Abstract
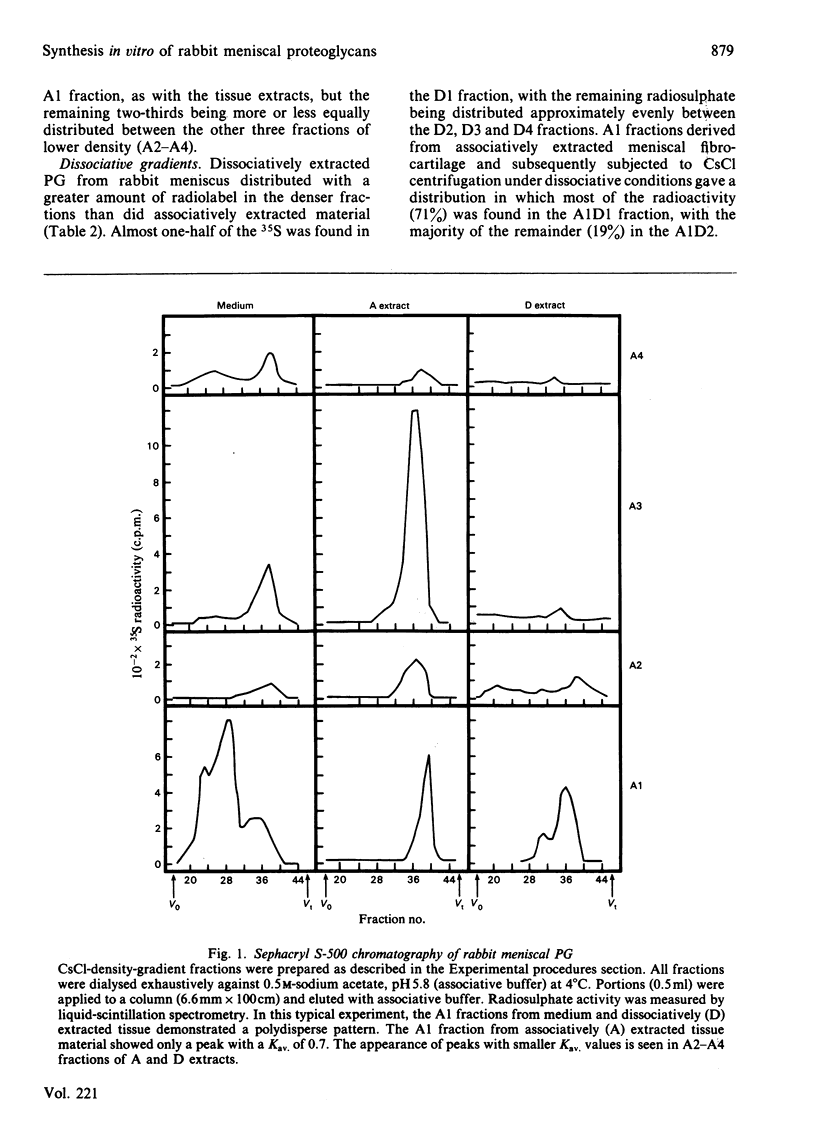
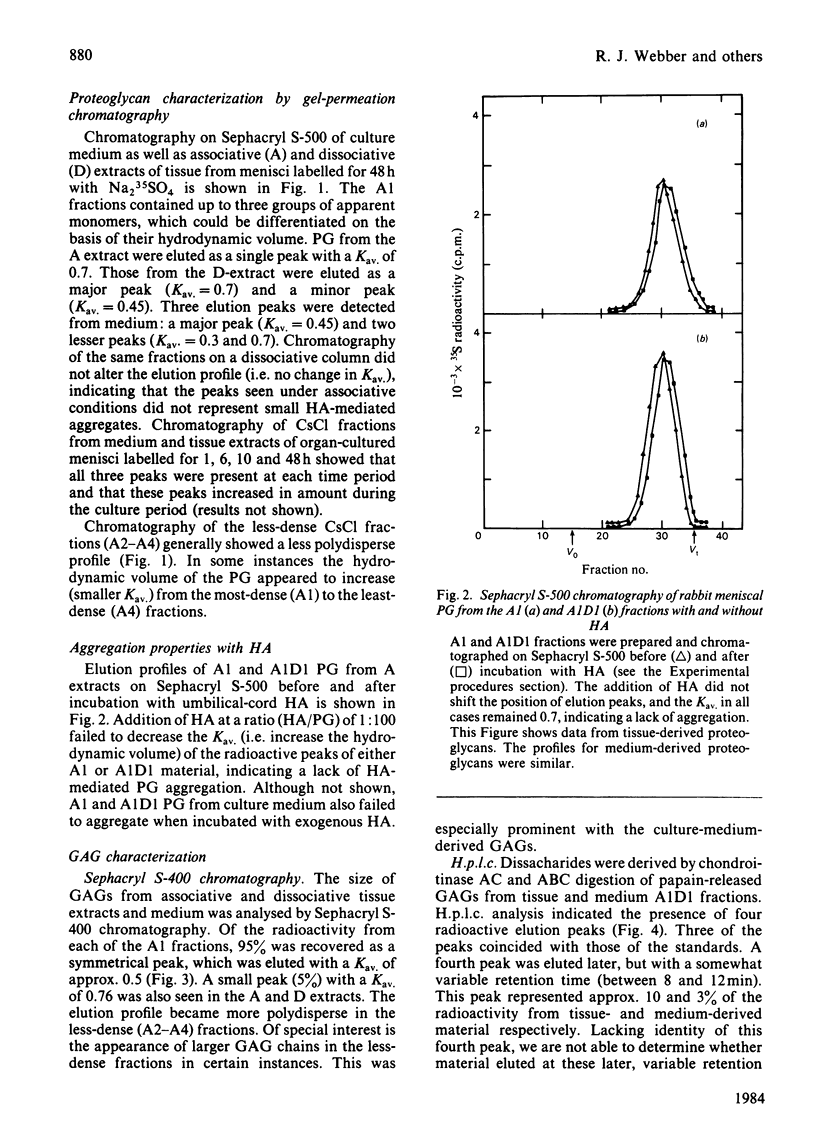
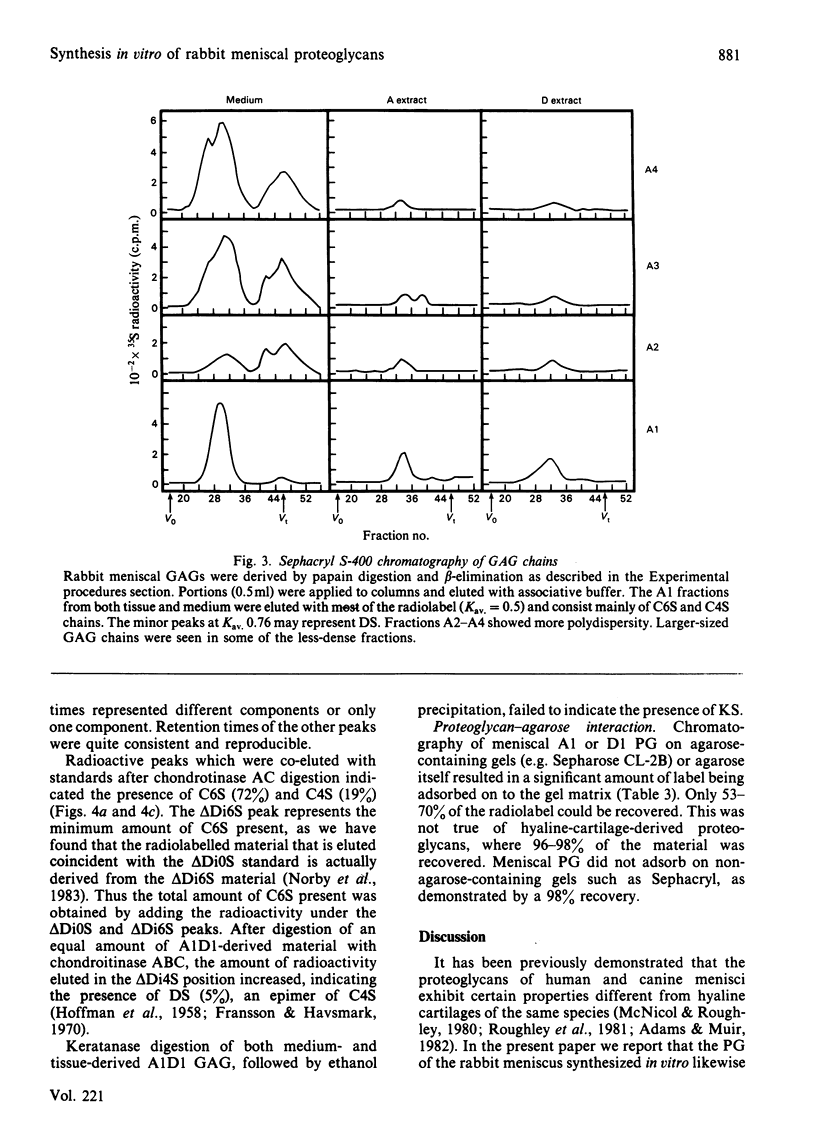
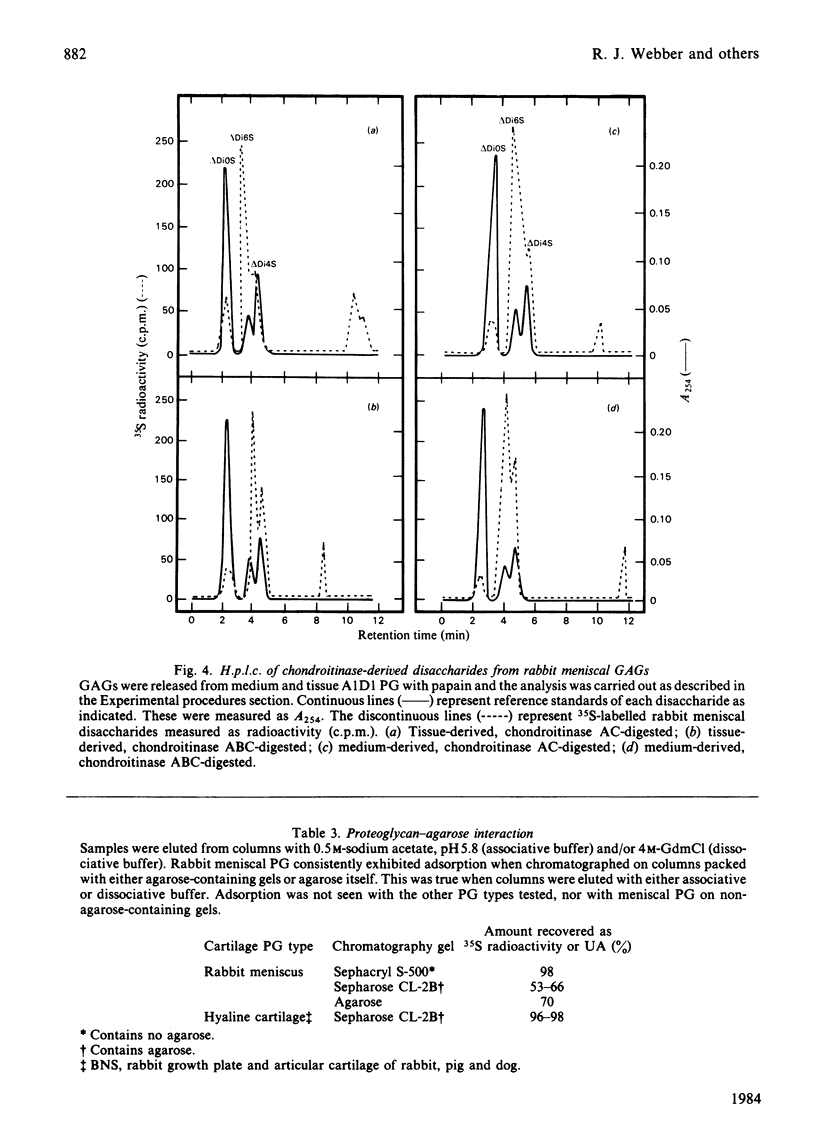
Rabbit menisci were incubated with Na2 35SO4 in short-term organ culture to label newly synthesized proteoglycans. The radioactive products present in both tissue and culture medium were characterized separately with respect to distribution after ultracentrifugation in CsCl isopycnic density gradients, hydrodynamic size, interaction with hyaluronic acid, and glycosaminoglycan composition (types, size and content). Analysis of proteoglycan size by gel-filtration chromatography of the most-dense CsCl fractions (A1) on Sephacryl S-500 (associative conditions) resolved three species. A peak with Kav. approx. 0.7 was present in each chromatogram, and constituted the principal component in tissue extracts. Two other peaks with Kav. values of approx. 0.2 and 0.45 were also found. When the A1 fraction from tissue was subjected to CsCl-density-gradient ultracentrifugation under dissociative conditions, 71% of the recovered radioactivity was present in the most dense (A1D1) fraction. Incubation with hyaluronic acid of either A1 or A1D1 fraction from associative extract did not alter the apparent size of the labelled product, indicating a lack of aggregate formation. Meniscal proteoglycans showed an unusual and marked tendency to adsorb irreversibly to agarose and agarose-containing gel-filtration-chromatography media. High-pressure liquid-chromatographic analyses indicated that the sulphated glycosaminoglycans consisted of chondroitin 6-sulphate (72%), chondroitin 4-sulphate (19%) and dermatan sulphate (5%). Endo-beta-galactosidase (keratanase) digestion of the material failed to detect the presence of keratan sulphate. Of the labelled glycosaminoglycans, 95% was eluted from Sephacryl S-400 as a single symmetrical peak with a Kav. of 0.5. The results of studies with tissue extracts and culture medium were similar.