Abstract
Stroke is a leading cause of death and disability worldwide. Several mechanisms are involved in the pathogenesis of ischemic stroke (IS). The contributory role of the inflammatory and immunity processes was demonstrated both in vitro and in animal models, and was confirmed in humans. IS evokes an immediate inflammatory response that involves complex cellular and molecular mechanisms. All components of the innate and adaptive immunity systems are involved in several steps of the ischemic cascade. In the early phase, inflammatory and immune mechanisms contribute to the brain tissue damage, whereas, in the late phase, they participate to the tissue repair processes. In particular, damage-associated molecular patterns (DAMPs) appear critical for the promotion of altered blood brain barrier permeability, leukocytes infiltration, tissue edema and brain injury. Conversely, the activation of regulatory T lymphocytes (Tregs) plays protective effects. The identification of specific cellular/molecular elements belonging to the inflammatory and immune responses, contributing to the brain ischemic injury and tissue remodeling, offers the advantage to design adequate therapeutic strategies. In this article, we will present an overview of the knowledge on inflammatory and immunity processes in IS, with a particular focus on the role of DAMPs and leukocytes infiltration. We will discuss evidence obtained in preclinical models of IS and in humans. The main molecular mechanisms useful for the development of novel therapeutic approaches will be highlighted. The translation of experimental findings to the human disease is still a difficult step to pursue. Further investigations are required to fill up the existing gaps.
Keywords: Ischemic stroke, Inflammation, Immunity, MCAO, Damps, Leukocytes
Introduction
Stroke is the second-leading cause of mortality after ischemic heart disease and one of the main causes of disability worldwide (Favate and Younger 2016; Benjamin et al. 2019). In most cases (85–87%), stroke is of ischemic type (ischemic stroke, IS) and is due to cerebral vessel occlusion by an embolus or a thrombus. The remaining type of stroke is hemorrhagic and occurs following the rupture of a cerebral vessel. In both situations, the blood flow is reduced or completely interrupted causing a failure of oxygen and nutrient supply to the tissue.
Several studies performed over the last decades highlighted the key role of inflammatory and immunity responses in the pathogenesis of IS in animal models (Schmidt-Pogoda et al. 2019; Iadecola and Anrather 2011).
The activation of both inflammatory and immunity responses occurs simultaneously to the ischemic brain damage and it amplifies the neuronal injury. All components of the innate immunity system are involved in several steps of the ischemic cascade including resident cells such as glia, microglia, endothelial cells and circulating inflammatory cells such as monocytes, macrophages and leukocytes. The latter cells, once activated, cross the blood brain barrier (BBB) and accumulate in the damaged brain tissue where they can exert either positive or negative effects (Kamel and Iadecola 2012). Numerous studies showed that the adaptive immunity is also activated in IS by the action of T and B lymphocytes and of the antigen presenting cells (Chamorro et al. 2012).
Studies in human populations analyzed the role of inflammatory markers, such as damage-associated molecular patterns (DAMPs), fibrinogen, white blood cell count and their correlation with stroke occurrence (Emerging Risk Factors et al. 2010; Goldstein et al. 2006). This correlation becomes more pronounced in vulnerable stroke patients such as those with atherosclerosis, obesity, infections, autoimmune diseases or in patients undergoing surgery. Numerous anti-inflammatory strategies were tested in recent years to reduce brain infarct volume, vascular injury and subsequent stroke in animal models. These anti-inflammatory strategies may have beneficial effects also for the treatment of human stroke (Muir et al. 2007).
In this article, we provide an overview of the current stand of knowledge on the role of immune and inflammatory responses in IS, with major emphasis on the involvement of DAMPs and of leukocytes infiltration. We discuss studies performed in commonly used animal models of IS and novel therapeutic approaches targeting DAMPs and leukocytes infiltration for the treatment of human IS.
Overview of Innate and Adaptive Immunity During IS
Innate immunity represents the first defense of the organism against pathogens and includes all mechanisms already existing before the encounter with the antigen. These mechanisms are unable to discriminate specific antigens and to activate an immunologic memory (Albiger et al. 2007). Inflammation acts as the integral part of the innate immune system and is triggered by several chemical mediators. Some of them directly derive from pathogens, like the pathogen-associated molecular patterns (PAMPs). Other mediators, such as DAMPs, are released from damaged cells and tissues. Both PAMPs and DAMPs activate the innate immune response (Newton and Dixit 2012). DAMPs are passively released from neuronal and non-neuronal dying brain cells and, through the binding with the Toll-like receptors (TLRs), they trigger a cascade of downstream signaling pathways that result in transcriptional changes and post-translational modifications (Gulke et al. 2018). DAMPs release induces a significant increase of other proinflammatory mediators, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, reactive oxygen species (ROS) and inducible nitric oxide syntase (iNOS), mainly secreted by activated microglia (Ritzel et al. 2015). These molecules exert detrimental effects contributing to the development of several diseases, such as autoimmune, neurodegenerative and cardiovascular diseases including IS (Scrivo et al. 2011).
During cerebral ischemia, oxygen and glucose deprivation may induce an irreversible brain injury, which depends from the duration of ischemia and from the interested brain area. IS evokes an immediate inflammatory response that involves complex cellular and molecular mechanisms (Schmidt-Pogoda et al. 2019).
Platelets aggregation and leukocytes infiltration represent important cellular mechanisms involved in the response of innate immunity during IS. They increase during brain ischemia and exacerbate blood flow occlusion and brain damage (Kamel and Iadecola 2012). Platelets activation generates pro-inflammatory signals whereas activated leukocytes interact with the endothelium by the binding with adhesion molecules [intercellular cell adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and selectins (Iadecola and Anrather 2011)]. Moreover, leukocytes may also migrate and infiltrate brain tissue. The latter occurs when the permeability of BBB is altered. BBB damage represents a key element of brain injury. Different mechanisms are involved in the BBB breakdown during an ischemic insult. Brain ischemia induces the upregulation of hypoxia-inducible factor 1 (HIF-1) and vascular-endothelial growth factor (VEGF), which in turn contribute to the degradation of tight junction proteins between brain endothelial cells (claudin, occludin and junctional adhesion molecules) (Engelhardt et al. 2014). Increased oxidative stress and DNA damage also increase endothelial cell inflammation leading to upregulation of ICAM-1, pro-inflammatory chemokines and metalloproteinases (MMPs), in particular MMP-2 and MMP-9. Alterations of the permeability of endothelial channels for Na+, K+ and Ca2+ and the upregulation of aquaporin 4 also contribute to worse cerebral edema (Vella et al. 2015). The structural changes of BBB components facilitate the infiltration of peripheral immune cells, such as T cells, macrophages and neutrophils to the brain with a further worsening of BBB damage (Yang and Rosenberg 2011).
Apart from innate immunity, many evidence suggests that adaptive immunity also plays a contributory role during IS. Generally, the adaptive or acquired immune system is activated to protect the organism by exposure to pathogens or toxins and has immunologic memory. Adaptive immunity is mediated by B and T cells. B lymphocytes produce and release antibodies against all possible antigens. On the other hand, T lymphocytes exert an effector role (Cytotoxic T cells, TC, that removes pathogens and infected host cells), a helper role (Helper T cells, TH, that collaborates with B cells and other immune cells), or a regulatory role (regulatory T cells, Tregs).
