Abstract
Patients on mechanical circulatory support are prone to infections, increasing morbidity and mortality. These circulatory support devices generate high mechanical shear stress (HMSS) that can causes trauma to blood. When leukocytes become damaged, their immune response function may be impaired or weakened, leading to increased infection vulnerability. This study examined neutrophil structural and functional alterations after exposure to 75, 125, and 175 Pa HMSS for 1 sec. Human blood was exposed to three levels of HMSS using a blood-shearing device. Neutrophil morphological alteration was characterized by examining blood smears. Flow cytometry assays were used to analyze expression levels of CD62L and CD162 receptors, activation level (CD11b), and aggregation (platelet-neutrophil aggregates). Neutrophil phagocytosis and rolling were examined via functional assays. The results show neutrophil structure (morphology and surface receptors) and function (activation, aggregation, phagocytosis, rolling) were significantly altered after HMSS exposure. These alterations include cell membrane damage, loss of surface receptors (CD62L and CD162), initiation of activation and aggregation, upregulation of phagocytic ability and increased rolling speed. The alterations were the most severe after 175 Pa exposure. HMSS caused damage and activation of neutrophils, potentially impairing normal neutrophil function, leading to weakened immune defense and increasing a patient’s vulnerability to infections.
Keywords: Neutrophil dysfunction, High mechanical shear stress, Ventricular Assist Devices, mechanical circulatory support
1. Introduction
Patients suffering from cardiac failure are placed on mechanically assisted circulation that helps provide hemodynamic support. Some examples of these mechanical circulatory support devices include left ventricular assisted devices (LVADs), extracorporeal membrane oxygenation (ECMO), and cardiopulmonary bypass. Patients on these treatments often experience myriad adverse events, including bleeding, thrombosis, and infection. Infection rates in LVAD patients range from 30% to 50%, making infections one of the most common complications and causes of death.1 Infections have been shown to have a five to six-fold increase in the 1-year mortality rate for patients on LVADs.2 Infections are also associated with increased morbidity or adverse events such as thrombosis, bleeding, hospital stay length, device replacement, and transplant failure.3 Infections can present as a local infection involving the driveline or pump pocket or as a systemic infection spread hematogenously to or from the device.4
LVADs and other blood-contacting pumps used in ECMO and cardiopulmonary bypass produce non-physiological high mechanical shear stress (HMSS) above 100Pa.5,6 Blood exposed to HMSS undergoes trauma that manifests as morphological alterations, shortened lifespan, biochemical changes, and complete destruction.7 The effects of HMSS on red blood cells (RBC) and platelets have been well studied.8,9 Research regarding leukocytes, on the other hand, has been widely overlooked. The few in-vitro studies that have been published show leukocyte morphological changes, reduced lifecycle, diminished cell immunity, surface receptor loss, activation, and cell fragmentation after exposure to HMSS.7,10–12 These leukocyte alterations have also been reported in vivo in clinical studies of LVAD patients. Reduced leukocyte count, compromised cellular immunity, increased leukocyte-platelet aggregates, cellular fragmentations, microparticle generation, and leukocyte activation were reported.13–18 These leukocyte structure and function alterations can impair or weaken the patient’s immune defense, making them more vulnerable to infections.
There are five subsets of leukocytes, with the most abundant being neutrophils. Neutrophils play a crucial role in immune defense. Neutrophils are recruited to sites of infections where they undergo various mechanisms, including phagocytosis, to kill bacterial and fungal pathogens. Information regarding neutrophil alterations after HMSS exposure is limited. This study aimed to quantify the effects of HMSS exposure that is relevant to circulatory support devices on neutrophils. Neutrophil structure (morphology, surface receptors) and function (activation, aggregation, phagocytosis, rolling) were examined after HMSS exposure at 75, 125, and 175 Pa for 1 sec.
2. Materials and Methods
2.1. Blood-shearing device
A customized centrifugal flow-through Couette device was used as the blood-shearing device (Fig. 1). This device used the CentriMag magnetic levitation and motor driving system (Abbott, Pleasanton, CA, USA). The parameters and setup of this device have been explained in a previous publication.11 Briefly, the device rotates from 500 to 5000 rpm and has a small gap (165 μm) between the rotor side walls and the housing. Inside this small gap, the shear stress is nearly uniform and larger than elsewhere in the device. The shear stress level is determined by the rotating speed and diameter of the rotor, blood viscosity, and gap size. Three HMSS levels representative of those observed within clinical centrifugal blood pumps were selected for this study (75, 125, and 175 Pa). The chosen exposure time was 1 second. The blood was pushed through the shearing device using a syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA, USA). The axial flow rate of 2.624 mL/min was used to achieve an exposure time of 1 second.
