM, lift‐off, subsidence), Knee Society clinical rating system and method of Ewald. Individual data were sent for the systematic review by the author (Åke Carlsson). | |
| Notes | Duration of follow‐up: 5 years. The cemented knees rotated less than uncemented knees with or without hydroxyapatite and migrated less than uncemented knees without hydroxyapatite. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization. |
| Allocation concealment (selection bias) | Low risk | The type of component to be used was written down on a piece of stiff paper that was folded and put inside another piece of stiff paper in a sealed envelope. What was written on the paper could not be read even if held against a lamp. During the operation, the envelope was opened in sequential order after the tibial resection. |
| Was the patient blinded? | Low risk | Single‐blind study |
| Was the surgeon blinded? | High risk | It is impossible to blind the surgeon to prosthesis fixation |
| Was the outcome assessor blinded? | High risk | Single‐blind study |
| Was the withdrawal/drop‐out rate described, acceptable (<20%), and comparable in both groups? | Low risk | Seven participants were lost (< 20%). |
| Selective reporting (reporting bias) | Low risk | The study protocol was available, and all of the study’s prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified manner. |
Ishii 2005b.
| Methods | RCT | |
| Participants | Osteoarthritis: 54 participants (56 knees), Rheumathoid arthritis: 3 participants (4 knees). Exclusion criteria: Peripherical vascular diseases or neurological problems. Average age of cementless group: 72 years. Average weight of cementless group: 59 kg. Average age of hybrid group: 72 years. Average weight of hybrid group: 61 kg. None participant lost. | |
| Interventions | The participants were randomly assigned to either the cementless or hybrid (cemented tibia and uncemented femur) group with a tourniquet pressure of 100 mm Hg above systolic blood pressure. The tourniquet used was the MT‐720 tourniquet system (Mizuho‐Ika, Tokyo, Japan). All the patients had spinal anesthesia. Esmarch’s bandage was used. A straight longitudinal midline skin incision was used with a medial parapatellar capsulotomy. In all cases, intramedullary femoral and extramedullary tibial resection guides were used. Each hole in the distal femur made for the intramedullary guides was filled with an autogenous bone plug. No lateral retinacular release or patellar arthroplasty was performed. During the arthroplasty, an effort was made to collect all the fluid lost in closed canisters using continuous wall suction. The amount of fluid that was administered for irrigation, using a bulb syringe, was recorded, and its volume was then subtracted from the total suction‐fluid volume to estimate the intraoperative blood loss. A Stryker CBCII Consta Vac Blood Conservation System (Stryker, Kalamazoo, Mich) was used in all the patients after surgery. The volume of blood collected was returned to the patients within 3 hours of surgery. No patient received medication for thromboembolism. The New Jersey LCS total knee system (DePuy, Warsaw, Ind) was used in all cases. |
|
| Outcomes | The red blood cell count, hemoglobin level, and hematocrit were recorded preoperatively and 1 week, 4 weeks, and 3 months postoperatively for each patient. The volumes of postoperative suction drainage within 3 hours and upon removal (first operative day) were recorded. Both the measured and calculated blood losses were evaluated using the maximum decrease in the hemoglobin level (1 week after surgery) and were normalized using each patient’s weight and height. | |
| Notes | The authors found no difference between the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal communication (Ishii 2005b) ‐ Random number table. |
| Allocation concealment (selection bias) | High risk | Personal communication (Ishii 2005b) ‐ The author used an open random number table. |
| Was the patient blinded? | Low risk | |
| Was the surgeon blinded? | High risk | It is impossible to blind the surgeon to prosthesis fixation. |
| Was the outcome assessor blinded? | High risk | The outcome assessor was the surgeon. |
| Was the withdrawal/drop‐out rate described, acceptable (<20%), and comparable in both groups? | Low risk | There were no withdrawals. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified outcomes that were of interest in the review were reported in the prespecified manner. |
Nilsson 1999.