Several studies suggest that T cell subtypes and their derived cytokines exhibit both adaptive and maladaptive effects in stroke (Papiernik et al. 1992; Selvaraj and Stowe 2017). Other T cell subtypes such as TH1 and TH17 cells, worse ischemic brain damage (Yilmaz et al. 2006; Kleinschnitz et al. 2010; Luo et al. 2015), whereas other subtypes like TH2 and Tregs may activate tissue repair mechanisms or induce anti-inflammatory cytokines release, such as TNF-β and IL-10. In addition, they may also inhibit T helper cells response and promote Tregs activation (Vignali et al. 2008; Liesz et al. 2009).
In middle cerebral artery occlusion (MCAO) murine models, CD8+cytotoxic T lymphocytes exacerbate ischemic brain damage through the release of different molecules secreted by their cytotoxic granules, such as perforin and the granzymes (Yilmaz et al. 2006; Kleinschnitz et al. 2010). They also produce pro-inflammatory cytokines, such as TNF-α and interferon (IFN)-γ, leading to a further increase of inflammation, impairment of brain injury and neurological deficits (Li et al. 2001; Yilmaz et al. 2006).
Differently from CD8+ T cells, Tregs act as suppressors of the activity of other T cells (Sakaguchi 2000; Bettelli et al. 2006) by releasing soluble inhibitory cytokines, such as transforming growth factor beta (TGF-β), interleukin 35 and interleukin 10 (Schmidt et al. 2012). Tregs can be distinguished from the other T cell subtypes by the expression of specific markers: CD4, CD25 and the transcription factor forkhead box (FoxP3) (Yan et al. 2009; Curiel 2007). Genetic depletion of Tregs profoundly increased brain damage and deteriorated functional outcome in MCAO mice, along with the post-ischemic activation of resident inflammatory cells, such as microglia and T cells. The latter represent the main sources of TNF-α and of cerebral IFN-γ, respectively. Early antagonization of TNF-α and IFN-γ prevented the infarct growth in Treg cells-depleted mice (Liesz et al. 2009).
Role of DAMPs in IS: Pre-clinical Evidence
In the presence of ischemic injury, innate immunity is activated in the early phase (Gelderblom et al. 2009). This process begins with the release of DAMPs. As previously mentioned, following ischemic damage DAMPs are released from various cell types including microglia and brain macrophages, brain endothelial cells and neurons (Chiba and Umegaki 2013; Gulke et al. 2018; Yang and Tracey 2010; Schmidt-Pogoda et al. 2019). Subsequently, by binding TLR4 and the receptor for advanced glycation end product (RAGE), DAMPs activate different pathways including TLRs and inflammasomes (Schaefer 2014; Zhang and Mosser 2008), thus exacerbating ischemic damage (Shichita et al. 2012a; Piccinini and Midwood 2010).
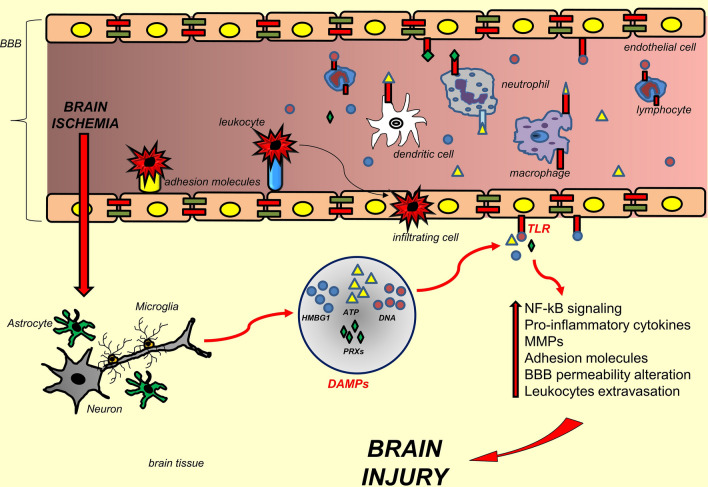
DAMPs include several molecules of different origin: high mobility group protein B1 (HMGB1), histones, DNA, RNA (nuclear compartment) (Goldstein et al. 2006), uric acid (Kono et al. 2010), ATP (Bours et al. 2006), heat shock proteins (cytosol) (Quintana and Cohen 2005), extracellular proteins (fibronectin, biglycan and tenascin C) and mitochondrial components (mtDNA). Once released, DAMPs induce a cascade of detrimental effects in the ischemic brain parenchyma (Kleinschnitz et al. 2010, 2013) (Fig. 1).
Fig. 1.
Summary of the main inflammatory mechanisms involved in the brain injury associated with IS. Schematic representation of the events related to DAMPs-induced inflammation. DAMPs are released from brain cells following an ischemic insult and they interact with TLRs. TLRs activation leads to inflammation, MMPs activation, BBB breakdown and leukocytes extravasation, all factors that belong to the innate immunity and exacerbate the brain injury. See text for further details. Abbreviations legend: ATP adenosine triphosphate, BBB blood brain barrier, DAMPs damaged-associated molecular patterns, HBMG1 high mobility protein B 1, MMPs metalloproteases, NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells, PRXs peroxiredoxins, TLRs toll-like receptors
Apart from IS, DAMPs were shown to play a crucial role in the pathogenesis of different human diseases by their ability to induce inflammation (Land 2015). DAMPs may also represent a promising target for the development of new therapeutic strategies (Table 1), as it will be discussed later (Richardson et al. 1986; Land 2020).
Table 1.
Therapeutics strategies targeting DAMPs and leukocytes infiltration in the middle cerebral artery occlusion (MCAO) murine model
| Therapeutic agent | Cell/molecular target | Mechanisms of action | References |
|---|---|---|---|
| Amlexanox (anti-HMGB1 antibody) | DAMPs |
HMGB1 inhibition Neuroprotective effect |
(Halder and Ueda 2018) |
| Anti-HMGB1 and anti-Prxs antibodies | DAMPs |
HMGB1 and Prxs inhibition ↓IL-23, IL-12, Il-6, Il-1β, TNFα ↓brain damage |
(Halder and Ueda 2018; Shichita et al. 2012b) |
| Phthalide derivative (CD21) | DAMPs |
Prx-1 inhibition DAMPs/TLR4 binding inhibition ↓IL-6, TNFα, IL-1β |
(Zou et al. 2020; Li et al. 2020) |
| Anti-selectins antibody | Leukocytes infiltration |
E-Selectins monoclonal antibody Neurological outcomes improvement BBB injury reduction |
(Huang et al. 2000) |
| TBC-1269 | Leukocytes infiltration |
P-Selectin inhibitor ↓brain myeloperoxidase and ischemic neurons ↑survival rate |
(Anaya-Prado et al. 2008) |
| ICAM-1 gene deletion | Leukocytes infiltration |
ICAM-1 inhibition Infarct size reduction Neurological functional improvement |
(Connolly et al. 1996) |
| Xanthotoxol | Leukocytes infiltration |
Anti-oxidant and anti-inflammatory natural compound ↓ICAM-1 and E-Selectin ↓brain edema, BBB damage ↓IL-1β, TNFα and IL-8 |
(He et al. 2013) |
| VCAM-1 gene deletion | Leukocytes infiltration |
VCAM-1 inhibition Infiltrating cells reduction Infarct size reduction |
(Liesz et al. 2011b) |
| Natalizumab | Leukocytes infiltration |
Anti-Integrin α4 monoclonal antibody Anti-inflammatory effect Functional and neurological outcomes improvement |
(Becker et al. 2001) |
| Fingolimod | Leukocytes infiltration |
Sphinogosine-1-phosphate receptor modulator Infarct size reduction Functional outcomes improvement |
(Liu et al. 2013) |
High Mobility Group Box 1
HMGB1 is a 215 amino-acid nuclear protein that in normal condition binds and modifies the transcription of DNA enhancing genes. In the presence of injury or infection, HMGB1 promotes inflammation (Lotze and Tracey 2005; Yang and Tracey 2005). High levels of systemic HMGB1 were detected in serum of animal models of stroke (Nakano et al. 2019; Muhammad et al. 2008).
