Abstract
Recent investigations have increased the interest on the connection between the microorganisms inhabiting the gut (gut microbiota) and human health. An imbalance of the intestinal bacteria representation (dysbiosis) could lead to different diseases, ranging from obesity and diabetes, to neurological disorders including Alzheimer’s disease (AD). The term “gut-brain axis” refers to a crosstalk between the brain and the gut involving multiple overlapping pathways, including the autonomic, neuroendocrine, and immune systems as well as bacterial metabolites and neuromodulatory molecules. Through this pathway, microbiota can influence the onset and progression of neuropathologies such as AD. This review discusses the possible interaction between the gut microbiome and AD, focusing on the role of gut microbiota in neuroinflammation, cerebrovascular degeneration and Aβ clearance.
Keywords: Gut microbiota, Alzheimer’s disease, Neuroinflammation, Blood-brain barrier, Beta-amyloid
Introduction
In recent years, there has been an increasing interest in the possible connection between the gut colonizing bacteria (gut microbiota) and human health. The gut microbiota is composed of more than 100 trillion bacteria in adults, nearly ten times as many as cells in the body, containing more than 1000 different species of bacteria (Bermon et al. 2015). The gut microbiota differs from one individual to another, conferring each one personal identity (Qin et al. 2010). Based on the data obtained by several studies, a healthy microbiota is the one that maintains community stability and species diversity, defining a good microbiota. Specifically, most abundant species (around 70–75%) are the anaerobic bacteria from the Firmicutes or Bacteroidetes phyla (Wang and Wang 2016) followed by Proteobacteria, Actinobacteria (such as Bifidobacterium), and Cyanobacteria (Qin et al. 2010). Interestingly, in the development of diseases ranging from obesity and diabetes to neurological disorders an imbalance in gut bacterial species, known as dysbiosis, has been observed (Sherwin et al.2018).
The numerous connections between the brain and the gut are known as the “gut-brain axis”. This crosstalk involves the participation of the autonomic, neuroendocrine, and immune systems together with metabolites and neuromodulatory molecules produced and released by gut bacteria (Quigley 2017). The gut microbiota has a central role in the regulation of the gut-brain axis, influencing not only gut but also central nervous system (CNS) activities. Indeed, several studies have demonstrated the direct connection between neuropsychiatric disorders and changes in the microbiome, microbiota-derived products and even exogenous antibiotics and probiotics. Data also indicate that microbiota, through the gut-brain axis, can influence brain plasticity and cognition, inducing the onset and progression of neuropathologies such as Alzheimer’s disease (AD).
AD is characterized by significant cognitive deficiencies, with amyloid-beta (Aβ) peptide aggregation and hyperphosphorylated tau tangles being the most prominent histopathological signs (Hyman et al. 2012; Montine et al. 2012; Menzies et al. 2015). Other hypotheses explaining AD pathogenesis include neuroinflammation, calcium signaling and energy metabolism imbalances, and vascular degeneration, but therapies based on these theories have failed in the past. This breakdown of therapeutic approaches based on current hypotheses has contributed to the need for a re-examination of AD pathogenesis from a new angle.
To date, the complexity of the impact of the gut microbiota in neuropathological disorders has not been understood (Bostanciklioğlu 2018). This review discusses the multiple ways the gut microbiome and AD may interact, focusing on the role of gut microbiota in neuroinflammation, cerebrovascular degeneration and Aβ clearance. Altogether, there seems to exist convincing evidence that modulation of the cross talk between gut and CNS through intestinal commensal microbiota control may be a potential treatment for neurodegenerative disorders such as AD (Bonfili et al. 2018).
Gut-Brain Axis and AD
The increasing knowledge obtained in the past years of the impact of the gut microbiota on the CNS has suggested the “brain-gut-microbiota axis” (Kowalski and Mulak 2019). The gut-brain axis is controlled bidirectionally. On one hand, the CNS modulating the enteric nervous system controls several aspects of the gut such us secretion, permeability, motility and immunity, and the efferent autonomic nervous pathways control muscle tissue and the mucus layer of the intestine (Carabotti et al. 2015). On the other hand, afferent signaling pathways and active molecules secreted by gut microbiota can alter brain functions (Burokas et al. 2015; Petra et al. 2015). Several studies have shown that changes in diet, the existence of pathogenic microorganisms, as well as the use of antibiotics and non-steroidal anti-inflammatory medications can induce the gut dysbiosis that can impact on brain cognitive function (Gareau 2014; Jiang et al. 2017). More specifically, there is convincing evidence for the interplay between AD and impaired gut microbial diversity, which is an incentive for suggesting a hypothesis on the pathogenesis of AD.
There are many processes that link gut microbiota function and AD. The gut microbiota has been shown to be essential for some brain processes like myelinization, neurogenesis and microglial activation and it has been closely related to behavioral, mood and cognitive modulation (Cenit et al. 2017).
Several groups have reported gut microbiome alterations in AD mouse models such as 5xFAD (Brandscheid et al. 2017) and APP/PS1 (Harach et al. 2017). In both models, gut microbiota dysbiosis has been described with an inverse correlation between the amount of Firmicutes and Bacteroidetes species, which means that when one is increased, the other is decreased. Very interestingly, it has been also demonstrated that APP/PS1 mice bred under sterile conditions show significantly lower levels of Aβ deposits compared to the same mice bred in non-sterile conditions (Harach et al. 2017). Similarly, when mice bred under sterile conditions are transplanted with normal condition-bred mice microbiota, a significant increase in brain Aβ deposits has been observed (Harach et al. 2017).
In a similar manner, altered microbiota diversity has been described in an amyloid transgenic Drosophila model (Wu et al. 2017). The impairment of the gut microbiota-induced neuroinflammation and cerebrovascular degeneration in this AD model suggests a connection between microbiota and brain damage.