Figure 1:
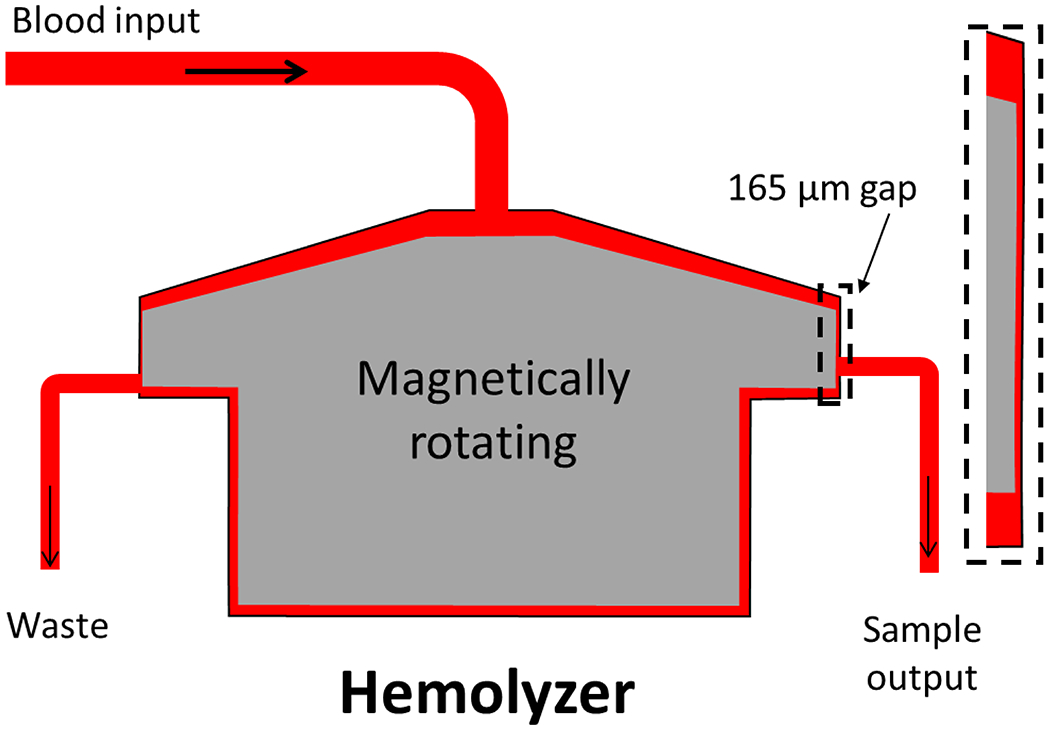
Overview of experimental set up using a Couette-type blood-shearing device (Hemolyzer).
2.2. Experimental Procedure
Ten Healthy human donors were recruited to participate in this study; all donors were informed about the study’s goal, and their written informed consent was given. One unit (450 mL) of blood was drawn from the antecubital vein into a collection bag. The bag was sterile and contained 3.2% sodium citrate (50 mL, 9:1 ratio). Blood viscosity was measured using the semi-micro viscometer (Cannon Instrument Company, State College, PA, USA). The rotating speed of the device was then calculated based on the viscosity of each HMSS level. Blood samples (n=10) exposed to the three levels of HMSS for 1 second in the narrow gap of the blood shearing device were collected for the analyses described below. The University of Maryland, Baltimore Institutional Review Board approved the protocol of obtaining blood from healthy donors for the study.
2.3. Blood Smears
After the samples were collected, 5 mL of blood was centrifuged at 200 g for 10 mins at RT. The plasma was discarded. The buffy coat was separated from the red blood cells. The smears were prepared from the buffy coat. The cells were stained using Shandon Kwik-Diff Stains (Fisher Scientific, Hampton, NH). Blood smears were placed in the fixative for 30 seconds and then allowed to dry. Next, they were placed in eosin for 15 sec followed by 5 sec in methylene blue. The smears were then imaged under the 100x bright field of a research microscope (IX71, Olympus). Images were used to examine neutrophil morphological alterations based on cell membrane integrity. Cells that did not have a continuous membrane, exposed cytoplasm, or those protruding cell membranes were considered damaged.