Apart from microglia, HMGB1 is secreted by immune cells, such as macrophages, monocytes and dendritic cells (Yilmaz and Granger 2010) and also by non-immune cells. HMGB1 binds different receptors such as TLRs, particularly TLR4 and TLR2, and RAGE that are expressed in the plasma membrane of different cell types (Yang et al. 2010; Qiu et al. 2008; Klune et al. 2008) (Fig. 1). The binding between HMGB1 and TLR4 is mediated by different adaptors including TIR domain-containing adaptor protein-inducing IFN-β (TRIF), TRIF-related adaptor molecule (TRAM) and myeloid differentiation factor 88 (My88) (Akira and Takeda 2004).The downstream effectors of HMGB1 include nuclear factor (NF)-kB, MAPK and type 1 INF-1, which contribute to a consequent release of TNF-α, IL-6, IL-8 and iNOS with further increase of inflammation (Takeuchi and Akira 2010).
In vitro experiments performed in microglia of MCAO rats and in primary neuronal and glial cell co-cultures confirmed that HMGB1 induced release of inflammatory mediators such as TNF-α, IL-1α, IL-1β, IL-6 and of other inflammatory proteins, increasing excitotoxicity and ischemic neuronal death (Kim et al. 2006).
In addition, in microglia derived from MCAO mouse model and treated with HMGB1 recombinant protein (rhHMGB1), the interaction between HMGB1 and TLR4 led to MAPK activation and NF-kB upregulation, increased production and release of cytokines, including TNF-α, IL-1β and IL-6. Conversely, these events were not found in the TLR4/MCAO-deficient mouse (TLR4−/−), demonstrating that TLR4 is essential to mediate the detrimental effects of rhHMGB1. Consistently, studies performed in transgenic mice lacking either TLR2 or TLR4 reported a reduction of the inflammatory response and of the brain injury (Caso et al. 2007; Sansing et al. 2011).
Similarly, when HMGB1 or TLR4 were neutralized with specific antibodies, a protective effect was revealed. In these conditions, levels of inflammatory mediators, including TNF-α, IL-1β and Cyclooxygenase-2 (Cox2), were significantly downregulated as expected (Caso et al. 2007; Yang et al. 2011).
Muhammad et al. demonstrated that the pro-inflammatory effect of HMGB1 was also due to the binding with RAGE receptor in a permanent MCAO mouse model. In this model, the authors found that anti-HMGB1 intraperitoneal administration significantly reduced the infarct volume, but it failed to do so when administered to RAGE deficient mice (RAGE−/−) (Liu et al. 2007; Muhammad et al. 2008). The effect of amlexanox, a HMGB1 inhibitor, was also evaluated in the MCAO model. Intracerebroventricular administration of amlexanox significantly protected the brain from ischemic damage (Halder and Ueda 2018). In the same model, treatment with an anti-HMGB1 antibody reduced levels of inflammatory cytokines (IL-23, IL-12, TNF-α, IL-6 and IL-1β) along with an improvement of ischemic brain damage (Halder and Ueda 2018).
Heat Shock Proteins
Heat shock proteins (HSPs) are molecules that normally act as chaperons and are involved in many biosynthetic pathways (Schaefer 2014). Some HSPs also induce inflammation through the activation of TLR2, TLR4 and CD91 (Zhou and Binder 2014; Schaefer 2014). Hsp70 is the most characterized HSP in IS. Depending on its localization, Hsp70 may exert either protective or detrimental effects. When intracellularly expressed, Hsp70 inhibits the expression of pro-inflammatory mediators such as NF-kB, MMPs and ROS. When expressed by the extracellular matrix, Hsp70 activates NF-kB signaling by its binding with TLRs on macrophages, dendritic cells and microglia (Gulke et al. 2018; Bartoletti-Stella et al. 2018; Guo et al. 2018).
Overexpression of Hsp70 by means of transgenes, viral constructs or fusion proteins was reported to exert neuroprotective effects in neurons and glia in preclinical models of stroke, to reduce cerebral infarct size in rats (Shevtsov et al. 2014; Zhan et al. 2010; Sharp et al. 2013) and to associate with the reduction of inflammatory mediators (TNF-α and IL-1β) in cerebral and endothelial cells (Kim and Yenari 2013).
Peroxiredoxins
Peroxiredoxins (Prxs) are a group of cytosolic DAMPs released in the extracellular space by necrotic brain cells (Fig. 1). Once released, Prxs interact with macrophages TLR2 and TLR4 leading to the production of pro-inflammatory cytokines such as IL-23 (Shichita et al. 2012b). IL-23 induces the release of IL-17 from T lymphocytes (γδ-T subtype), which in turn contributes to neuronal cell death (Shichita et al. 2009; Konoeda et al. 2010). In addition, Prxs promote a more pronounced macrophages tissue infiltration compared to HMGB1 (Shichita et al. 2012b). Interestingly, administration of a gastrodin derivative, a phenolic glycoside extract from tuber of Gastrodiae rhizome, was reported to reduce post-ischemic expression of Prxs (mainly Prx-1, Prx-2 and Prx-4) and of inflammation in the MCAO model (Mao et al. 2017). Other studies showed the neuroprotective and anti-inflammatory effects of the phthalide derivative CD21, a natural compound isolated from the rhizome of L. porteri. This molecule acts as a DAMPs/TLR4 pathway inhibitor in the MCAO model. In the specific, administration of CD21 reduced Prx-1 expression and TLR4/NF-kB activity and suppressed the inflammatory responses mediated by IL-6, TNF-α and IL-1β. These effects were associated with an overall reduction of infarct volume and of neurological deficits (Zou et al. 2020; Li et al. 2020).In the MCAO mouse, Shichita et al. reported that anti-Prxs antibody administration 12 h after stroke was able to attenuate ischemic brain damage and to improve neurological score, along with a reduction of inflammatory cytokines expression (Shichita et al. 2012b). Interestingly, the authors demonstrated that Prxs were produced in the acute phase of IS (from 12 to 24 h after stroke onset) whereas HMGB1 production was observed in the hyperacute phase (within 6 h following stroke). The latter evidence suggests that Prxs display a longer therapeutic time window compared to HMGB1.