AD patients show gut dysbiosis with significant changes in the composition of the intestinal microbiome when compared to healthy subjects (Vogt et al. 2017; Bostanciklioğlu 2019). Specifically, AD patients have decreased levels of bacteria in Actinobacteria (specifically, bacteria of the genus Bifidobacterium) and in the phylum Firmicutes. Noteworthy, decreased levels in Firmicutes have also been reported in the microbiome of individuals with type 2 diabetes (T2DM) (Larsen et al. 2010) as well as obesity and interestingly, diabetes and insulin resistance are defined risk factors for the developing of AD (Ott et al. 1999; de la Monte and Wands 2005; Rawlings et al. 2014).
In the same study, subjects with AD showed an increase in the phylum Bacteroidetes, reflected by increased Bacteroidaceae family and elevated Bacteroides at the genus level. The phylum Bacteroidetes encompasses a diverse and abundant group of gram-negative commensal bacteria in the gut (Rajilić-Stojanović M and de Vos 2014), including the genus Bacteroides, which has been detected at higher levels in the gut of individuals with T2DM (Larsen et al. 2010). The major outer membrane component of gram-negative bacteria is lipopolysaccharide (LPS), which is capable of triggering systemic inflammation and the release of pro-inflammatory cytokines after translocation from the gut to systemic circulation (Cani et al. 2007).
Additionally, compared to control subjects, AD patients showed reduced Actinobacteria. These differences were mostly driven by changes in Bifidobacterium. Actinobacteria, particularly the Bifidobacterium genus, are an important bacterial inhabitant of the human gut, and their beneficial health effects have been extensively described (O’Callaghan and van Sinderen 2016; Arboleya et al. 2016). Specifically, certain species of Bifidobacterium are associated with anti-inflammatory properties and decreased intestinal permeability (Underwood et al. 2015). Moreover, Bifidobacterium supplement has been shown to decrease LPS levels in the intestine and improve gut mucosal barrier properties in mice (Griffiths et al. 2004; Wang et al. 2006). Interestingly, in germ-free mice colonized with human gut microbiota, increased levels of Bifidobacterium are associated with decreased bacterial translocation to systemic circulation, while increased levels of Bacteroides have been shown to increase bacterial translocation (Rommond et al. 2008). Considering all these findings, increased Bacteroides and decreased Bifidobacterium in AD participants may represent a gut microbial phenotype with particular propensity for translocation of pro-inflammatory bacterial components.
Noteworthy, a tight correlation has been described between gut microbiome genera and AD biomarkers, i.e., Aβ42/Aβ40, p-tau and the Aβ/p-tau ratio in the cerebrospinal fluid (CSF) (Vogt et al. 2017). A significant association in AD subjects between CSF YKL-40 and abundance of Bacteroides, Turicibacter, and SMB53 (family Clostridiaceae) was observed. Moreover, marked variation in the composition of bowel bacteria has been also observed in AD subjects (Zhuang et al. 2018). However, the species that changed differ compared to the study performed by Vogt et al. (2017). Many factors could be behind those differences; ethnicity, comorbidities, dietary preferences and lifestyle are some of those factors (Tasnim et al. 2017).
The findings of these experiments indicate that variations in the intestinal microbiota may affect brain functions. Published data also provide signs of the impact of the gut microbiome on amyloid pathology, and suggest the possible function of the gut microbiome as one of the AD pathogenesis factors. In addition, the gut microbiota is extremely susceptible to lifestyle and aging as a complex modifiable system. Therefore, in the following sections we will discuss the impact of contemporary lifestyle and aging on the gut microbiota and the relationship to AD pathology.
Gut Microbiota Alterations Induced by AD Risk Factors
The most important factors that impact and modulate the gut microbiome are dietary changes (Pistollato et al. 2016). In addition, many of the factors that can modulate gut microbiota composition, such as aging, stress or obesity, are also considered risk factors for AD.
As already mentioned, gut microbiota composition can vary in different pathological conditions like depression (Wang et al. 2017) or neurodegenerative diseases (Brandscheid et al. 2017; Parashar and Udayabanu 2017). However, this issue raises an important question: is it the disease per se that changes the microbiota or are the disease risk factors the ones affecting the gut microbiome? Deleterious lifestyle conditions in our actual societies have been considered the significant risk factors for the onset and development of AD (van Praag 2018). The most remarkable feature of epidemiological studies is that significant rise of AD in developing countries is associated with dietary changes (Grant 2014). Furthermore, apart from the main AD risk factor, that is aging, in modern culture there are several unhealthy lifestyle conditions that lead to the development of AD. These factors include unhealthy diets and stress, and simultaneously, the gut microbiome is highly sensitive to these factors. From this point of view, the assessment of the purported connections between aging, modern lifestyle, gut microbiota and AD is a considerable issue that requires special attention.
Aging
As advanced age is a significant risk factor for AD, age-related changes in the gut microbiota could play a central role in the development of neuropsychopathologies. In this line, several studies have shown that gut microbiota composition suffers significant variations in the aging process (Salazar et al. 2017; Nagpal et al. 2018).
Several studies show that gut composition differs between old and young people. However, it is not possible to set a specific point where the microbiome gets altered as it occurs gradually over time (O’Toole and Jeffery 2015; Zapata and Quagliarello 2015).
Colonization of the gut may start in utero from the placenta. Most of infant microbiome is acquired during birth and follows with feeding and exposure to environment (Quigley 2017). The gut microbiota evolves rapidly the first three years and at the age of 3 years old, microbiota composition becomes similar to that of adults (Zapata and Quagliarello 2015; Quigley 2017). Childhood and adolescence are two critical periods for gut microbiota composition and neuronal development (Cenit et al. 2017) which get more diverse with dietary changes.
Aging is closely related with reduced microbiota diversity and this fact has been related to frailty while healthy aging is associated with a diverse microbiome (Cenit et al. 2017). Several authors have described that in elderly people the amount of Bifidobacterium and Lactobacillus is decreased (Gavini et al. 2001; Hopkins and Macfarlane 2002). These variations in the number and composition of the gut microbiota could be due to decreased bacterial adhesion to the intestinal wall related to the alterations in the structure of the colon mucous membrane (He et al. 2001). Moreover, in the aging process it has been observed that Bacteroides species diversity vary (Bartosch et al. 2004; Layton et al. 2006) and proteolytic bacteria (Fusobacteria, Propionibacteria, and Clostridia) increase, leading to putrefactive processes. It should be noticed that the impact of aging on the microbiome is intimately associated with diet and lifestyle, so it remains uncertain if aging per se, independent of external influences, is able to alter the microbiome (Quigley 2017). For instance, while bacterial cells do not age per se, people getting older start to take antibiotics, other drugs and begin to present comorbidities affecting the gut.