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Muir H. The glycosaminoglycans of canine menisci. Biochem J. 1981 Aug 1;197(2):385–389. doi: 10.1042/bj1970385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson I., Heinegård D. Characterization of the keratan sulphate proteoglycans from bovine corneal stroma. Biochem J. 1978 Mar 1;169(3):517–530. doi: 10.1042/bj1690517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Nimni M. E. The stability of the collagen phenotype during stimulated collagen, glycosaminoglycan, and DNA synthesis by articular cartilage organ cultures. Arch Biochem Biophys. 1979 Feb;192(2):327–335. doi: 10.1016/0003-9861(79)90100-0. [DOI] [PubMed] [Google Scholar]
- Christner J. E., Brown M. L., Dziewiatkowski D. D. Interactions of cartilage proteoglycans with hyaluronate. Inhibition of the interaction by modified oligomers of hyaluronate. J Biol Chem. 1979 Jun 10;254(11):4624–4630. [PubMed] [Google Scholar]
- Faltz L. L., Caputo C. B., Kimura J. H., Schrode J., Hascall V. C. Structure of the complex between hyaluronic acid, the hyaluronic acid-binding region, and the link protein of proteoglycan aggregates from the swarm rat chondrosarcoma. J Biol Chem. 1979 Feb 25;254(4):1381–1387. [PubMed] [Google Scholar]
- Fransson L. A., Havsmark B. Structure of dermatan sulfate. VII. The copolymeric structure of dermatan sulfate from horse aorta. J Biol Chem. 1970 Sep 25;245(18):4770–4783. [PubMed] [Google Scholar]