Leukocytes Infiltration in IS: Preclinical Evidence
Evidence obtained in MCAO models demonstrated that both cytotoxic T lymphocytes and Tregs are involved in leukocytes infiltration following IS. In the MCAO rat model, cytotoxic T lymphocytes were strongly implicated in the neutrophils infiltration that occurs after reperfusion by producing and releasing pro-inflammatory cytokines, mainly IFN-γ, with an increase of ICAM-1 expression (Schroeter et al. 1994; Jander et al. 1995). Autocrine production of IFN-γ improved also both motility and function of CD8+ cytotoxic cells whereas inhibition of IFN-γ markedly reduced cytotoxic function, motility and cell survival of CD8+ cells (Bhat et al. 2017).
Conversely, systemic administration of purified Tregs derived from donor mice in both MCAO mouse and rat models reduced leukocytes infiltration and peripheral inflammatory cells (dendritic cells, macrophages and neutrophils) in the brain lesions and attenuated BBB damage. In fact, both in vivo and in vitro studies demonstrated that Tregs treatment after ischemia improved BBB integrity, by preserving the ultrastructure of tight junctions and of basement membranes, through the suppression of MMP-9 production (Abdulkareem et al. 2013; Li et al. 2013).
Endothelial selectins and integrins also participate to the process of leukocytes infiltration during IS. When activated, P-selectin and E-selectin translocate to the endothelial surface where they interact with leukocytes. On the other hand, L-selectin is expressed on leukocytes surface and is essential for leukocytes recruitment to the site of injury (Bargatze et al. 1994). Additional proteins, such as integrins and adhesion molecules, including ICAM-1 and VCAM-1, are involved in leukocytes rolling (Edwards and Bix 2019).
Both genetic and pharmacological inhibition of E-selectin led to an improvement of neurological outcome in the MCAO mouse model (Huang et al. 2000), whereas P-selectin-deficient mice showed decreased BBB breakdown (Jin et al. 2010). Administration of the selectin inhibitor TBC-1269 reduced brain myeloperoxidase level, number of ischemic neurons and improved survival rates (80% in TBC-1269 treated rats vs. 40% in controls rats) in a rat model of global cerebral ischemia (Anaya-Prado et al. 2008).
The adhesion molecules ICAM-1 and VCAM-1 were widely investigated in IS (Stanimirovic et al. 1997). Inhibition of ICAM-1 associated with decreased brain damage and leukocytes infiltration (Kitagawa et al. 1998; Kanemoto et al. 2002; Vemuganti et al. 2004). ICAM-1 knockout mice also displayed reduced infarct size, as well as an improvement of neurological function (Connolly et al. 1996). Xanthotoxol is a biologically active linear furocoumarin that occurs in a large number of plants and is mainly extracted from the fruit of Cnidium monnieri cusson (He et al. 2007). It exerts several pharmacological activities such as anti-inflammatory, anti-oxidant and neuroprotective effects (Ng et al. 2000). In a rat model of focal cerebral ischemia/reperfusion, xanthotoxol administration correlated with reduction of brain edema, decreased ICAM-1 and E-selectin levels and inhibition of neutrophils infiltration. Xanthotoxol treatment also attenuated BBB disruption and reduced levels of pro-inflammatory cytokines (IL-1β, TNF-α, IL-8) (He et al. 2013).
The downregulation of VCAM-1 improved stroke outcomes in pre-clinical models (Zhang and Wei 2003; Cervera et al. 2004). MCAO mice carrying genetic knockdown of VCAM-1 showed reduced T lymphocytes infiltration and reduced infarct volume (Liesz et al. 2011b). However, intravenous injection of anti-VCAM-1 antibody failed to show a neuroprotective effect in other experimental conditions (Justicia et al. 2006).
Different integrins were targeted for potential pharmacological therapies, such as lymphocyte function-associated antigen 1 (LFA-1), macrophage-1 antigen (Mac-1) and, more recently, antigen-4 (VLA-4). Studies performed in animal models of stroke demonstrated that the administration of monoclonal antibody against VLA-4 was able to reduce leukocytes adhesion to the activated endothelium and to prevent lymphocytes migration to the inflamed tissue (Becker et al. 2001). In addition, integrins downregulation reduced infarct volume and neurological deficits (Arumugam et al. 2004; Chen et al. 1994; Prestigiacomo et al. 1999).
Finally, administration of fingolimod, a sphingosine-1-phosphate receptor modulator widely used for the treatment of multiple sclerosis, was found to inhibit extravascular migration of leukocytes in animal models of cerebral ischemia (Massberg and von Andrian 2006; Liesz et al. 2011a; Liu et al. 2013). The main therapeutic strategies targeting leukocytes infiltration in the MCAO model are reported in Table 1.
DAMPs and Leukocytes Infiltration in Human IS
Recently, Malone et al. highlighted the need to develop novel therapeutic treatments in addition to thrombolysis and thrombectomy, particularly for those patients who, for different reasons, cannot receive these therapies (Malone et al. 2019b). The novel therapies should reduce post-ischemic brain damage and, at the same time, activate the mechanisms of tissue remodelling. Therefore, they could improve the functional outcomes by increasing patients’ survival.
Due to the role played by immunity in each stroke phase, a wide range of immune-targeted therapies was developed and is currently being tested (Malone et al. 2019a; Neuhaus et al. 2017) (Table 2).
Table 2.
Therapeutics strategies targeting DAMPs and leukocytes infiltration in human IS
| Type of study | Therapeutic agent | Cell/molecular target | Mechanisms of action | References |
|---|---|---|---|---|
| IS patients | Olmesartan | DAMPs |
Angiotensin II type 1 receptor blocker ↓Prxs |
(Tada et al. 2015) |
|
Enlimomab Acute Stroke Phase III clinical trial |
Enliomab | Leukocytes infiltration |
ICAM-1 antibody No beneficial effects in humans were found |
(del Zoppo 2010; Enlimomab Acute Stroke Trial 2001) |
|
LeukArrest Phase III and ASTIN clinical trials |
Rovelzumab (Hu23F2G) | Leukocytes infiltration |
Anti-Integrin Mac-1 monoclonal antibody No beneficial effects in humans were found |
(Liesz et al. 2011b; Becker 2002) |
|
ACTION Phase II clinical trial |
Natalizumab | Leukocytes infiltration |
Anti-Integrin α4 monoclonal antibody No beneficial effects in humans were found |
(Becker et al. 2001; Elkins et al. 2017) |
| IS patients | Fingolimod | Leukocytes infiltration |
Sphinogosine-1-phosphate receptor modulator Improvement of neurological functions Decreased microvascular permeability Lower circulating lymphocytes; Smaller lesion volumes and hemorrhage |
(Fu et al. 2014; Zhu et al. 2015) |
IS ischemic stroke, ASTIN acute stroke therapy by inhibition of neutrophils
The pre-clinical evidence described above suggests that DAMPs release and leukocytes infiltration are pivotal mechanisms of the immune response during IS, and they also represent interesting therapeutic targets for the treatment of human stroke. On the other hand, they may also represent useful predictors of both stroke onset and progression.