Stress
CNS stress levels may affect gut physiology and alter gut composition (Pistollato et al. 2016). Stress may provoke the release of hormones and neurotransmitters that could influence gut physiology and alter microbiome habitat, favoring the growth of certain strains (Cenit et al. 2017). Moreover, stress may cause huge changes in microbiota and intestinal physiology such us greater macromolecular permeability and increased secretory state. Stress has also been related to bacterial attachment and internalization in the epithelium: indeed, several studies have showed that the use of probiotics can reverse and normalize those stress-associated changes (Zareie et al. 2006).
Infection with Citrobacter rodentium, a non-invasive murine pathogen has been associated with chronic physical stress, supporting the hypothesis that stress can produce changes in microbiota. However, infection with Citrobacter rodentium alone was not able to produce changes in memory or cognition in wild type mice. When mice were exposed to stress, impairments in memory and cognition could be observed in infected mice but not in the uninfected. This impairment was related to lower levels of c-fos and BDNF and it was still present after clearance of the pathogen, thus indicating a long lasting effect. Furthermore, administration of probiotics one week prior to Citrobacter rodentium infection was able to prevent stress-associated changes (Gareau et al. 2011).
Early life stress in rodents such as that the produced by maternal separation (MS) has been related to changes in microbiota in early life including lower levels of Lactobacillus, Bacteroides and increased levels of Clostridium (Gareau et al. 2006, 2007; Wang and Wang 2016), and these modifications are still present even in adulthood (O’Mahony et al. 2009). These variations in the microbiota originating from stress have been related to increased severity and risk for colitis and infections with parasites like Nippostrongylus brasiliensis (Barreau et al. 2004, 2006). Moreover, these changes have been also observed in rhesus monkeys in which prenatal stress induced a decrease in levels of Bifidobacterium and Lactobacillus (Wang and Wang 2016).
Obesity
Obesity is a worldwide problem that has drastic long-term impacts on people's health (González-Muniesa et al. 2017). According to a large number of studies, obese people have major risk of developing neurodegenerative diseases (Profenno et al. 2010). Clinical and experimental data, for instance, suggest that high fat diets (HFD) and obesity are related to memory and learning deficiencies (Elias et al. 2005; Sabia et al. 2009), and presumably also brain atrophy (Enzinger et al. 2005). Furthermore, mounting data suggest that mid-life obesity raises the risk of suffering dementias such as AD later in life (Anstey et al. 2011).
Studies through the literature show positive associations between consuming healthy food and better cognitive performance and unhealthy food with worse performances even in short time interventions. A study performed in people in their sixties showed that an increase of unhealthy foods intake for four years was related to smaller hippocampus volumes (Jacka et al. 2015). On the other hand, another study showed that healthy food ingestion was associated with better cognitive performance (Wu et al. 2019). Vegetable and fruit ingestion has been also related to lower risk of age-related cognitive deficiencies (Wu et al. 2018). Moreover, higher intakes of fish were associated with greater cognitive performance (Okubo et al. 2017).
Some dietary patterns such as the Mediterranean diet have been related to lower cardiovascular risk and decreased age-related cognitive impairment (Samieri et al. 2013; Wu et al. 2019). However, dietary patterns characterized by higher intakes of meat, fried food and processed products and lower intakes of whole grains have been associated with greater pro-inflammatory status and decreased cognition (Shivappa et al. 2015; Leigh and Morris 2020). Diets high in saturated fats have also been related to decreased memory and cognition performances (Eskelinen et al. 2008). In this line, HFD or high in fat and sugar (HFHS) diets have been related with cognitive deficiency in adulthood and greater risk of dementia (Leigh and Morris 2020). This issue has been extensively studied in the literature showing that diet-induced obesity impairs cognition in healthy controls and worsens it in neurodegenerative models.
In view of the high number of obese subjects, there is an urgent need for a better comprehension of the pathophysiological processes involved in the process of obesity and their effects on cognitive performance. HFHS diet intake affects the composition of microbiota and may contribute to an imbalanced microbial environment in the intestine. Therefore, it has been recently suggested that the gut microbiome may be part of a mechanistic connection between consumption of HFD and other unbalanced diets and impaired cognition. The literature has showed us that short-term diet exposure is able to change microbiota composition in humans (David et al. 2014) and mice (Carmody et al. 2015) within days and that these changes may be relevant to early onset of cognitive impairment (Leigh and Morris 2020).
Indeed, several preclinical studies have demonstrated that HFD is able to induce gut microbiota alterations and induce the progression of dementia (Studzinski et al. 2009; Nam et al. 2017; Sah et al. 2017; Sanguinetti et al. 2018). Focusing specifically on AD, a study performed in 12 months old APP23 mice showed that HFD-induced gut microbiota changes were related to increased levels of Aβ (Nam et al. 2017).
In this line, a study performed in the 3xTg-AD AD mouse model showed that HFD and genetic predisposition to neurodegenerative diseases share similar abnormalities in the gut microbiome (Sanguinetti et al. 2018). The analysis of serum and fecal metabolites of those mice revealed a decrease in choline and unsaturated fatty acids, and elevated levels of lactate, amino acids, ketone bodies, trimethylamine (TMA), and trimethylamine N-oxide (TMAO) in HFD fed 3xTg-AD mice, which analogously are associated with impaired cognition (Janeiro et al. 2018).
In the same way, some studies have shown the benefits of using probiotics in combination with HFD, even in healthy subjects. Most of these studies have used Bifidobacteria or Lactobacillus strains for their research reporting restored cognition impairment (induced by HFD) and improvements in some cognitive tests like MWM or fear conditioning (Savignac et al. 2015; Chunchai et al. 2018; Ishikawa et al. 2019; Romo-Araiza et al. 2018).