2.4. Flow Cytometry Assays
Neutrophil activation, aggregation, and receptor shedding were measured using flow cytometry assays. V421-labeled anti-human CD66b (clone G10F5, IgMκ), FITC-labeled anti-human CD62L (L-selectin, Clone DREG56, IgG1κ), PE-labeled anti-human activated CD11b (MAC-1, clone CBRM1/5, IgG1κ), and APC-labeled anti-human CD41a (clone HIP8, IgG1κ) were all obtained from BD Biosciences (San Jose, CA, USA). PerCP-labeled CD45 (clone 2D1, IgG1κ) and APC-labeled anti-human CD162 (clone KPL-1, IgG1κ) were acquired from BioLegend (San Diego, CA, USA). Blood samples (50 μL) were stained with 0.2 μL of CD45-PerCP, 2 μL of CD66b-V421, 5 μL of CD62L-FITC, 2.5 μL of CD11b-PE, and 2.5 μL of CD41a-APC, or 1 μL of CD162-APC. Samples were incubated in the dark for 30 mins. Blood samples were also stained with co-responded isotypes and incubated. Samples were fixed with 1% paraformaldehyde (1 mL) for 30 mins, followed by centrifugation at 250 g/min for 5 mins. Red blood cells were lysed with BD lysing buffer (1.5 mL) for 15 mins at RT, followed by centrifugation at 1500 rpm/min for 5 mins. Cells were washed with FC-PBS (PBS, 0.5%-1% BSA, 0.1% NaN3 sodium azide) and resuspended in 300 μL of PBS. Flow cytometry data for these prepared samples were acquired on the BD LSRII (BD Bioscience, San Jose, CA, USA) and Amnis FlowSight (Luminex, Austin, TX, USA) flow cytometers. Leukocytes were gated based on CD45 expression versus side scatter (SSC). Neutrophils were identified as CD66b+ cells.
2.5. Phagocytosis Assay
A commercial phagocytosis assay kit (Cayman Chemical, Ann Arbor, MI, USA) was used. The kit uses fluorescently labeled rabbit IgG-coated latex beads as the phagocytic target. The beads coated with DyLight 633 IgG were added (0.5 μL) to whole blood (50 μL). The samples were incubated at 37°C for 1 hour, and the negative controls were placed at 4°C. Phagocytosis was stopped after incubation by placing them on ice for 5 minutes. The neutrophils were then labeled with 5 μL of PE anti-CD66b Ab (clone G10F5, IgMκ, BD Biosciences) for 30 mins on ice. RBCs were lysed with BD lysing buffer for 10 mins and then washed with cold FC-PBS. After centrifugation, the samples were resuspended in 200 μL of PBS. Samples were then analyzed on the BD Calibur Flow cytometer (BD Bioscience). Based on the size, forward scatter (FSC) versus side scatter (SSC) was used for initial gating. Subsequently, neutrophils were identified as CD66b+ cells, and over 10,000 CD66b+ events were recorded. The amount of DyLight 633 IgG+ events among CD66+ cells was used to indicate neutrophil phagocytosis capacity.
2.6. Neutrophil Rolling on P-selectin
Microfluidic channels were prepared using master templates that were fabricated using standard negative photolithography processes. The channels were 1000 μm x 100 μm (width x height). Microchannels were fabricated using polydimethylsiloxane (PDMS; Sylgard® 184 Silicone Elastomer, Electron Microscopy Sciences, Hatfield, PA). The PDMS prepolymer and crosslinker were mixed (10:1 weight), degassed, and poured over the photoresist templates. The PDMS solution was cured at 70°C for two hours. After curing the PDMS stamps were cut out and reservoirs were punched out by using a 2mm biopsy punch. The PDMS stamps were sealed over glass coverslips following plasma treatment for 30 seconds using a Plasma cleaner (Harrick Plasma Inc, Ithaca, NY).
Recombinant human P-selectin/CD62P Fc Chimera Protein, CF (R&D Systems, Minneapolis, MN) was coated onto the microchannel surface. P-selectin (25 μL) at 10 μg/mL was injected into the microchannel and were incubated at 4°C overnight to allow protein adhesion. Microchannels were then blocked using 1% BSA solution for 1 hr at RT. Following blocking they were washed with PBS.