| Methods | RCT and opaque envelopes. The primary outcome measure in this study was RSA. For the secondary outcome measure, i.e. the clinical data, the assessor was not blinded. | |
| Participants | Inclusion: Osteoarthrosis: 43 participants (45 knees). RA: 13 participants (15 knees). No exclusion criteria were specified. Average age: 69 years. Twenty‐four participants were lost. | |
| Interventions | Randomized tibial component fixation: Cemented (only under the tibial plateau) or uncemented with hydroxyapatite. The femoral components were fixed identically to the corresponding tibial components (Nilsson 1999); however, the uncemented bones were not hydroxyapatite coated. A cemented all‐polyethylene patellar component was used in all of the knees. All of the knees received a Tricon II (Smith & Nephew) total knee prosthesis. Bone cement (Palacos cum Gentamicin, Shering‐Plough, Kenilworth, NJ) was used. The cut proximal tibia was cleaned with a high‐pressure lavage before the bone cement was injected via a delivery nozzle to improve the penetration of the cement into the trabecular bone. |
|
| Outcomes | RSA (absolute values of rotation around the three orthogonal axes, MTPM, lift‐off and subsidence), Knee Society knee score, Knee Society pain score, Knee Society function score, HKA, range of motion and radiolucent lines analysis at the tibial component interface, as described by the Knee Society. | |
| Notes | Duration of follow‐up: 5 years. No difference between the two types of fixation. Some data were obtained by personal communication (Nilsson 1999). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | After cutting the proximal tibia and prior to inserting the tibial component, a telephone call was made from the operating room to a secretary at the orthopedic department. She opened a sealed envelope and read the contents, i.e. to use a cemented component or an uncemented component. The envelopes were placed in a locked location. |
| Was the patient blinded? | Unclear risk | This item was not mentioned in the trial report. |
| Was the surgeon blinded? | High risk | It is impossible to blind the surgeon to prosthesis fixation. |
| Was the outcome assessor blinded? | Unclear risk | This item was not mentioned in the trial report. |
| Was the withdrawal/drop‐out rate described, acceptable (<20%), and comparable in both groups? | Low risk | The withdrawal/drop‐out rate was described and comparable in both groups but was not acceptable. Missing outcome data were balanced in numbers among intervention groups with similar reasons for missing data among groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified manner. |
Toksvig‐Larsen 1998.
| Methods | RCT, sealed envelopes. The primary outcome measure in this study was RSA. For the secondary outcome measure, i.e. the clinical data, the assessor was not blinded. | |
| Participants | Inclusion: Osteoarthrosis: 27 participants (28 knees). No exclusion criteria were specified. Average age: 71 years. Average body weight: 79 kg. Five participants were lost. | |
| Interventions | Randomized tibial component fixation: Cemented or uncemented without hydroxyapatite. The femoral component was inserted without cement, and the patella was not resurfaced. The cement was applied to the horizontal cut surface but not around the stem. All knees received a porous‐coated anatomic total knee prosthesis (PCA Universal; Howmedica) with a porous‐coated anatomic modular cruciform stem tibial component. The cementing technique included vacuum‐mixing of the cement, lavage, and pressurization. | |
| Outcomes | RSA (MTPM, subsidence and inducible displacement), HKA, Hospital for Special Surgery, extension, flexion, and walking distance. | |
| Notes | Duration of follow‐up: 2 years. The prostheses that was inserted with cement subsided 0.0 ± 0.1 millimeter, and the prostheses that was inserted without cement subsided 0.5 ± 0.1 millimeter at the two year follow‐up evaluation (P = 0.008). Some data were obtained by personal communication (Toksvig‐Larsen 1998). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In a personal communication (Toksvig‐Larsen 1998), the author stated: "All knees were randomized, patients with bilateral surgery were operated with an interval of more than 3 months." |
| Allocation concealment (selection bias) | Low risk | Personal communication (Toksvig‐Larsen 1998) ‐ The allocation was performed using sealed envelopes. |
| Was the patient blinded? | Unclear risk | This item was not mentioned in the trial report. |
| Was the surgeon blinded? | High risk | It is impossible to blind the surgeon to prosthesis fixation. |
| Was the outcome assessor blinded? | Unclear risk | This item was not mentioned in the trial report. |
| Was the withdrawal/drop‐out rate described, acceptable (<20%), and comparable in both groups? | High risk | The withdrawal/drop‐out rate was described, was not acceptable and was not comparable between the groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified manner. |
Uvehammer 2007.