Exercise and Sedentary Lifestyle
Intimately related to unhealthy nutrition, sedentary lifestyle is another important condition in obese individuals. Because of being associated with a variety of chronic health problems, sedentary lifestyle is emerging as an important public health issue in many countries (Owen et al. 2010). Mounting evidence shows that sedentary habits may be a risk factor for cognitive impairment (Wheeler et al. 2017), while regular exercise can be an useful method for dementia prevention (Fenesi et al. 2017).
Increasing data indicate that the gut microbiome can be influenced by exercise (Fernandez et al. 2018), and this effect is particularly noticeable in sedentary subjects and obese people (Bressa et al. 2017; Allen et al. 2018). Moreover, in a study performed in APP/PS1 mice (Abraham et al. 2019), the authors showed that physical activity induced an increase in butyrate-producing bacteria, and a reduction in pro-inflammatory and H2O2-generating bacteria. These changes in microbiota were accompanied by a reduction of Aβ levels in brain and a slowdown of progression of AD symptoms.
How Dysbiosis Could Contribute to AD?
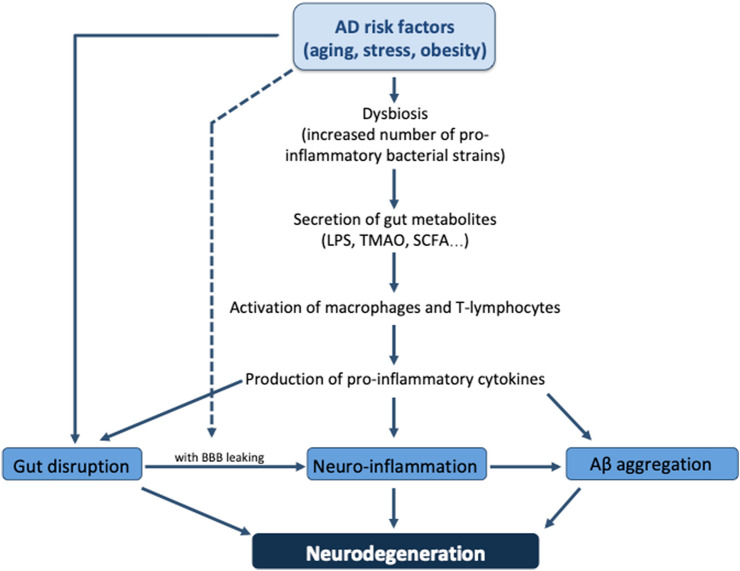
As mentioned before, AD is characterized by significant deficiencies in cognition, with Aβ peptide aggregation and hyperphosphorylated tau tangles being the most prominent histopathological signs. Several mechanisms triggered by dysbiosis may affect the gut-brain axis and could be involved in AD onset and progression, with inflammation, gut permeability and Aβ accumulation being the ones gaining major interest (Fig. 1).
Fig. 1.
Dysbiosis as a possible link between AD risk factors and neurodegeneration. Aging, stress and obesity can contribute per se to gut disruption, that in a situation with a pathological blood-brain barrier (BBB) leaking, could lead to neuroinflammation. Moreover, these factors can lead to a dysbiosis that could further cause a major neuroinflammation due to increased pro-inflammatory cytokine production and secretion of inflammatory gut metabolites like TMAO or LPS. Finally, a link between gut dysbiosis and brain Aβ accumulation has also been suggested
Gut Permeability
Gut microbiota dysbiosis induces a reduction in the expression of tight junction proteins in epithelial colon cells, inducing intestinal inflammation. The alteration of gut barrier function causes the release of microbial metabolites to the circulatory system, which promotes inflammation. Consequently, elevated amounts of circulating pro-inflammatory cytokines could enter the CNS in BBB leaking situations and induce neuroinflammation through activation of microglia (Erny et al. 2015) and astrocytes (Rothhammer et al. 2016).
It has been hypothesized that gut leaking could be the underlying mechanism linking obesity and AD. According to this hypothesis, in a study performed in mice under a HFD, a profound gut microbiota alteration was observed (Cani et al. 2008). Moreover the group treated with HFD showed elevated gut permeability, presumably due to the decreased expression of tight junction proteins, i.e. zonulin (ZO-1) and occluding (OCL), increasing the circulating levels of the bacterial LPS and inflammatory markers like TNF-α and IL-1. Interestingly, the administration of oral antibiotics normalized gut microbiota diversity and gut permeability by increasing the expression of both ZO-1 and OCL (Cani et al. 2008). In line with this idea, another study found that subjects with neurodegeneration had increased circulating LPS and monocyte activation levels compared to controls (Zhang et al. 2009). Since it is recognized that the BBB is disrupted in AD and this can result in anomalous microglial activation and brain inflammation, the combination of impaired gut permeability could also initiate pathogenic signals between the gut microbiota and the CNS. Indeed, microbial dysbiosis that alters the permeability of the gut can cause a systemic inflammatory state that can strengthen the usual neuro-inflammatory reactions observed in AD (Calsolaro and Edison 2016; Zhao et al. 2017a, b; Spielman et al. 2018).
Apart from the impact on gut permeability, it has been suggested that the intestinal microbiome could have a direct effect on AD biomarkers too (Spielman et al. 2018). Indeed, a recent study found a positive association between a pattern of around 50 microbial metabolites and the onset of AD and cognitive impairment (Xu and Wang 2016). Noteworthy, TMAO was among the most elevated microbial metabolites; TMAO is a molecule which has been linked to the consumption of animal fats, therefore supporting the idea that HFD can be considered as a risk factor for AD so they can induce gut microbial dysbiosis, which subsequently exerts several pro-inflammatory and pathogenetic functions.
Inflammation
According to a recent hypothesis, the composition of the gut microbiota is able to influence AD neuroinflammation. Specifically, it has been suggested that in AD pathology, the alteration of gut microbiota, altering the intestinal permeability, can induce an inflammatory condition not only in the gut, but also in the CNS, since the pro-inflammatory cytokines can get into the bloodstream and impact the brain (Kelly et al. 2015; Luca et al. 2015).
