Abstract
Responses to vasopressin were studied in human platelets loaded with the fluorescent Ca2+ indicator, quin2. In the presence of 1 mM external Ca2+, vasopressin caused a transient rise in [Ca2+]i from the basal level near 100nM to about 700 nM; peak [Ca2+]i was reached in a few seconds and the level then declined towards resting over several minutes. In the absence of external Ca2+ there was a much smaller rise of similar time-course, suggesting that vasopressin increases [Ca2+]i mainly by stimulated-influx across the plasma membrane but also by partly releasing internal Ca2+. Inhibition of thromboxane A2 formation somewhat reduced the peak [Ca2+]i in the presence of external Ca2+, but had no effect on the response attributed to release of internal Ca2+. With external Ca2+, vasopressin stimulated shape-change, secretion and aggregation. Secretion and aggregation were decreased by about half following blockage of thromboxane production. The ability of vasopressin to induce shape-change and secretion even at near basal [Ca2+]i suggests that activators other than Ca2+ are involved.
Full text
PDF
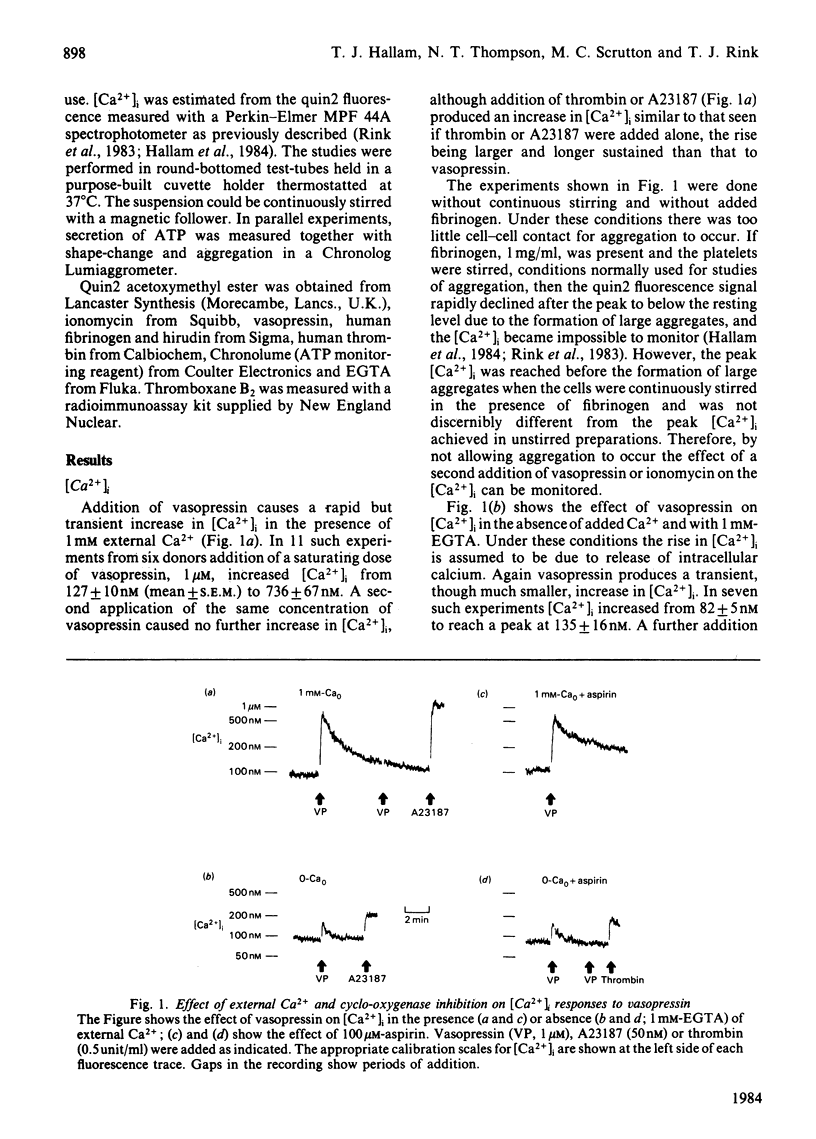
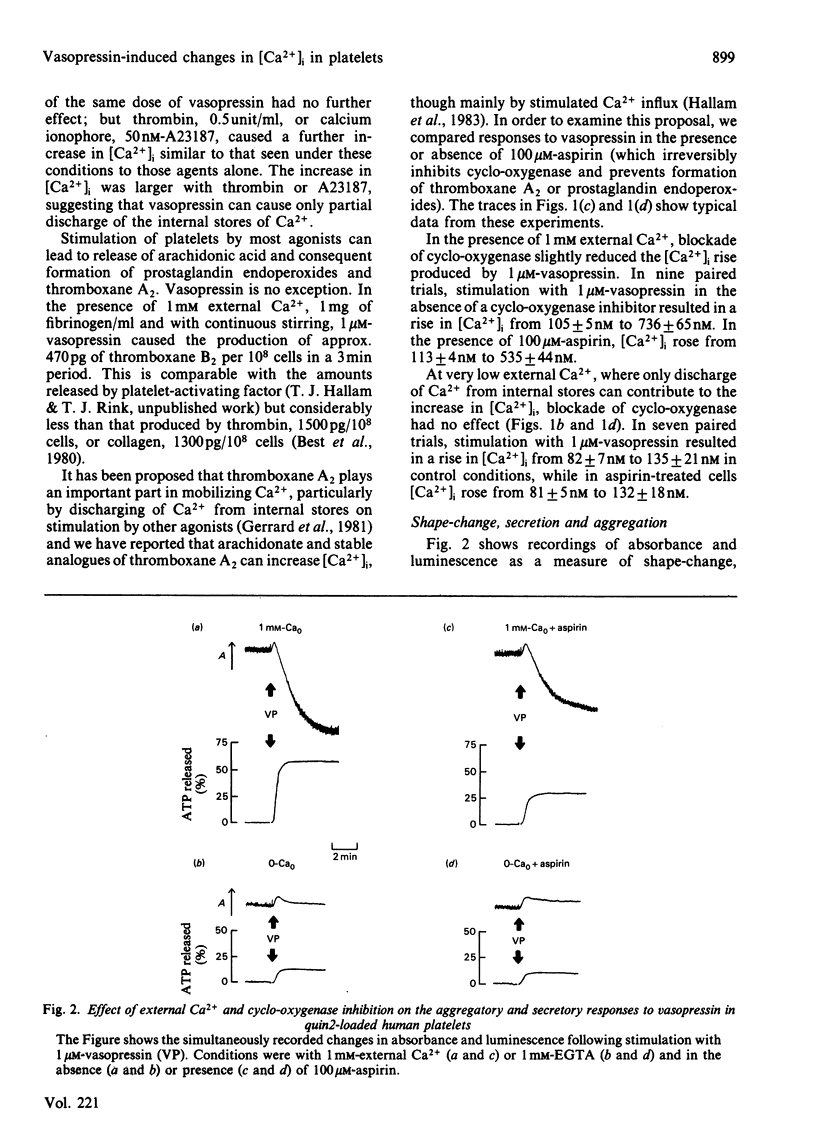


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best L. C., Holland T. K., Jones P. B., Russell R. G. The interrelationship between thromboxane biosynthesis, aggregation and 5-hydroxytryptamine secretion in human platelets in vitro. Thromb Haemost. 1980 Feb 29;43(1):38–40. [PubMed] [Google Scholar]
- Billah M. M., Michell R. H. Phosphatidylinositol metabolism in rat hepatocytes stimulated by glycogenolytic hormones. Effects of angiotensin, vasopressin, adrenaline, ionophore A23187 and calcium-ion deprivation. Biochem J. 1979 Sep 15;182(3):661–668. doi: 10.1042/bj1820661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Shukla S. D., Hanahan D. J., Olson M. S. Stimulation of hepatic glycogenolysis by acetylglyceryl ether phosphorylcholine. J Biol Chem. 1984 Feb 10;259(3):1468–1471. [PubMed] [Google Scholar]