| Methods | RCT. Unknown randomization technique. Blinding not stated. | |
| Participants | Inclusion: Osteoarthrosis: 50 participants (54 knees). All of the femoral components were manufactured with an 80 mm stem and were only available in one size (size 2/medium). This made the series more homogeneous regarding biomechanical factors but excluded selection of participants with larger or smaller knees. Average age: 72 years. Fifteen participants were lost (16 knees). | |
| Interventions | The femoral component fixation (cemented or uncemented with hydroxyapatite) and prosthesis design (three variations of the Freeman‐Samuelson knee replacement (Finsbury Orthopaedics Ltd): one with a rotating tibial insert, one with a medial femoral condyle representing part of a sphere articulating against a corresponding concave tibial polyethylene insert (FS1000), and the original standard version) were randomized, and the participants were randomized into one of the six treatment options. All of the tibial components were cemented. In 44 of the knees, a patellar component was inserted. Refobacin‐Palacos R bone cement (Biomet Orthopaedics Inc., Warsaw, Indiana) was prepared by vacuum mixing. |
|
| Outcomes | RSA (absolute values of rotation around the three orthogonal axes), HKA, and Hospital for Special Surgery knee score. | |
| Notes | Duration of follow‐up: 2 years. No difference between the two types of fixation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This item was not mentioned in the trial report. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were opened before the operation. |
| Was the patient blinded? | Unclear risk | This item was not mentioned in the trial report. |
| Was the surgeon blinded? | High risk | It is impossible to blind the surgeon to prosthesis fixation. |
| Was the outcome assessor blinded? | Unclear risk | This item was not mentioned in the trial report. |
| Was the withdrawal/drop‐out rate described, acceptable (<20%), and comparable in both groups? | Low risk | The withdrawal/drop‐out rate was described, was comparable in both groups and was not acceptable. Missing outcome data were balanced in numbers among intervention groups with similar reasons for missing data among the groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified manner. |
RCT = randomoized controlled trial
RSA = roentgen stereophotogrammetric analysis
MTPM = maximum total point motion
RA = rheumatoid arthritis
HKA = hip‐knee‐ankle‐angle
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albrektsson 1992 | Personal communication (Albrektsson 1992) ‐ The author included post‐traumatic osteoarthritis participants |
| Baker 2007 | Personal communication (Khaw 2002) ‐ The author did not differentiate between post‐traumatic and non post‐traumatic osteoarthritis |
| Beaupré 2007 | Personal communication (Beaupré 2007) ‐ The author did not differentiate between post‐traumatic and non post‐traumatic osteoarthritis |
| Ishii 2005a | Personal communication (Ishii 2005a) ‐ Non‐randomized controlled trial |
| Keblish 1993 | Personal communication (Keblish 1993) ‐ Non‐randomized controlled trial |
| Khaw 2002 | Personal communication (Khaw 2002) ‐ The author did not differentiate between post‐traumatic and non post‐traumatic osteoarthritis |
| Nilsson 2006 | Personal communication (Nilsson 2006) ‐ The author included post‐traumatic osteoarthritis participants |
| Önsten 1998 | The study by Önsten 1998 is the same as the study by Carlsson 2005 with a shorter follow‐up |
Characteristics of studies awaiting assessment [ordered by study ID]
Demey 2010.
| Methods | RCT. Computer‐generated randomization. Single‐blind. |
| Participants | Osteoarthritis and chondrocalcinosis: 107 participants. There were no significant differences between the two groups with respect to anthropometric or demographic data (sex, age, body mass index, weight, height, diagnosis). No patient was lost. |
| Interventions | The operations were performed using the HLS Noetos® prosthesis (Tornier, Saint‐Ismier, France) and were randomized to cemented or hybrid (cementless femoral implant and cemented tibial implant) fixation of the components. |
| Outcomes | Hemoglobin and hematocrit levels, volumes of postoperative suction drainage and the rate of blood transfusion |
| Notes | No differences were found between the two groups |
Dunbar 2009.
| Methods | RCT, computer‐generated randomization. The primary outcome measured in this study was RSA, which is a radiographic "measuring tool" and is not influenced by the assessor. For the secondary outcome measure, i.e. the clinical data, blinding was not stated. |
| Participants | Seventy participants. Inclusion and exclusion criteria were not described. Age: 60.2 ± 8.3 years (trabecular metal group) and 61.1 ± 9.4 years (cemented group). Twenty‐one participants (21 knees) were lost. |
| Interventions | Participants were randomized to receive either the trabecular metal or the cemented tibial component of the same design (Zimmer, Warsaw, Indiana). The surgery was performed using a standardized protocol consisting of a resection of the posterior cruciate ligament, patellar resurfacing with a cemented inlay component, cementing of the femoral component, and placement of 0.8 mm marker beads for the radiostereometric analysis. Four, five, or six tantalum markers were placed around the periphery of the polyethylene component, and eight to twenty tantalum markers were inserted into the proximal part of the tibia. |
| Outcomes | RSA and WOMAC. |
| Notes | Duration of follow‐up: 2 years. No significant difference with regard to WOMAC was found between the groups. A subset of the trabecular metal components migrated extensively in the postoperative period but stabilized by one year, and the proportion considered to be at risk for early aseptic loosening was 0.0 (95% CI, 0.0 to 0.12) in the entire group. Four cemented components were considered to be at risk for early aseptic loosening (proportion at risk, 0.19; 95% confidence interval, 0.08 to 0.4). |
Nelissen 1998.