A recent study conducted in APP/PS1 mice found that the amount of circulating inflammatory cytokines released by gut bacteria was modified by a high-dose antibiotic cocktail (Minter et al. 2016). Furthermore, the authors assessed some of those cytokine levels in lymphocyte and splenic cells and concluded that there was no change in their levels, which reinforces the idea that the signal molecules generated by the gut microbiota influence brain homeostasis.
Similarly, Cattaneo et al. investigated the connection between gut bacteria and AD, demonstrating a connection between cognitive deficiencies, brain amyloid levels and the presence of inflammatory markers in the circulation (Cattaneo et al. 2017). According to this study, AD patients with cognitive impairment had greater circulating levels of pro-inflammatory cytokines, i.e., CXCL2, IL-6, IL-1β and NLRP3 and decreased levels of the anti-inflammatory cytokine IL-10. Interestingly, these cytokine level alterations correlated positively with Escherichia/Shigella amount. In consequence, they concluded that gut dysbiosis, with higher levels of pro-inflammatory (Escherichia/Shigella) and lower levels of anti-inflammatory (Eubacterium rectale) bacteria, may play a crucial role in AD cognitive alterations (Cattaneo et al. 2017).
An important issue is that many gut bacteria secrete LPS. LPS is the key component of gram-negative bacteria's outer cell membrane, which may induce neuro-inflammatory reactions in the case of penetration from the intestinal cavity into the bloodstream. Although in healthy conditions LPS is unable to access the brain, in situations in which BBB permeability is increased, such as neurodegenerative diseases, LPS could reach the CNS. In this line, post-mortem studies performed in AD patients have shown that LPS levels in the hippocampus and cortex are two to three times greater than in elderly people of the same age without cognitive alterations (Zhao et al. 2017a, b). Moreover, intraventricular administration of LPS in mice over 4 weeks may cause nerve cell death, chronic neuroinflammation and synaptic plasticity impairment of the neurons of the hippocampus (Hauss-Wegrzyniak and Wenk 2002).
Microglia, cells that are considered the brain resident macrophages, play a crucial role on brain inflammation. LPS released by bacteria can activate NFκB in human primary microglial cells (Zhao and Lukiw 2018), a pro-inflammatory transcription factor involved in the onset and development of AD. NFκB is able to induce transcription of pro-inflammatory miRNAs like miRNA-155, miRNA-146a, miRNA-125b, miRNA-34a and miRNA-9, inducing neuro-inflammatory factors and inhibiting phagocytosis (Zhao and Lukiw 2018). Indeed, it has been shown that micro-RNA-34a inhibits TREM2 expression, disrupting microglia phagocytic potential and increasing Aβ42 accumulation (Bhattacharjee et al. 2016).
Inflammatory mediators enter the CNS via the circulatory system as well as the lymphatic system linking the CNS and the digestive system (Louveau et al. 2015; Saksida et al. 2017). It has been shown that in the 5xFAD AD mouse model the number of T helper (Th) CD4+ cells is significantly increased in gut-associated lymphoid tissues (GALT) (Saksida et al. 2017). Besides this phenotypic characterization, the authors observed that the concentration of IL-17 per cell in transgenic mice was substantially lower. IL-17-expressing Th cells migrate to the CNS throughout the GALT; and this is essential for neurodegeneration because of their interactions with microglia that can contribute to the clearance of Aβ molecules and tau aggregation (Koutrolos et al. 2014; Wekerle 2016).
Having demonstrated the interaction between the gut microbiota and the inflammatory profile, it has been questioned whether the restoration of gut microbiota by the administration of probiotics could be an efficient strategy for AD treatment. This issue has been studied in different AD experimental models. For example, the administration of SLAB51, a probiotic formulation, to the 3xTg-AD mouse model over 16 weeks decreased pro-inflammatory (IL1a, IL1b, IL2, IL12, IFNɣ and TNFα) and elevated anti-inflammatory cytokine (IL4, IL6….) plasma levels (Bonfili et al. 2017). Other studies have also linked probiotics to a decrease in levels of pro-inflammatory cytokines such as IL-6, IL-1b and TNF-α (Rincón et al. 2014; Wang et al. 2015). They were also associated with increased levels of natural killer cells, activated lymphocytes, and phagocytosis (Pistollato et al. 2016). In addition, another study evaluated the effects of Fructooligosaccharide (FOS) on inflammation, Aβ accumulation and behavioral disorders, energy metabolism and the antioxidative system in Sprague-Dawley AD models (Chen et al. 2017). This work found that elevated rates of pro-inflammatory cytokines were restored with FOS therapy, promoting the development of Bifidobacteria and Lactobacilli, whose metabolites may increase plasma levels of anti-inflammatory cytokines (Bonfili et al. 2017). Moreover, the use of probiotics containing Lactobacillus has been shown to ameliorate anxiety and decrease memory defects related to Western diets (Gareau 2014).
Abeta Acumulation
The most important hallmarks of AD pathogenesis are tau aggregations and Aβ. Several studies have suggested a plausible link between gut microbiota and brain Aβ accumulation.
In a previously mentioned study performed by Minter et al. (2016) (see Sect. 4.1.), the authors evaluated the effects of the altered gut microbiome on Aβ accumulation and found that APP/PS1 mice treated with an antibiotics cocktail exhibit reduced Aβ load and plaque size, while the soluble Aβ peptide appeared elevated, which suggest insoluble Aβ plaque degradation increased.