In this section, we discuss relevant human studies that corroborate the findings obtained in preclinical models of IS.
DAMPs
In patients with cerebral ischemia, elevated HMGB1 levels were found to correlate with severe stroke size (Huang et al. 2013; Schulze et al. 2013; Sapojnikova et al. 2014; Goldstein et al. 2006). In a study performed in 338 patients with IS, plasma level of HMGB1 was reported to correlate with elevated mortality and unfavourable outcome after 1-year of follow-up. This evidence suggests that plasma HMGB1 level may represent an important biomarker for predicting the clinical outcomes of IS after 1-year (Huang et al. 2013).
High plasma level of Prx-1 and low level of Prx-5 were found in patients with IS soon after the onset of symptoms (Kunze et al. 2014; Richard et al. 2016; Mao et al. 2017). Interestingly, in a study of 98 patients the level of Prx-5 was lower in the presence of severe stroke and was inversely correlated with markers of inflammation. The observed reduction of circulating Prx-5 in severe stroke may be explained by its premature degradation or reduced synthesis. Further studies are needed to define the potential role of Prx-5 in stroke. The role of other DAMPs, such as HSPs, should also be evaluated in humans (Kunze et al. 2014).
Some studies reported that DAMPs inhibition represents an efficacious strategy also in human IS. Tada et al. demonstrated that administration of olmesartan, an Angiotensin II type I receptor blocker, showed antioxidant and anti-inflammatory properties, with a significant reduction of plasma levels of Prxs and of the oxidized low-density lipoprotein/β-2-glycoprotein-I complex (oxLDL/β2GPI) after 12 weeks of treatment in stroke patients (Tada et al. 2015). To the best of our knowledge, specific inhibitors of DAMPs have not yet been tested in humans.
Leukocytes Infiltration
Leukocytes infiltration was observed in specimens of stroke patients. In this regard, T lymphocytes, mainly CD8+ cytotoxic cells, and macrophages accumulation were observed in human stroke lesions (Zrzavy et al. 2018). Increased blood levels of CD4+CD28nullT cells were found to correlate with stroke severity and serum levels of proinflammatory cytokines in IS patients (Tuttolomondo et al. 2015).
As described above, selectins, adhesion molecules and integrins, expressed on the endothelial cell surface play a pivotal role in leukocytes extravasation. Their suppression represents a potential strategy for conferring protection against IS, as shown in preclinical models.
Fu et al. reported that the oral administration of fingolimod showed beneficial effects on cerebral infarct size, rates of haemorrhagic transformation and neurological function in patients with IS (Fu et al. 2014).The protective effects of fingolimod, administered in combination with alteplase, a fibrinolytic agent, were also observed in a separate trial by Zhu et al. (2015). At the molecular level, the fingolimod prevented normal egress of lymphocytes from lymphoid organs and also inhibited leukocytes and NK cells infiltration into the central nervous system (Chun and Hartung 2010).The efficacy of natalizumab has been also evaluated in the phase II “ACTION” clinical trial in patients with acute IS. Although natalizumab administration did not affect infarct volume, some neurological improvements were reported (Elkins et al. 2017).
Despite the promising evidence that inhibition of leukocytes infiltration could exert protective effects in humans, no other positive results were obtained in this regard. For instance, the inhibition of ICAM-1 by using a specific antibody, enlimomab, did not show beneficial effects in a phase III clinical trial. In fact, patients treated with enlimomab reported higher mortality, increased incidence of serious adverse events and more frequent infections (Enlimomab Acute Stroke Trial 2001; del Zoppo 2010). Rovelzumab (also known as Hu23F2G), a monoclonal antibody targeting the integrin Mac1, also failed to show neuroprotective effects in the human phase III clinical trial “LeukArrest” (Becker 2002). The same negative result was reported by the “Acute Stroke Therapy by Inhibition of Neutrophils” (ASTIN) trial (Krams et al. 2003).
Conclusions
The evidence discussed in our review suggests that DAMPs-induced inflammation and innate immunity mechanisms represent key elements in the early stage of IS. In fact, studies performed in animal models of IS revealed that BBB disruption and the consequent leukocytes extravasation exacerbated ischemic injury. DAMPs also enhanced leukocytes extravasation, thus creating synergistic deleterious effects in the cerebral ischemic area. Therefore, the inhibition of DAMPs and of leukocytes infiltration appears as a promising therapeutic approach for IS. In this regard, we reviewed studies demonstrating that specific antibodies or inhibitors able to target these mechanisms improved neurological outcomes and reduced infarct size both in preclinical models and in IS patients. However, further efforts should be performed in this field. First of all, additional drugs targeting DAMPs and leukocytes infiltration should be characterized in order to achieve a better site-specific action. Some compounds that have been shown to be efficacious in pre-clinical models of stroke failed to exert the same beneficial effects in clinical trials. To overcome this issue, pre-clinical models which best mimic the human immune and inflammatory responses during stroke should be developed. The therapeutic time window for drug administration should also be better defined in humans.
Overall, further human studies are needed to solve the current controversial findings and uncertainties.
Author Contributions
Conceptualization: [RS, SR]; Writing—original draft preparation: [RS]; Writing—review and editing: [RS, MF, SR]; Literature search: [RS, MC, FB, SM]; Critical revision: [SR]. All authors read and approved the final manuscript.
Funding
This work was supported by a Grant from the Italian Ministry of Health.
Compliance with Ethical Standards
Conflict of interest
None to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rosita Stanzione and Maurizio Forte have equally contributed.
Contributor Information
Rosita Stanzione, Email: stanzione@neuromed.it.
Speranza Rubattu, Email: rubattu.speranza@neuromed.it.