- Franzén A., Björnsson S., Heinegård D. Cartilage proteoglycan aggregate formation. Role of link protein. Biochem J. 1981 Sep 1;197(3):669–674. doi: 10.1042/bj1970669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg H. G., Swann D. A. Age-related changes in the chemical composition of bovine articular cartilage. The structure of high-density proteoglycans. Biochem J. 1981 Feb 1;193(2):459–468. doi: 10.1042/bj1930459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg V. M., Lance E. M., Davis P. Experimental immune synovitis in the rabbit. Relative roles of cell mediated and humoral immunity. Arthritis Rheum. 1974 Nov-Dec;17(6):993–1005. doi: 10.1002/art.1780170612. [DOI] [PubMed] [Google Scholar]
- HOFFMAN P., LINKER A., MEYER K. Chondroitin sulfates. Fed Proc. 1958 Dec;17(4):1078–1082. [PubMed] [Google Scholar]
- Habuchi H., Yamagata T., Iwata H., Suzuki S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J Biol Chem. 1973 Sep 10;248(17):6019–6028. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hassell J. R., Newsome D. A., Hascall V. C. Characterization and biosynthesis of proteoglycans of corneal stroma from rhesus monkey. J Biol Chem. 1979 Dec 25;254(24):12346–12354. [PubMed] [Google Scholar]
- Ito K., Kimata K., Sobue M., Suzuki S. Altered proteoglycan synthesis by epiphyseal cartilages in culture at low SO4(2-) concentration. J Biol Chem. 1982 Jan 25;257(2):917–923. [PubMed] [Google Scholar]
- Keiser H. D., Malemud C. J. A comparison of the proteoglycans produced by rabbit articular chondrocytes in monolayer and spinner culture and those of bovine nasal cartilage. Connect Tissue Res. 1983;11(4):273–284. doi: 10.3109/03008208309004860. [DOI] [PubMed] [Google Scholar]
- Krause W. R., Pope M. H., Johnson R. J., Wilder D. G. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976 Jul;58(5):599–604. [PubMed] [Google Scholar]