The gut microbiome, which is mainly located in the colon and the ileum, is able to produce short-chain fatty acids (SCFAs) which are biologically active and may cross the BBB, by fermenting fibrous foods. Several SCFAs have been studied and it has been observed that they trigger the pathogenesis of AD by altering the aggregations of tau and Aβ (Cummings et al. 1987; Macfarlane and Macfarlane 2012). In an in vitro study (Ho et al. 2018) the authors found that butyric acid, propionic acid and valeric acid were able to inhibit Aβ40 oligomerization, valeric acid showing the strongest inhibitory effect. In addition, the study determined that only valeric acid administration completely inhibited Aβ42 oligomer formation; afterwards, the authors discovered that both butyric acid and valeric acid were able to inhibit the conversion of Aβ40 monomers to Aβ fibrils. In conclusion, this study demonstrated that reduction of the amount of the anti-inflammatory bacteria and the subsequent decrease in the levels of secreted beneficial metabolites might enhance Aβ accumulation in the brain. Apart from the in vitro study, an in vivo study (Kobayashi et al. 2017) found that behavioral and working memory deficits in an AD model induced by intracerebral Aβ25-35 injection were ameliorated by acetate. SCFA-induced changes in Aβ accumulation could be due to the fact that valeric acid and isovaleric acid, isobutyric acid and butyric acid, propionic acid, acetic acid and formic acid have been found to disturb the activation of microglia. The gut microbiota can control microglial maturation and activation and thus in cases having impaired gut microbiota, microglia maturation and phagocytosis capacity for tau and Aβ decreases (Erny et al. 2015). Martins and Binosha Fernando (2014) have claimed that butyric acid can normalize the excessive histone acetylation by inhibiting histone deacetylase in microglia; butyric acid thereby may increase the number of microglia, which leads to a decrease in aberrant levels of inflammation and could increase Aβ phagocytosis.
Moreover, Aβ42 levels in mice increase with the intraperitoneal administration of LPS (Kahn et al. 2012), that could be due to the disruption of Aβ transport through the BBB, raising its influx and reducing efflux (Jaeger et al. 2009). Furthermore, in vitro studies showed that endotoxins secreted by Escherichia coli strains accelerate fibril formation and Aβ aggregation (Asti and Gioglio 2014). Finally, it has also been shown that intraventricular administration of LPS in combination with ascorbic acid increases the intraneuronal Aβ (Hauss-Wegrzyniak and Wenk 2002).
Although these studies show compelling evidence, further studies are necessary to demonstrate the gut microbiota metabolites’ effects on Aβ accumulation, brain inflammation and vascular degeneration, so that the multiple ways intestinal metabolites and AD interact can be fully understood.
Conclusion
The present review highlights the hypothesis wherein the gut microbiome may affect the pathogenesis of AD. Emerging evidence shows that there is a relationship between the biology of AD risk factors, such as aging or obesity, and the pathophysiology of AD. Risk factors can induce microbiota dysbiosis and raise pro-inflammatory bacteria levels over anti-inflammatory bacteria levels. At that point, bacterial metabolites promote gut leaking, inducing systemic and CSN inflammation, which results in Aβ accumulation and subsequent neurodegeneration (Fig. 1). There is emerging evidence in the literature showing the effect that global microbiome composition or targeted strains can exert on cognition. Based in this idea, it is tempting to speculate that restoring gut microbiota with probiotics or by fecal microbiota transplantation could be a promising therapeutic strategy for neurodegenerative diseases. Indeed, numerous studies suggest that a healthy microbiota helps to maintain brain homeostasis by lowering inflammation in the CNS, vascular pathology and the aggregations of misfolded proteins. Nevertheless, more human studies are required to check if the benefits observed in animals are also applicable in humans and demonstrate if these beneficial effects are due to changes in the microbiome or the action that probiotics exert as dietary supplements.
Acknowledgements
MH Janeiro is a recipient of a fellowship from Ministerio de Ciencia, Innovación y Universidades (FPU).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham D, Feher J, Scuderi GL, Szabo D, Dobolyi A, Cservenak M et al (2019) Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp Gerontol 115:122–131 [DOI] [PubMed] [Google Scholar]
- Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA et al (2018) Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 50(4):747–757 [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M, Young J (2011) Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 12(5):e426-37 [DOI] [PubMed] [Google Scholar]
- Arboleya S, Watkins C, Stanton C, Ross RP (2016) Gut bifidobacteria populations in human health and aging. Front Microbiol 7:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asti A, Gioglio L (2014) Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimer’s Dis 39(1):169–79 [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L (2004) Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 53(4):501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F, de Lahitte JD, Ferrier L, Frexinos J, Bueno L, Fioramonti J, (2006) Neonatal maternal deprivation promotes Nippostrongylus brasiliensis infection in adult rats. Brain Behav Immun 20(3):254–60 [DOI] [PubMed] [Google Scholar]
- Bartosch S, Fite A, Macfarlane GT, McMurdo MET (2004) Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 70(6):3575–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermon S, Petriz B, Kajeniene A, Prestes J, Castell L, Franco OL (2015) The microbiota: An exercise immunology perspective. Exerc Immunol Rev 21:70–9 [PubMed] [Google Scholar]
- Bhattacharjee S, Zhao Y, Dua P, Rogaev EI, Lukiw WJ (2016) MicroRNA-34α-mediated down-regulation of the microglial-enriched triggering receptor and phagocytosis-sensor TREM2 in age-related macular degeneration. PLoS One 11(3):e0150211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C et al (2017) Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 7(1):2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Cuccioloni M, Angeletti M, Berardi S, Scarpona S et al (2018) SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol Neurobiol 55(10):7987–8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanciklioğlu M (2018) Intestinal bacterial flora and Alzheimer’s disease. Neurophysiology 50:140–148 [Google Scholar]
- Bostanciklioğlu M (2019) The role of gut microbiota in pathogenesis of Alzheimer’s disease. J Applied Microbiol 127(4):954–967 [DOI] [PubMed] [Google Scholar]
- Brandscheid C, Schuck F, Reinhardt S, Schäfer KH, Pietrzik CU, Grimm M et al (2017) Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. J Alzheimer’s Dis 56(2):775–788 [DOI] [PubMed] [Google Scholar]
- Bressa C, Bailén-Andrino M, Pérez-Santiago J, González-Soltero R, Pérez M, Montalvo-Lominchar MG et al (2017) Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One 12(2):e0171352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A, Moloney RD, Dinan TG, Cryan JF (2015) Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol 91:1–62 [DOI] [PubMed] [Google Scholar]
- Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement 12(6):719–32 [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772 [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM et al (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57(6):1470–81 [DOI] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209 [PMC free article] [PubMed] [Google Scholar]
- Carmody RN, Gerber GK, Luevano JM, Gatti DM, Somes L, Svenson KL et al (2015) Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 17(1):72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C et al (2017) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68 [DOI] [PubMed] [Google Scholar]