References
- Abdulkareem N, Smelt J, Jahangiri M (2013) Bicuspid aortic valve aortopathy: genetics, pathophysiology and medical therapy. Interact Cardiovasc Thorac Surg 17(3):554–559. 10.1093/icvts/ivt196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7):499–511. 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- Albiger B, Dahlberg S, Henriques-Normark B, Normark S (2007) Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med 261(6):511–528. 10.1111/j.1365-2796.2007.01821.x [DOI] [PubMed] [Google Scholar]
- Anaya-Prado R, Perez-Gomez N, Toledo-Pereyra LH, Walsh J, Jordan J, Ward PA (2008) Small molecule selectin inhibitor in global cerebral ischemia and controlled hemorrhagic shock. J Trauma 65(3):678–684. 10.1097/TA.0b013e3181843f3a [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Salter JW, Chidlow JH, Ballantyne CM, Kevil CG, Granger DN (2004) Contributions of LFA-1 and Mac-1 to brain injury and microvascular dysfunction induced by transient middle cerebral artery occlusion. Am J Physiol Heart Circ Physiol 287(6):H2555–2560. 10.1152/ajpheart.00588.2004 [DOI] [PubMed] [Google Scholar]
- Bargatze RF, Kurk S, Butcher EC, Jutila MA (1994) Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med 180(5):1785–1792. 10.1084/jem.180.5.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti-Stella A, Baiardi S, Stanzani-Maserati M, Piras S, Caffarra P, Raggi A, Pantieri R, Baldassari S, Caporali L, Abu-Rumeileh S, Linarello S, Liguori R, Parchi P, Capellari S (2018) Identification of rare genetic variants in Italian patients with dementia by targeted gene sequencing. Neurobiol Aging. 10.1016/j.neurobiolaging.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Becker KJ (2002) Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin 18(Suppl 2):s18–s22. 10.1185/030079902125000688 [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, Relton J, Harlan J, Winn R (2001) Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke 32(1):206–211. 10.1161/01.str.32.1.206 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139(10):e56–e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Bhat P, Leggatt G, Waterhouse N, Frazer IH (2017) Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis 8(6):e2836. 10.1038/cddis.2017.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112(2):358–404. 10.1016/j.pharmthera.2005.04.013 [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I (2007) Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115(12):1599–1608. 10.1161/CIRCULATIONAHA.106.603431 [DOI] [PubMed] [Google Scholar]
- Cervera A, Justicia C, Reverter JC, Planas AM, Chamorro A (2004) Steady plasma concentration of unfractionated heparin reduces infarct volume and prevents inflammatory damage after transient focal cerebral ischemia in the rat. J Neurosci Res 77(4):565–572. 10.1002/jnr.20186 [DOI] [PubMed] [Google Scholar]
- Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R (2012) The immunology of acute stroke. Nat Rev Neurol 8(7):401–410. 10.1038/nrneurol.2012.98 [DOI] [PubMed] [Google Scholar]
- Chen H, Chopp M, Zhang RL, Bodzin G, Chen Q, Rusche JR, Todd RF 3rd (1994) Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol 35(4):458–463. 10.1002/ana.410350414 [DOI] [PubMed] [Google Scholar]
- Chiba T, Umegaki K (2013) Pivotal roles of monocytes/macrophages in stroke. Mediat Inflamm 2013:759103. 10.1155/2013/759103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hartung HP (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33(2):91–101. 10.1097/WNF.0b013e3181cbf825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly ES Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ (1996) Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest 97(1):209–216. 10.1172/JCI118392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ (2007) Tregs and rethinking cancer immunotherapy. J Clin Invest 117(5):1167–1174. 10.1172/JCI31202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ (2010) Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci 1207:143–148. 10.1111/j.1749-6632.2010.05761.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DN, Bix GJ (2019) The inflammatory response after ischemic stroke: targeting beta2 and beta1 integrins. Front Neurosci 13:540. 10.3389/fnins.2019.00540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, Lansberg MG, Tang W, Chang I, Muralidharan K, Gheuens S, Mehta L, Elkind MSV (2017) Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol 16(3):217–226. 10.1016/S1474-4422(16)30357-X [DOI] [PubMed] [Google Scholar]
- Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J (2010) C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375(9709):132–140. 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt S, Patkar S, Ogunshola OO (2014) Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol 171(5):1210–1230. 10.1111/bph.12489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlimomab Acute Stroke Trial I (2001) Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 57(8):1428–1434. 10.1212/wnl.57.8.1428 [DOI] [PubMed] [Google Scholar]
- Favate AS, Younger DS (2016) Epidemiology of ischemic stroke. Neurol Clin 34(4):967–980. 10.1016/j.ncl.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, Han W, Xue R, Liu Q, Hao J, Yu C, Shi FD (2014) Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci USA 111(51):18315–18320. 10.1073/pnas.1416166111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40(5):1849–1857. 10.1161/STROKEAHA.108.534503 [DOI] [PubMed] [Google Scholar]
- Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, Czura CJ, Lee DC, Ward MF, Bruchfeld AN, Wang H, Lesser ML, Church AL, Litroff AH, Sama AE, Tracey KJ (2006) Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 25(6):571–574. 10.1097/01.shk.0000209540.99176.72 [DOI] [PubMed] [Google Scholar]
- Gulke E, Gelderblom M, Magnus T (2018) Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord. 10.1177/1756286418774254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Ma J, Li T, Yan L (2018) Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-kappaB pathway by targeting FOXO3. Exp Cell Res 369(1):34–42. 10.1016/j.yexcr.2018.04.027 [DOI] [PubMed] [Google Scholar]
- Halder SK, Ueda H (2018) Amlexanox inhibits cerebral ischemia-induced delayed astrocytic high-mobility group box 1 release and subsequent brain damage. J Pharmacol Exp Ther 365(1):27–36. 10.1124/jpet.117.245340 [DOI] [PubMed] [Google Scholar]
- He W, Zhang BL, Zhou SY, Sun XL, Zhang SY (2007) Facile total synthesis of xanthotoxol. Synth Commun 37(3):361–367. 10.1080/00397910601038616 [Google Scholar]
- He W, Chen W, Zhou Y, Tian Y, Liao F (2013) Xanthotoxol exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. Cell Mol Neurobiol 33(5):715–722. 10.1007/s10571-013-9939-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES Jr (2000) Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke 31(12):3047–3053 [PubMed] [Google Scholar]
- Huang JM, Hu J, Chen N, Hu ML (2013) Relationship between plasma high-mobility group box-1 levels and clinical outcomes of ischemic stroke. J Crit Care 28(5):792–797. 10.1016/j.jcrc.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17(7):796–808. 10.1038/nm.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G (1995) Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab 15(1):42–51. 10.1038/jcbfm.1995.5 [DOI] [PubMed] [Google Scholar]
- Jin AY, Tuor UI, Rushforth D, Kaur J, Muller RN, Petterson JL, Boutry S, Barber PA (2010) Reduced blood brain barrier breakdown in P-selectin deficient mice following transient ischemic stroke: a future therapeutic target for treatment of stroke. BMC Neurosci 11:12. 10.1186/1471-2202-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, Chamorro A, Planas AM (2006) Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab 26(3):421–432. 10.1038/sj.jcbfm.9600198 [DOI] [PubMed] [Google Scholar]