- Lane J. M., Brighton C. T. In vitro rabbit articular cartilage organ model. I. Morphology and glycosaminoglycan metabolism. Arthritis Rheum. 1974 May-Jun;17(3):235–243. doi: 10.1002/art.1780170306. [DOI] [PubMed] [Google Scholar]
- Langenskiöld A., Michelsson J. E., Videman T. Osteoarthritis of the knee in the rabbit produced by immobilization. Attempts to achieve a reproducible model for studies on pathogenesis and therapy. Acta Orthop Scand. 1979 Feb;50(1):1–14. doi: 10.3109/17453677909024083. [DOI] [PubMed] [Google Scholar]
- Lee G. J., Tieckelmann H. High-performance liquid chromatographic determinations of disaccharides resulting from enzymatic degradation of isomeric chondroitin sulfates. Anal Biochem. 1979 Apr 1;94(1):231–236. doi: 10.1016/0003-2697(79)90814-5. [DOI] [PubMed] [Google Scholar]
- Malemud C. J., Norby D. P., Sokoloff L. Explant culture of human and rabbit articular chondrocytes. Connect Tissue Res. 1978;6(3):171–179. doi: 10.3109/03008207809152628. [DOI] [PubMed] [Google Scholar]
- McNicol D., Roughley P. J. Extraction and characterization of proteoglycan from human meniscus. Biochem J. 1980 Mar 1;185(3):705–713. doi: 10.1042/bj1850705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz R. W., Davis W., Sammarco J., Martens M., Baker J., Mayor M., Burstein A. H., Frankel V. H. Experimentally induced degenerative joint lesions following partial meniscectomy in the rabbit. Arthritis Rheum. 1973 May-Jun;16(3):397–405. doi: 10.1002/art.1780160317. [DOI] [PubMed] [Google Scholar]
- Norby D. P., Goldberg V. M., Moskowitz R. W., Malemud C. J. Buffer induced modification of 6-sulfated disaccharide in chondroitin lyase digestions. Anal Biochem. 1983 Jun;131(2):324–332. doi: 10.1016/0003-2697(83)90177-x. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Kjellén L., Hök M. Cell-surface heparan sulfate. Isolation and characterization of a proteoglycan from rat liver membranes. J Biol Chem. 1979 Sep 10;254(17):8505–8510. [PubMed] [Google Scholar]