| Methods | RCT. Unknown randomization technique. Double‐blind. |
| Participants | Twenty‐one participants: osteoarthrosis (5 knees) and rheumatoid arthritis (26 knees). Average age: 68 years. Three participants (3 knees) were lost. |
| Interventions | Fixation with cement, without cement, or with a hydroxyapatite coating (without cement) was randomly chosen during the operation. All of the prostheses were posterior cruciate ligament‐retaining total condylar implants (Interax; Howmedica, Rutherford, New Jersey). A patellar component made of ultra‐high molecular weight polyethylene was used in all but one total knee arthroplasty. No patellar components were used in one knee because the remaining patellar bone was too thin. |
| Outcomes | RSA, Knee Society score, conventional roentgenograms, length of surgery (in minutes) and femorotibial angle. |
| Notes | Duration of follow‐up: 2 years. A significant difference with regard to micromotion was found between the non‐coated components fixed without cement and the hydroxyapatite‐coated components fixed without cement and between the non‐coated components fixed without cement and the components fixed with cement (P < 0.001, analysis of variance). The hydroxyapatite‐coated components fixed without cement and the components fixed with cement both had far less micromotion along the longitudinal axis (subsidence) throughout the follow‐up period than the non‐coated components fixed without cement. |
Nilsson 1992.
| Methods | RCT. Unknown randomization technique. Blinding not stated. |
| Participants | Osteoarthrosis and rheumatoid arthritis: 39 participants. Average age: 68 years. Eleven participants’ knees were lost to follow‐up. |
| Interventions | The operations were performed using the Tricon‐M knee prosthesis (Smith and Nephew Richards) and were randomized to cemented or cementless fixation of the components. |
| Outcomes | Scintimetry, RSA, HKA and HSS knee score. |
| Notes | Duration of follow‐up: 2 years. No difference between the two types of fixation. |
Parker 2001.
| Methods | RCT. Unknown randomization technique. Blinding not stated. |
| Participants | Osteoarthrosis: 99 participants (100 knees). Average age: 66.7 years. Thirty‐three participants were lost. |
| Interventions | One hundred MGI total knee replacements (Zimmer, Warsaw, Indiana) were performed in 99 participants randomized to either cementless or hybrid fixation. In all of the cases, femoral fixation was a cementless porous design. Tibial fixation was randomized to either cemented or cementless, and patellar fixation paralleled the tibial component. |
| Outcomes | HSS knee score, Knee Society knee score, failure rate and reasons for failure. |
| Notes | Duration of follow‐up: 12 years. Differences in the outcome between the two groups were not sufficiently significant to recommend one method of fixation over another. |
RCT = randomoized controlled trial
RSA = roentgen stereophotogrammetric analysis
WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
HKA = hip‐knee‐ankle‐angle
HSS = Hospital for Special Surgery knee score
MGI total knee replacement = Miller‐Galante I total knee replacement
Differences between protocol and review
We included the Current Controlled Trials in the electronic search strategies and the risk of bias and summary of findings tables were completed.
Contributions of authors
Gilberto Yoshinobu Nakama ‐ Protocol and review writing, search procedures, studies selection, inclusion criteria. Gustavo JM Almeida ‐ Protocol writing, search procedures, studies selection, inclusion criteria. Maria Stella Peccin ‐ Protocol writing, search procedures, inclusion criteria. Ozorio A Lira Neto ‐ Protocol writing, search procedures, studies selection, inclusion criteria.
Antônio Altenor B. Queiroz ‐ Protocol writing. Ricardo Dizioli Navarro ‐ Protocol writing and review writing. (The author is deceased (July 2010), and the living authors did not make substantive changes to the review beyond the deceased author's contribution).