- Kamel H, Iadecola C (2012) Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol 69(5):576–581. 10.1001/archneurol.2011.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto Y, Nakase H, Akita N, Sakaki T (2002) Effects of anti-intercellular adhesion molecule-1 antibody on reperfusion injury induced by late reperfusion in the rat middle cerebral artery occlusion model. Neurosurgery 51(4):1034–1041; discussion 1041–1032. 10.1097/00006123-200210000-00033 [DOI] [PubMed] [Google Scholar]
- Kim JY, Yenari MA (2013) The immune modulating properties of the heat shock proteins after brain injury. Anat Cell Biol 46(1):1–7. 10.5115/acb.2013.46.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK (2006) HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 26(24):6413–6421. 10.1523/JNEUROSCI.3815-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M, Yanagihara T (1998) Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab 18(12):1336–1345. 10.1097/00004647-199812000-00008 [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G (2010) Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115(18):3835–3842. 10.1182/blood-2009-10-249078 [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H (2013) Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 121(4):679–691. 10.1182/blood-2012-04-426734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A (2008) HMGB1: endogenous danger signaling. Mol Med 14(7–8):476–484. 10.2119/2008-00034.Klune [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Chen CJ, Ontiveros F, Rock KL (2010) Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest 120(6):1939–1949. 10.1172/JCI40124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoeda F, Shichita T, Yoshida H, Sugiyama Y, Muto G, Hasegawa E, Morita R, Suzuki N, Yoshimura A (2010) Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochem Biophys Res Commun 402(3):500–506. 10.1016/j.bbrc.2010.10.058 [DOI] [PubMed] [Google Scholar]
- Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA, Investigators AS (2003) Acute stroke therapy by inhibition of neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke 34(11):2543–2548. 10.1161/01.STR.0000092527.33910.89 [DOI] [PubMed] [Google Scholar]
- Kunze A, Zierath D, Tanzi P, Cain K, Becker K (2014) Peroxiredoxin 5 (PRX5) is correlated inversely to systemic markers of inflammation in acute stroke. Stroke 45(2):608–610. 10.1161/STROKEAHA.113.003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land WG (2015) The role of damage-associated molecular patterns (DAMPs) in human diseases: part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J 15(2):e157–170 [PMC free article] [PubMed] [Google Scholar]
- Land WG (2020) Use of DAMPs and SAMPs as therapeutic targets or therapeutics: a note of caution. Mol Diagn Ther 24(3):251–262. 10.1007/s40291-020-00460-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Kostulas N, Huang YM, Xiao BG, van der Meide P, Kostulas V, Giedraitas V, Link H (2001) IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. J Neuroimmunol 116(1):5–14. 10.1016/s0165-5728(01)00264-8 [DOI] [PubMed] [Google Scholar]
- Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X (2013) Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol 74(3):458–471. 10.1002/ana.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shi MQ, Chen C, Du JR (2020) Phthalide derivative CD21 ameliorates ischemic brain injury in a mouse model of global cerebral ischemia: involvement of inhibition of NLRP3. Int Immunopharmacol 86:106714. 10.1016/j.intimp.2020.106714 [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R (2009) Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 15(2):192–199. 10.1038/nm.1927 [DOI] [PubMed] [Google Scholar]
- Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, Bauer H, Sommer C, Veltkamp R (2011a) FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS ONE 6(6):e21312. 10.1371/journal.pone.0021312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R (2011b) Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 134(Pt 3):704–720. 10.1093/brain/awr008 [DOI] [PubMed] [Google Scholar]
- Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M (2007) Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J 21(14):3904–3916. 10.1096/fj.07-8770com [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang C, Tao W, Liu M (2013) Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int J Neurosci 123(3):163–169. 10.3109/00207454.2012.749255 [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5(4):331–342. 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhou Y, Xiao W, Liang Z, Dai J, Weng X, Wu X (2015) Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res 1597:86–94. 10.1016/j.brainres.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Malone K, Amu S, Moore AC, Waeber C (2019a) The immune system and stroke: from current targets to future therapy. Immunol Cell Biol 97(1):5–16. 10.1111/imcb.12191 [DOI] [PubMed] [Google Scholar]
- Malone K, Amu S, Moore AC, Waeber C (2019b) Immunomodulatory therapeutic strategies in stroke. Front Pharmacol 10:630. 10.3389/fphar.2019.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao XN, Zhou HJ, Yang XJ, Zhao LX, Kuang X, Chen C, Liu DL, Du JR (2017) Neuroprotective effect of a novel gastrodin derivative against ischemic brain injury: involvement of peroxiredoxin and TLR4 signaling inhibition. Oncotarget 8(53):90979–90995. 10.18632/oncotarget.18773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, von Andrian UH (2006) Fingolimod and sphingosine-1-phosphate–modifiers of lymphocyte migration. N Engl J Med 355(11):1088–1091. 10.1056/NEJMp068159 [DOI] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M (2008) The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci 28(46):12023–12031. 10.1523/JNEUROSCI.2435-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir KW, Tyrrell P, Sattar N, Warburton E (2007) Inflammation and ischaemic stroke. Curr Opin Neurol 20(3):334–342. 10.1097/WCO.0b013e32813ba151 [DOI] [PubMed] [Google Scholar]
- Nakano T, Nakamura Y, Matsuyama K, Irie K, Yasumura M, Hirata Y, Yamasaki M, Misumi K, Yamashita Y, Myose T, Matsuo K, Sano K, Kamimura H, Ishikura H, Egawa T, Mishima K (2019) Long-term treatment with thrombomodulin improves functional outcomes after cerebral ischemia even if administration is delayed. Thromb Haemost 119(3):467–478. 10.1055/s-0038-1677532 [DOI] [PubMed] [Google Scholar]
- Neuhaus AA, Couch Y, Hadley G, Buchan AM (2017) Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 140(8):2079–2092. 10.1093/brain/awx126 [DOI] [PubMed] [Google Scholar]
- Newton K, Dixit VM (2012) Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TB, Liu F, Wang ZT (2000) Antioxidative activity of natural products from plants. Life Sci 66(8):709–723. 10.1016/s0024-3205(99)00642-6 [DOI] [PubMed] [Google Scholar]
- Papiernik E, Goujon H, Demeulemeester R, Mezin R (1992) Evaluation of a public health measure for the amelioration of birth safety in Martinique: 1977–1984. J Gynecol Obstet Biol Reprod (Paris) 21(4):347–354 [PubMed] [Google Scholar]
- Piccinini AM, Midwood KS (2010) DAMPening inflammation by modulating TLR signalling. Mediat Inflamm. 10.1155/2010/672395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestigiacomo CJ, Kim SC, Connolly ES Jr, Liao H, Yan SF, Pinsky DJ (1999) CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke 30(5):1110–1117. 10.1161/01.str.30.5.1110 [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA (2008) Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 28(5):927–938. 10.1038/sj.jcbfm.9600582 [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR (2005) Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol 175(5):2777–2782. 10.4049/jimmunol.175.5.2777 [DOI] [PubMed] [Google Scholar]
- Richard S, Lapierre V, Girerd N, Bonnerot M, Burkhard PR, Lagerstedt L, Bracard S, Debouverie M, Turck N, Sanchez JC (2016) Diagnostic performance of peroxiredoxin 1 to determine time-of-onset of acute cerebral infarction. Sci Rep 6:38300. 10.1038/srep38300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, Riopelle RJ (1986) Distribution of neuronal receptors for nerve growth factor in the rat. J Neurosci 6(8):2312–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD (2015) Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflamm 12:106. 10.1186/s12974-015-0329-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S (2000) Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101(5):455–458. 10.1016/s0092-8674(00)80856-9 [DOI] [PubMed] [Google Scholar]
- Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K (2011) Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann Neurol 70(4):646–656. 10.1002/ana.22528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapojnikova N, Kartvelishvili T, Asatiani N, Zinkevich V, Kalandadze I, Gugutsidze D, Shakarishvili R, Tsiskaridze A (2014) Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochim Biophys Acta 1842(9):1379–1384. 10.1016/j.bbadis.2014.04.031 [DOI] [PubMed] [Google Scholar]
- Schaefer L (2014) Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem 289(51):35237–35245. 10.1074/jbc.R114.619304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Oberle N, Krammer PH (2012) Molecular mechanisms of treg-mediated T cell suppression. Front Immunol 3:51. 10.3389/fimmu.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Pogoda A, Minnerup J, Kleinschnitz C (2019) Immunology of stroke: from animal models to clinical trials. Ther Adv Neurol Disord 12:1756286419830862. 10.1177/1756286419830862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G (1994) Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol 55(2):195–203. 10.1016/0165-5728(94)90010-8 [DOI] [PubMed] [Google Scholar]
- Schulze J, Zierath D, Tanzi P, Cain K, Shibata D, Dressel A, Becker K (2013) Severe stroke induces long-lasting alterations of high-mobility group box 1. Stroke 44(1):246–248. 10.1161/STROKEAHA.112.676072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivo R, Vasile M, Bartosiewicz I, Valesini G (2011) Inflammation as "common soil" of the multifactorial diseases. Autoimmun Rev 10(7):369–374. 10.1016/j.autrev.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Selvaraj UM, Stowe AM (2017) Long-term T cell responses in the brain after an ischemic stroke. Discov Med 24(134):323–333 [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Zhan X, Liu DZ (2013) Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res 4(6):685–692. 10.1007/s12975-013-0271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov MA, Nikolaev BP, Yakovleva LY, Dobrodumov AV, Dayneko AS, Shmonin AA, Vlasov TD, Melnikova EV, Vilisov AD, Guzhova IV, Ischenko AM, Mikhrina AL, Galibin OV, Yakovenko IV, Margulis BA (2014) Neurotherapeutic activity of the recombinant heat shock protein Hsp70 in a model of focal cerebral ischemia in rats. Drug Des Dev Ther 8:639–650. 10.2147/DDDT.S62024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A (2009) Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med 15(8):946–950. 10.1038/nm.1999 [DOI] [PubMed] [Google Scholar]
- Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H (2012a) Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem 123(Suppl 2):29–38. 10.1111/j.1471-4159.2012.07941.x [DOI] [PubMed] [Google Scholar]
- Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, Ishii T, Takahashi H, Mori S, Nishibori M, Kuroda K, Akira S, Miyake K, Yoshimura A (2012b) Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med 18(6):911–917. 10.1038/nm.2749 [DOI] [PubMed] [Google Scholar]
- Stanimirovic D, Shapiro A, Wong J, Hutchison J, Durkin J (1997) The induction of ICAM-1 in human cerebromicrovascular endothelial cells (HCEC) by ischemia-like conditions promotes enhanced neutrophil/HCEC adhesion. J Neuroimmunol 76(1–2):193–205. 10.1016/s0165-5728(97)00057-x [DOI] [PubMed] [Google Scholar]
- Tada Y, Yagi K, Uno M, Matsushita N, Kanematsu Y, Kuwayama K, Shimada K, Nishi K, Hirasawa M, Satomi J, Kitazato KT, Kageji T, Matsuura E, Nagahiro S (2015) Improvement of plasma biomarkers after switching stroke patients from other angiotensin II type I receptor blockers to olmesartan. J Stroke Cerebrovasc Dis 24(7):1487–1492. 10.1016/j.jstrokecerebrovasdis.2015.03.015 [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140(6):805–820. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A, Pecoraro R, Casuccio A, Di Raimondo D, Butta C, Clemente G, Della Corte V, Guggino G, Arnao V, Maida C, Simonetta I, Maugeri R, Squatrito R, Squadrito R, Pinto A (2015) Peripheral frequency of CD4+ CD28- cells in acute ischemic stroke: relationship with stroke subtype and severity markers. Medicine (Baltimore) 94(20):e813. 10.1097/MD.0000000000000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella J, Zammit C, Di Giovanni G, Muscat R, Valentino M (2015) The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci 9:108. 10.3389/fncel.2015.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuganti R, Dempsey RJ, Bowen KK (2004) Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke 35(1):179–184. 10.1161/01.STR.0000106479.53235.3E [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8(7):523–532. 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Greer JM, Etherington K, Cadigan GP, Cavanagh H, Henderson RD, O'Sullivan JD, Pandian JD, Read SJ, McCombe PA (2009) Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol 206(1–2):112–117. 10.1016/j.jneuroim.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA (2011) Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42(11):3323–3328. 10.1161/STROKEAHA.110.608257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Tracey KJ (2005) High mobility group box 1 (HMGB1). Crit Care Med 33(12 Suppl):S472–474. 10.1097/01.ccm.0000187005.81616.a9 [DOI] [PubMed] [Google Scholar]
- Yang H, Tracey KJ (2010) Targeting HMGB1 in inflammation. Biochim Biophys Acta 1799(1–2):149–156. 10.1016/j.bbagrm.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q, Lu FL, Xiang J (2010) High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cereb Blood Flow Metab 30(2):243–254. 10.1038/jcbfm.2009.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, Li JC, Fang CQ, Wang JZ (2011) HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab 31(2):593–605. 10.1038/jcbfm.2010.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN (2010) Leukocyte recruitment and ischemic brain injury. Neuromol Med 12(2):193–204. 10.1007/s12017-009-8074-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN (2006) Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113(17):2105–2112. 10.1161/CIRCULATIONAHA.105.593046 [DOI] [PubMed] [Google Scholar]
- Zhan X, Ander BP, Liao IH, Hansen JE, Kim C, Clements D, Weisbart RH, Nishimura RN, Sharp FR (2010) Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke 41(3):538–543. 10.1161/STROKEAHA.109.572537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LH, Wei EQ (2003) Neuroprotective effect of ONO-1078, a leukotriene receptor antagonist, on transient global cerebral ischemia in rats. Acta Pharmacol Sin 24(12):1241–1247 [PubMed] [Google Scholar]
- Zhang X, Mosser DM (2008) Macrophage activation by endogenous danger signals. J Pathol 214(2):161–178. 10.1002/path.2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YJ, Binder RJ (2014) The heat shock protein-CD91 pathway mediates tumor immunosurveillance. Oncoimmunology 3:e28222. 10.4161/onci.28222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD (2015) Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation 132(12):1104–1112. 10.1161/CIRCULATIONAHA.115.016371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Yang XJ, Gan YM, Liu DL, Chen C, Duan W, Du JR (2020) Neuroprotective effect of phthalide derivative CD21 against ischemic brain injury: involvement of MSR1 mediated DAMP peroxiredoxin1 clearance and TLR4 signaling inhibition. J Neuroimmune Pharmacol. 10.1007/s11481-020-09911-0 [DOI] [PubMed] [Google Scholar]
- Zrzavy T, Machado-Santos J, Christine S, Baumgartner C, Weiner HL, Butovsky O, Lassmann H (2018) Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol 28(6):791–805. 10.1111/bpa.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]