- Cenit MC, Sanz Y, Codoñer-Franch P (2017) Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol 23(30):5486–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Yang X, Yang J, Lai G, Yong T, Tang X et al (2017) Prebiotic effect of Fructooligosaccharides from Morinda officinalis on Alzheimer’s disease in rodent models by targeting the microbiota-gut-brain axis. Front Aging Neurosci 9:403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunchai T, Thunapong W, Yasom S et al (2018) Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation 15(1):11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch HWJ, Naylor CPE, MacFarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28(10):1221–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484):559–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR (2005) Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis 7:45–61 [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB (2005) Obesity, diabetes and cognitive deficit: The Framingham heart study. Neurobiol Aging 26(Suppl 1):11–6 [DOI] [PubMed] [Google Scholar]
- Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S et al (2005) Risk factors for progression of brain atrophy in aging: Six-year follow-up of normal subjects. Neurology 64(10):1704–11 [DOI] [PubMed] [Google Scholar]
- Erny D, De Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen MH, Ngandu T, Helkala E-L, Tuomilehto J, Nissinen A, Soininen H et al (2008) Fat intake at midlife and cognitive impairment later in life: A population-based CAIDE study. Int J of Geriatr Psychiatry 23(7):741–7 [DOI] [PubMed] [Google Scholar]
- Fenesi B, Fang H, Kovacevic A, Oremus M, Raina P, Heisz JJ (2017) Physical exercise moderates the relationship of apolipoprotein E (APOE) genotype and dementia risk: a population-based study. J Alzheimer’s Dis 56(1):297–303 [DOI] [PubMed] [Google Scholar]
- Fernandez DM, Clemente JC, Giannarelli C (2018) Physical activity, immune system, and the microbiome in cardiovascular disease. Front in Physiol 9:763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG (2014) (2014) Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol. 817:357–71 [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Yang PC, Macqueen G, Perdue MH (2006) Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res 59(1):83–8 [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH (2007) Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56(11):1522–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ et al (2011) Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60(3):307–17 [DOI] [PubMed] [Google Scholar]
- Gavini F, Cayuela C, Antoine JM, Lecoq C, Lefebvre B, Membré JM et al (2001) Differences in the distribution of bifidobacterial and enterobacterial species in human faecal microflora of three different (children, adults, elderly) age groups. Microb Ecol Health Dis 13(1):40–45 [Google Scholar]
- González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF et al (2017) Obesity. Nat Rev Dis Prim 3:17034 [DOI] [PubMed] [Google Scholar]
- Grant WB (2014) Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimer’s Dis 38(3):611–20 [DOI] [PubMed] [Google Scholar]
- Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leavens A et al (2004) In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci 49:579–589 [DOI] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G et al (2017) Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7:41802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Wenk GL (2002) Beta-amyloid deposition in the brains of rats chronically infused with thiorphan or lipopolysaccharide: The role of ascorbic acid in the vehicle. Neurosci Lett 322(2):75–8 [DOI] [PubMed] [Google Scholar]
- He F, Ouwehand AC, Isolauri E, Hosoda M, Benno Y, Salminen S (2001) Differences in composition and mucosal adhesion of bifidobacteria isolated from healthy adults and healthy seniors. Curr Microbiol 43(5):351–4 [DOI] [PubMed] [Google Scholar]
- Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM (2018) Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 18(1):83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins MJ, Macfarlane GT (2002) Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol 51(5):448–454 [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R, Fukushima H, Nakakita Y, Kado H, Kida S (2019) Dietary heat-killed Lactobacillus brevis SBC8803 (SBL88TM) improves hippocampus-dependent memory performance and adult hippocampal neurogenesis. Neuropsychopharmacol Rep 39(2):140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, Cherbuin N, Anstey KJ, Sachdev P, Butterworth P (2015) Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Med 13:215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA et al (2009) Lipopolysaccharide alters the blood-brain barrier transport of amyloid β protein: A mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun 23(4):507–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M (2018) Implication of trimethylamine N-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 10(10):1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Li G, Huang P, Liu Z, Zhao B (2017) The gut microbiota and Alzheimer’s disease. J Alzheimer’s Dis 58(1):1–15 [DOI] [PubMed] [Google Scholar]
- Kahn MS, Kranjac D, Alonzo CA, Haase JH, Cedillos RO, McLinden KA et al (2012) Prolonged elevation in hippocampal Aβ and cognitive deficits following repeated endotoxin exposure in the mouse. Behav Brain Res 229(1):176–84 [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP (2015) Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front in Cell Neurosci 9:392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A et al (2017) Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep 7(1):13510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutrolos M, Berer K, Kawakami N, Wekerle H, Krishnamoorthy G (2014) Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol Commun 2:163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski K, Mulak A (2019) Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil 25(1):48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK et al (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G (2006) Development of Bacteroides 16S rRNA gene taqman-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol 72(6):4214–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh SJ, Morris MJ (2020) Diet, inflammation and the gut microbiome: Mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta Mol Basis Dis 1866(6):165767 [DOI] [PubMed]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca M, Luca A, Calandra C (2015) The role of oxidative damage in the pathogenesis and progression of Alzheimer’s disease and vascular dementia. Oxid Med Cell Longev 2015:504678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GT, Macfarlane S (2012) Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 95(1):50–60 [DOI] [PubMed] [Google Scholar]
- Martins IJ, Binosha Fernando WMAD (2014) High fibre diets and Alzheimer’s disease. Food Nutr Sci 5:15 [Google Scholar]
- Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16(6):345–57 [DOI] [PubMed] [Google Scholar]
- Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P et al (2016) Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep 6:30028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2012) National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 123(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K et al (2018) Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging 4(4):267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KN, Mounier A, Wolfe CM, Fitz NF, Carter AY, Castranio EL et al (2017) Effect of high fat diet on phenotype, brain transcriptome and lipidome in Alzheimer’s model mice. Sci Rep 7(1):4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan A, van Sinderen D (2016) Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 7:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A-M, Quigley EMM et al (2009) Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiat 65(3):263–7 [DOI] [PubMed] [Google Scholar]