The flow rate of 17.15 μL/min in the microchannel was calculated using the height and width of the microchannel, blood viscosity, and the selected wall shear stress (WSS) of 6 dyn/cm2. This WSS was selected because it is within the range of WSS observed in post capillary venules where leukocytes normally undergo rolling and adhesion.19,20 The microchannel was placed over the observation window of the fluorescent microscope (IX71, Olympus) and connected to a syringe pump (PHD2000, Harvard Apparatus, Holliston, MA, USA). Blood samples were stained (200:1 volume ratio) with acridine orange for 2-5 mins. The samples were then perfused through the protein coated microchannels at a controlled flow rate for 5 mins in the dark. Leukocyte rolling was recorded with the fluorescent microscope equipped with an Olympus DP80 digital camera. One-minute videos were taken using the Olympus cellSens software (Olympus, Tokyo, Japan). The videos were analyzed for rolling velocity using TackMate on Fiji (NIH, Bethesda, MD).
2.7. Data Analysis
FlowJo V10.7.2 software (BD Biosciences) was used to analyze flow cytometry data measured on LSRII and FACSCaliber flow cytometers. IDEAS software (Luminex) was used to analyze data measured on the Amnis Flowsight flow cytometer. The data is presented as mean ± SE (standard error of the mean), and the sample size was ten unless otherwise stated. Prism (GraphPad Software Inc La Jolla, CA) was used for one-way ANOVA statistical analysis; a P value ≤ 0.05 is considered statistically significant.
3. Results
3.1. Morphological Alternation
The HMSS at the levels in this study caused morphological changes in neutrophils. Alterations such as loss of cell membrane integrity, fragmentations, and complete rupture of cells are examples. Neutrophil morphological alterations after HMSS exposure were characterized by examining the blood smears that were evaluated under a light microscope. All leukocytes contained in the buffy coat were stained using the differential stain. Neutrophils were identified due to their multi-lobed nucleus and their pale red cytoplasm. Other leukocytes have different nuclear structures and cytoplasm coloring. Neutrophils have a circular morphology at the base with a distinct multi-lobed nucleus. At 75 Pa, the morphology is still circular, but vacuoles were seen in the cell cytoplasm. At 125 Pa, even more, vacuoles were present. Finally, at 175 Pa, the neutrophils lost their membrane integrity, and the circular morphology was lost; vacuoles could also be seen. Representative images are shown in Fig. 2A. The percentage of damaged cells based on cell membrane integrity is demonstrated in Fig. 2B. The percentage of damaged cells significantly increased with higher HMSS; after 175 Pa exposure, 60% of the cells displayed some morphological damage.
Figure 2:
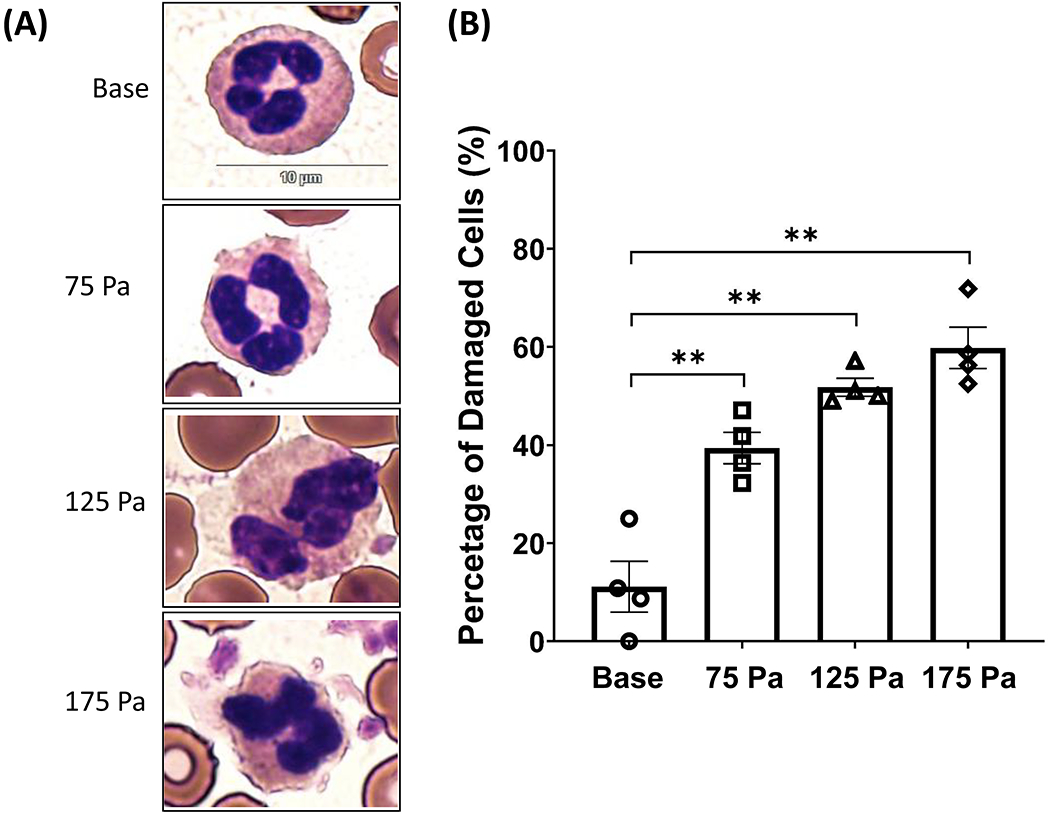
(A) Representative images of neutrophil morphology before and after shear stress exposure of 75, 125, and 175 Pa for 1 sec. (B) The percentage of cell damaged was analyzed based on membrane integrity (mean ± SE), n=4. **P< 0.05.