Sources of support
Internal sources
-
Brazilian Cochrane Centre, Brazil.
Technical support
-
Orthopaedic and Trauma Department, Universidade Federal de São Paulo, São Paulo, Brazil.
Technical support
External sources
No sources of support supplied
Declarations of interest
None known.
Ricardo Dizioli Navaro deceased; declarations of interest published in the protocol: "none known".
Deceased July 2010
New
References
References to studies included in this review
Carlsson 2005 {published and unpublished data}
- Carlsson A. Personal communication July 2008.
- Carlsson A, Björkman A, Besjakov J, Onsten I. Cemented tibial component fixation performs better than cementless fixation: a randomized radiostereometric study comparing porous‐coated, hydroxyapatite‐coated and cemented tibial components over 5 years. Acta Orthopaedica 2005 Jun;76(3):362‐9. [PubMed] [Google Scholar]
Ishii 2005b {published and unpublished data}
- Ishii Y. Personal communication January 2012.
- Ishii Y, Matsuda Y. Perioperative blood loss in cementless or hybrid total knee arthroplasty without patellar resurfacing. Journal of Arthroplasty 2005;20(8):972‐6. [DOI] [PubMed] [Google Scholar]
Nilsson 1999 {published and unpublished data}
- Nilsson KG. Personal communication May 2008.
- Nilsson KG, Kärrholm J, Carlsson L, Dalén T. Hydroxyapatite coating versus cemented fixation of the tibial component in total knee arthroplasty: prospective randomized comparison of hydroxyapatite‐coated and cemented tibial components with 5‐year follow‐up using radiostereometry. Journal of Arthroplasty 1999;14(1):9‐20. [DOI] [PubMed] [Google Scholar]
Toksvig‐Larsen 1998 {published and unpublished data}
- Toksvig‐Larsen S. Personal communication May 2008.
- Toksvig‐Larsen S, Ryd L, Lindstrand A. Early inducible displacement of tibial components in total knee prostheses inserted with and without cement: a randomized study with roentgen stereophotogrammetric analysis. Journal of Bone and Joint Surgery ‐ American Volume 1998 Jan;80(1):83‐9. [PubMed] [Google Scholar]
Uvehammer 2007 {published data only (unpublished sought but not used)}
- Uvehammer J, Kärrholm J, Carlsson L. Cemented versus hydroxyapatite fixation of the femoral component of the Freeman‐Samuelson total knee replacement: a radiostereometric analysis. Journal of Bone and Joint Surgery ‐ British Volume 2007 Jan;89(1):39‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albrektsson 1992 {published and unpublished data}
- Albrektsson BE. Personal communication November 2007.
- Albrektsson BE, Carlsson LV, Freeman MA, Herberts P, Ryd L. Proximally cemented versus uncemented Freeman‐Samuelson knee arthroplasty. A prospective randomised study. Journal of Bone and Joint Surgery ‐ British Volume 1992 Mar;74(2):233‐8. [DOI] [PubMed] [Google Scholar]
Baker 2007 {published and unpublished data}
- Baker PN, Khaw FM, Kirk LM, Esler CN, Gregg PJ. A randomised controlled trial of cemented versus cementless press‐fit condylar total knee replacement: 15‐year survival analysis. Journal of Bone and Joint Surgery ‐ British Volume 2007;89(12):1608‐14. [DOI] [PubMed] [Google Scholar]
- Khaw FM. Personal communication October 2007.
Beaupré 2007 {published and unpublished data}
- Beaupré LA. Personal communication July 2008.
- Beaupré LA, al‐Yamani M, Huckell JR, Johnston DW. Hydroxyapatite‐coated tibial implants compared with cemented tibial fixation in primary total knee arthroplasty. A randomized trial of outcomes at five years. Journal of Bone and Joint Surgery ‐ American Volume 2007;89(10):2204‐11. [DOI] [PubMed] [Google Scholar]
Ishii 2005a {published and unpublished data}
- Ishii Y. Personal communication September 2007.
- Ishii Y, Matsuda Y, Sakata S, Onda N, Omori G. Primary total knee arthroplasty using the Genesis I total knee prosthesis: a 5‐ to 10‐year follow‐up study. The Knee 2005 Oct;12(5):341‐5. [DOI] [PubMed] [Google Scholar]
Keblish 1993 {published and unpublished data}
- Keblish P, Udvarhelyi I, Váczi G. Low contact stress knee prosthesis system. Results and complications. Magyar traumatológia, ortopédia, kézsebészet, plasztikai sebészet 1993;36(3):243‐51. [PubMed] [Google Scholar]
- Váczi G. Personal communication October 2007.