- O’Toole PW, Jeffery IB (2015) Gut microbiota and aging. Science 350(6265):1214–5 [DOI] [PubMed] [Google Scholar]
- Okubo H, Inagaki H, Gondo Y, Kamide K, Ikebe K, Masui Y et al (2017) Association between dietary patterns and cognitive function among 70-year-old Japanese elderly: A cross-sectional analysis of the SONIC study. Nutr J 16(1):56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 53:1937–1942 [DOI] [PubMed] [Google Scholar]
- Owen N, Sparling PB, Healy GN, Dunstan DW, Matthews CE (2010) Sedentary behavior: Emerging evidence for a new health risk. Mayo Clin Proc 85(12):1138–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar A, Udayabanu M (2017) Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat Disord 38:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC (2015) Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin ther 37(5):984–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F, Cano SS, Elio I, Vergara MM, Giampieri F, Battino M (2016) Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev 74(10):624–34 [DOI] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV (2010) Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatr 67(6):505–12 [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley EMM (2017) Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep 17(12). [DOI] [PubMed]
- Rajilić-Stojanović M, de Vos WM (2014) The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38:996–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D et al (2014) Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 161:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón D, Vaquero J, Hernando A et al (2014) Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int 34(10):1504–1512 [DOI] [PubMed] [Google Scholar]
- Romo-Araiza A, Gutiérrez-Salmeán G, Galván EJ et al (2018) Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci 10:416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond MB, Colavizza M, Mullié C, Kalach N, Kremp O, Mielcarek C et al (2008) Does the intestinal bifidobacterial colonisation affect bacterial translocation? Anaerobe 14:43–48 [DOI] [PubMed] [Google Scholar]
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L et al (2016) Type i interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22(6):586–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A (2009) Body mass index over the adult life course and cognition in late midlife: the Whitehall II cohort study. Am J Clin Nutr 89(2):601–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah SK, Lee C, Jang JH, Park GH (2017) Effect of high-fat diet on cognitive impairment in triple-transgenic mice model of Alzheimer’s disease. Biochem Biophys Res Commun 493(1):731–736 [DOI] [PubMed] [Google Scholar]
- Saksida T, Koprivica I, Vujičić M, Stošić-Grujičić S, Perović M, Kanazir S et al (2017) Impaired IL-17 production in gut-residing immune cells of 5xFAD mice with Alzheimer’s disease pathology. J Alzheimer’s Dis 61(2):619–630 [DOI] [PubMed] [Google Scholar]
- Salazar C, Valdés-Varela L, González S, Gueimondede los Reyes-Gavilán MCG (2017) Nutrition and the gut microbiome in the elderly. Gut Microbes 8(2):82–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samieri C, Okereke OI, Devore E, Grodstein F (2013) Long-term adherence to the mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr 143(4):493–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti E, Collado MC, Marrachelli VG, Monleon D, Selma-Royo M, Pardo-Tendero MM et al (2018) Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci Rep 8(1):4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF (2015) Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 287:59–72 [DOI] [PubMed] [Google Scholar]
- Sherwin E, Dinan TG, Cryan JF (2018) Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci 1420(1):5–25 [DOI] [PubMed] [Google Scholar]
- Shivappa N, Hébert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E et al (2015) Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr 113(4):665–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman LJ, Gibson DL, Klegeris A (2018) Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int 120:149–163 [DOI] [PubMed] [Google Scholar]
- Studzinski CM, Li F, Bruce-Keller AJ, Fernandez-Kim SO, Zhang L, Weidner AM et al (2009) Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP x PS1 knock-in mice. J Neurochem 108(4):860–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasnim N, Abulizi N, Pither J, Hart MM, Gibson DL (2017) Linking the gut microbial ecosystem with the environment: Does gut health depend on where we live? Front Microbiol 8:1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MA, German JB, Lebrilla CB, Mills DA (2015) Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res 77:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H (2018) Lifestyle factors and Alzheimer’s disease. Brain Plast 4(1):1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC et al (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7(1):13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Wang YP (2016) Gut microbiota-brain axis. Chin Med J (Engl) 129(19):2373–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z (2006) The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma 61:650–657 [DOI] [PubMed] [Google Scholar]
- Wang IK, Wu YY, Yang YF et al (2015) The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef Microbes 6(4):423–430 [DOI] [PubMed] [Google Scholar]
- Wang S, Huang XF, Zhang P, Newell KA, Wang H, Zheng K et al (2017) Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci Rep 7(1):12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H (2016) The gut-brain connection: Triggering of brain autoimmune disease by commensal gut bacteria. Rheumatol 55(suppl 2):ii68–ii75 [DOI] [PubMed] [Google Scholar]
- Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ et al (2017) Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement 3(3):291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Cao ZS, Chang KM, Juang JL (2017) Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat Commun 8(1):24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Fisher-Hoch SP, Reininger BM, McCormick JB (2018) Association between fruit and vegetable intake and symptoms of mental health conditions in mexican americans. Heal Psychol 37(11):1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Song X, Chen GC, Neelakantan N, Van Dam RM, Feng L et al (2019) Dietary pattern in midlife and cognitive impairment in late life: a prospective study in Chinese adults. Am J Clin Nutr 110(4):912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Wang QQ (2016) Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol 10(Suppl 3):63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata HJ, Quagliarello VJ (2015) The microbiota and microbiome in aging: Potential implications in health and age-related diseases. J Am Geriatr Soc 63(4):776–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM et al (2006) Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55(11):1553–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M et al (2009) Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol 206(1–2):121–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Lukiw WJ (2018) Bacteroidetes Neurotoxins and Inflammatory Neurodegeneration. Mol Neurobiol 55(12):9100–9107 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol 7:318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cong L, Jaber V, Lukiw WJ (2017) Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Front Immunol 8:1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L et al (2018) Gut Microbiota is altered in patients with Alzheimer’s disease. J Alzheimer’s Dis 63(4):1337–1346 [DOI] [PubMed] [Google Scholar]