- Damen F. J., Mier P. D. Cytochrome P-450-dependent O-dealkylase activity in mammalian skin. Br J Pharmacol. 1982 Jan;75(1):123–127. doi: 10.1111/j.1476-5381.1982.tb08764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Ruggles P. A., Scrutton M. C., Wallis R. B. Desensitisation in human and rabbit blood platelets. Thromb Haemost. 1982 Jun 28;47(3):278–284. [PubMed] [Google Scholar]
- Hallam T. J., Sanchez A., Rink T. J. Stimulus-response coupling in human platelets. Changes evoked by platelet-activating factor in cytoplasmic free calcium monitored with the fluorescent calcium indicator quin2. Biochem J. 1984 Mar 15;218(3):819–827. doi: 10.1042/bj2180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., Rosson G. M. Aggregation of human blood platelets by vasopressin. Am J Physiol. 1972 Oct;223(4):958–967. doi: 10.1152/ajplegacy.1972.223.4.958. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Baughan A. K. Fibrinogen binding to human platelet plasma membranes. Identification of two steps requiring divalent cations. J Biol Chem. 1983 Sep 10;258(17):10240–10246. [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Buxton D. B., Olson M. S., Hanahan D. J. Acetylglyceryl ether phosphorylcholine. A potent activator of hepatic phosphoinositide metabolism and glycogenolysis. J Biol Chem. 1983 Sep 10;258(17):10212–10214. [PubMed] [Google Scholar]
- Siess W., Siegel F. L., Lapetina E. G. Arachidonic acid stimulates the formation of 1,2-diacylglycerol and phosphatidic acid in human platelets. Degree of phospholipase C activation correlates with protein phosphorylation, platelet shape change, serotonin release, and aggregation. J Biol Chem. 1983 Sep 25;258(18):11236–11242. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Marks J. S., Coll K. E., Williamson J. R. Quantitation and early kinetics of inositol lipid changes induced by vasopressin in isolated and cultured hepatocytes. J Biol Chem. 1983 May 10;258(9):5716–5725. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]