Khaw 2002 {published and unpublished data}
- Khaw FM. Personal communication October 2007.
- Khaw FM, Kirk LM, Morris RW, Gregg PJ. A randomised, controlled trial of cemented versus cementless press‐fit condylar total knee replacement. Ten‐year survival analysis. Journal of Bone and Joint Surgery ‐ British Volume 2002;84(5):658‐66. [DOI] [PubMed] [Google Scholar]
Nilsson 2006 {published and unpublished data}
- Nilsson KG. Personal communication September 2007.
- Nilsson KG, Henricson A, Norgren B, Dalén T. Uncemented HA‐coated implant is the optimum fixation for TKA in the young patient. Clinical Orthopaedics and Related Research 2006 Jul;448:129‐39. [DOI] [PubMed] [Google Scholar]
Önsten 1998 {published data only}
- Önsten I, Nordqvist A, Carlsson AS, Besjakov J, Shott S. Hydroxyapatite augmentation of the porous coating improves fixation of tibial components. A randomised RSA study in 116 patients. Journal of Bone and Joint Surgery ‐ British Volume 1998;80‐B:417‐25. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Demey 2010 {published data only}
- Demey G, Servien E, Pinaroli A, Lustig S, Aït Si Selmi T, Neyret P. The influence of femoral cementing on perioperative blood loss in total knee arthroplasty: a prospective randomized study. Journal of Bone and Joint Surgery ‐ American Volume 2010;92(3):536‐41. [DOI] [PubMed] [Google Scholar]
Dunbar 2009 {published data only}
- Dunbar MJ, Wilson DA, Hennigar AW, Amirault JD, Gross M, Reardon GP. Fixation of a trabecular metal knee arthroplasty component. A prospective randomized study. Journal of Bone and Joint Surgery ‐ American Volume 2009;91(7):1578‐86. [DOI] [PubMed] [Google Scholar]
Nelissen 1998 {published data only (unpublished sought but not used)}
- Nelissen RG, Valstar ER, Rozing PM. The effect of hydroxyapatite on the micromotion of total knee prostheses. A prospective, randomized, double‐blind study. Journal of Bone and Joint Surgery ‐ American Volume 1998;80(11):1665‐72. [DOI] [PubMed] [Google Scholar]
Nilsson 1992 {published data only (unpublished sought but not used)}
- Nilsson KG, Björnebrink J, Hietala SO, Kärrholm J. Scintimetry after total knee arthroplasty. Prospective 2‐year study of 18 cases of arthrosis and 15 cases of rheumatoid arthritis. Acta Orthopaedica Scandinavica 1992 Apr;63(2):159‐65. [DOI] [PubMed] [Google Scholar]
Parker 2001 {published data only (unpublished sought but not used)}
- Parker DA, Rorabeck CH, Bourne RB. Long‐term follow up of cementless versus hybrid fixation for total knee arthroplasty. Clinical Orthopaedics and Related Research 2001 Jul;388:68‐76. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1995
- Altman DG, Bland JM. Statistics notes: The normal distribution. BMJ 1995;310:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brassard 2006
- Brassard MF, Insall JN, Scuderi GR, Faris PM. Complications of total knee arthroplasty. In: Insall JN, Scott WN editor(s). Surgery of the knee. 4th Edition. Vol. 2, New York: Churchill Livingstone, 2006:1716‐60. [Google Scholar]
Castro 1999
- Castro AA, Clark OA, Atallah AN. Optimal search strategy for clinical trials in the Latin American and Caribbean Health Science Literature Database (LILACS database): Update [Letter]. São Paulo Medical Journal = Revista Paulista de Medicina 1999;117(3):138‐9. [DOI] [PubMed] [Google Scholar]
Cates 2004
- Cates C. Visual Rx 2.0 NNT Calculator. Dr Chris Cates EBM Website ‐ www.nntonline.net.