3.2. Receptor Loss
Neutrophils migrate to the sites of infection through a controlled cascade process. The initial step of this migration cascade is to capture and roll neutrophils on endothelial cells. This step is governed by selectins such as CD62L (L-selectin) and CD162 (P-selectin glycoprotein ligand-1). Circulating neutrophils constitutively express these receptors. The expression levels of these receptors were measured before and after HMSS exposure (75, 125, and 175 Pa for 1 second). Since the fluorescent intensity of the flow cytometry data is proportional to fluorophores conjugated to the antibody bound to available copies of a receptor on a cell, the mean fluorescent intensity (MFI) of the channel for CD62L or CD162 was used to represent the relative quantity of the receptors of CD62L and CD162 on the neutrophil surface, respectively. Figure 3A shows the MFI for CD62L surface expression levels in the blood samples at the base and three HMSS conditions (75, 125, and 175Pa). The MFI for CD62L surface expression was 5134 (arbitrary unit - AU) at the base and decreased to 4416, 4121, and 3862 for 75, 125, and 175 Pa samples. The surface expression of CD62L on neutrophils significantly decreased with increasing HMSS. The most drastic change can be seen at 175Pa, where the L-selectin decreased by 25% compared to the base. The decrease in the MFI for CD162 receptor expression can also be seen in Fig. 3B. The surface expression of CD162 on neutrophils decreased with increasing HMSS as well. The MFI for CD162 surface expression after 175 Pa exposure was significantly lower when compared to that at the base. The MFI for CD162 expression decreased from 11302 (AU) to 10522, 10216, and 9576 for 75, 125, and 175 Pa samples, respectively.
Figure 3:

(A) Surface expression of CD62L on CD66b+ neutrophils before and after shear stress exposure for 1 second at 75, 125, and 175 Pa. (B) Surface expression of CD162 on CD66b+ neutrophils before and after shear stress exposure of 75, 125, and 175 Pa for 1 sec. **P< 0.05.
3.3. Activation and Aggregation
When neutrophils are activated, they begin the process of extravasation. During extravasation, integrins such as MAC-1 (CD11b/CD18) are upregulated due to their part in assisting neutrophils to sites of inflammation. MAC-1 expression was used as a marker for activation by measuring CD11b surface expression on neutrophils. The gating strategy for identifying neutrophils and CD11b positive neutrophils on flow cytometry data is shown in Fig. 4A. The expression level of CD11b was significantly upregulated after HMSS exposure. All the sheared samples had substantially higher CD11b expression compared to that at the base. The MFI for CD11b expression increased from 266 (AU) to 514, 1262, and 2137 for 75, 125, and 175 Pa samples (Fig. 4B), with the 175 Pa sample having the most drastic increase. Representative images after HMSS exposure in bright-field and fluorescent CD11b+ signal obtained with Amnis imagining flow cytometry (20x) are shown in Fig. 4C. Platelet-neutrophil aggregates are also a marker for neutrophil activation because activated neutrophils can induce the aggregation. Platelet-neutrophil aggregates were examined before and after exposure to HMSS at 75, 125, and 175 Pa for 1 second. The CD66b-CD41a aggregates were significantly increased after exposure to HMSS, with the 175 Pa sample having the most drastic increase (Fig 5A). The percentage of platelet-neutrophil aggregates (CD41a+ and CD66b+) increased from 4.6% to 21%, 56%, and 76% for 75, 125, and 175 Pa samples, respectively. Representative merged fluorescent images of neutrophil-platelet aggregates (both CD66b+ and CD41+) after HMSS exposure (75, 125, and 175 Pa) obtained with Amnis imagining flow cytometry (20x) are shown in Fig. 5B.