- Rostand K. S., Baker J. R., Caterson B., Christner J. E. Isolation and characterization of mouse articular cartilage proteoglycans using preformed CsCl density gradients in the Beckman Airfuge. A rapid semi-micro procedure for proteoglycan isolation. J Biol Chem. 1982 Jan 25;257(2):703–707. [PubMed] [Google Scholar]
- Roughley P. J., McNicol D., Santer V., Buckwalter J. The presence of a cartilage-like proteoglycan in the adult human meniscus. Biochem J. 1981 Jul 1;197(1):77–83. doi: 10.1042/bj1970077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLHEIM K. THE GLYCOSAMINOGLYCANS OF HUMAN SEMILUNAR CARTILAGE. J Oslo City Hosp. 1965 Jun;15:127–132. [PubMed] [Google Scholar]
- Santer V., White R. J., Roughley P. J. Proteoglycans from normal and degenerate cartilage of the adult human tibial plateau. Arthritis Rheum. 1981 May;24(5):691–700. doi: 10.1002/art.1780240510. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Nieduszynski I. A., Phelps C. F. Self-association of proteoglycan subunits from pig laryngeal cartilage. Biochem J. 1978 Apr 1;171(1):109–114. doi: 10.1042/bj1710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V. M., MaleMud C. J., Sokoloff L. Chondroid expression by lapine articular chondrocytes in spinner culture following monolayer growth. Connect Tissue Res. 1974;2(2):127–136. doi: 10.3109/03008207409152098. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Biosynthesis of proteoglycans by rat granulosa cells cultured in vitro. J Biol Chem. 1979 Dec 25;254(24):12355–12364. [PubMed] [Google Scholar]
- Yanagishita M., Rodbard D., Hascall V. C. Isolation and characterization of proteoglycans from porcine ovarian follicular fluid. J Biol Chem. 1979 Feb 10;254(3):911–920. [PubMed] [Google Scholar]