Dickersin 1994
- Dickersin K, Scherer R, Levefre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fehring 2006
- Fehring TK, Mason JB. Cemented total knee replacement: The gold standard. In: Insall JN, Scott WN editor(s). Surgery of the knee. 4th Edition. New York: Churchill Livingstone, 2006:1626‐30. [Google Scholar]
Gandhi 2009
- Gandhi R, Tsvetkov D, Davey JR, Mahomed NN. Survival and clinical function of cemented and uncemented prostheses in total knee replacement: a meta‐analysis. Journal of Bone and Joint Surgery ‐ British Volume 2009;91(7):889‐95. [DOI] [PubMed] [Google Scholar]
Gicquel 2000
- Gicquel P, Kempf JF, Gastaud F, Schlemmer B, Bonnomet F. Comparative study of fixation mode in total knee arthroplasty with preservation of the posterior cruciate ligament. Revue de Chirurgie Orthopédique et Réparatrice de l'Appareil Moteur 2000;86:240‐9. [PubMed] [Google Scholar]
Harris 1976
- Harris WH, Schiller AL, Scholler JM, Freiberg RA, Scott R. Extensive localized bone resorption in the femur following total hip replacement. Journal of Bone and Joint Surgery ‐ American Volume 1976;58(5):612‐8. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huiskes 1998
- Huiskes R. The causes of failure for hip and knee arthroplasties [Oorzaken van falen van heup‐ en knieartroplastieken]. Nederlands Tijdschrift voor Geneeskunde 1998;142(37):2035‐40. [PubMed] [Google Scholar]
Kärrholm 2006
- Kärrholm J, Gill RH, Valstar ER. The history and future of radiostereometric analysis. Clinical Orthopaedics and Related Research 2006;448:10‐21. [DOI] [PubMed] [Google Scholar]
Maloney 1996
- Maloney WJ, Woolson ST. Increasing incidence of femoral osteolysis in association with uncemented Harris‐Galante total hip arthroplasty: a follow‐up report. Journal of Arthroplasty 1996;11:130‐4. [DOI] [PubMed] [Google Scholar]
McCaskie 1998
- McCaskie AW, Deehan DJ, Green TP, Lock KR, Thompson JR, Harper WM, et al. Randomised, prospective study comparing cemented and cementless total knee replacement: results of press‐fit condylar total knee replacement at five years. Journal of Bone and Joint Surgery ‐ British Volume 1998;80(6):971‐5. [DOI] [PubMed] [Google Scholar]
McCormack 2009
- McCormack K, Cody DJ, Murray D, Ramsay CR, Campbell MK. Metal versus non‐metal backing of the tibial component for total knee replacement for osteoarthritis and/or rheumatoid arthritis. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004801] [DOI] [Google Scholar]
Mirra 1976
- Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clinical Orthopaedics and Related Research 1976;117:221‐40. [PubMed] [Google Scholar]
Morais 2012
- Morais JF. The relationship among the standard deviation of a dataset and the standard deviation and average two part of this set. arXiv: 1202.0850v1[stat.ME] 2012.
Nilsson 1993
- Nilsson KG, Karrholm J. Increased varus‐valgus tilting of screw‐fixated knee prostheses: stereoradiographic study of uncemented versus cemented tibial components. The Journal of Arthroplasty 1993;8:529‐40. [PubMed] [Google Scholar]
Ryd 1995
- Ryd L, Albrektsson BE, Carlsson L, Dansgård F, Herberts P, Lindstrand A, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. Journal of Bone and Joint Surgery ‐ British Volume 1995;77(3):377‐83. [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group, 2009. Available from http://www.cc‐ims.net/gradepro.
Valstar 2005
- Valstar ER, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthopaedica 2005;76(4):563‐72. [DOI] [PubMed] [Google Scholar]
Whiteside 2006
- Whiteside LA. Cementless total knee designs. In: Insall JN, Scott WN editor(s). Surgery of the knee. 4. New York: Churchill Livingstone, 2006:1613‐25. [Google Scholar]
Wilco 2001
- Jacobs W, Anderson PG, van Limbeek J, Wymenga AAB. Mobile bearing vs fixed bearing prostheses for total knee arthroplasty for post‐operative functional status in patients with osteoarthritis and rheumatoid arthritis. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003130.pub2] [DOI] [PubMed] [Google Scholar]
Wilco 2005
- Jacobs W, Clement DJ, Wymenga AAB. Retention versus sacrifice of the posterior cruciate ligament in total knee replacement for treatment of osteoarthritis and rheumatoid arthritis. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004803.pub2] [DOI] [PubMed] [Google Scholar]