Figure 4:
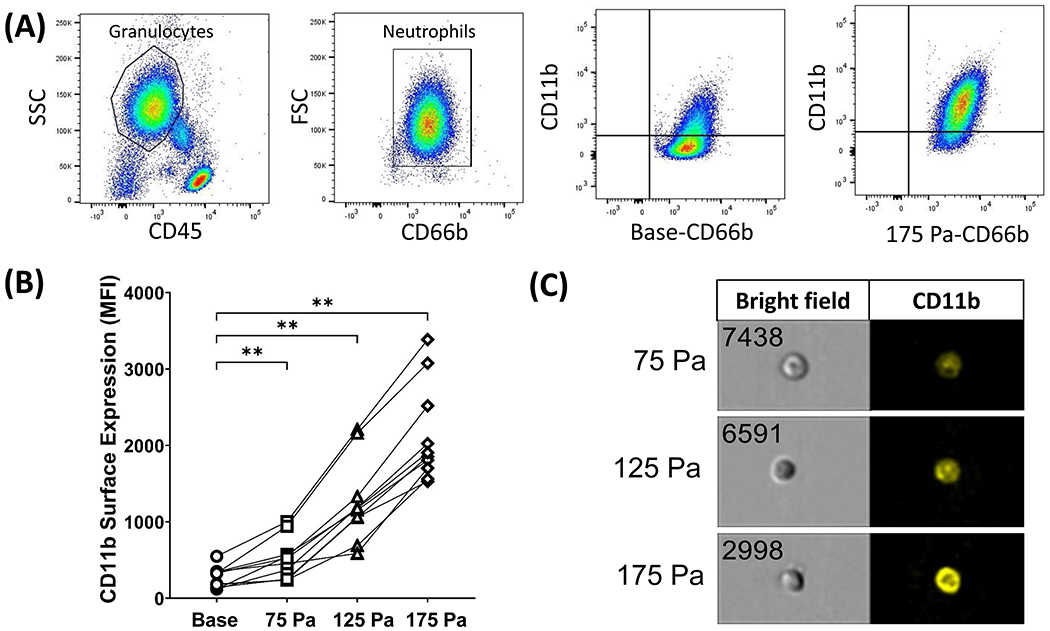
(A) Flow cytometry gating strategy for CD11b expression on CD66b+ neutrophils and representative plots from one experiment for base and 175 Pa samples. (B) Surface expression of CD11b on CD66b+ neutrophils before and after shear stress exposure of 75, 125, and 175 Pa for 1 sec. ** P< 0.05. (C) Representative FlowSight intensity images showing bright field and fluorescent CD11b+ cell images (20X) from Amnis image flow cytometry.
Figure 5:

(A) Percentage of CD66b+ neutrophils that expressed CD41a (platelet marker) before and after shear stress exposure of 75, 125, and 175 Pa for 1 sec. ** P< 0.05. (B) Representative neutrophil-platelet aggregate images for 75, 125, and 175 Pa samples showing bright field and fluorescent CD66b+ and CD41a+ cell images (20X) from Amnis image flow cytometry.
3.4. Phagocytosis
One of the neutrophils’ primary functions is phagocytosis, which removes bacterial and fungal pathogens. This ability was probed by measuring the percentage of neutrophils that engulfed rabbit IgG-coated latex beads before and after HMSS (75, 125, 175 Pa) exposure. Figure 6A shows dot pots of the flow cytometry data gating strategy and representative plots for the base and 175 Pa samples. Neutrophil phagocytic ability increased after HMSS exposure, Fig. 5B. The percentage of Neutrophils that underwent phagocytosis increased significantly after HMSS exposure at 125 and 175 Pa. The percentage of neutrophils that experienced phagocytosis rose from 8.3% to 14%, 18%, and 19% for 75, 125, and 175 Pa samples, respectively.
Figure 6:
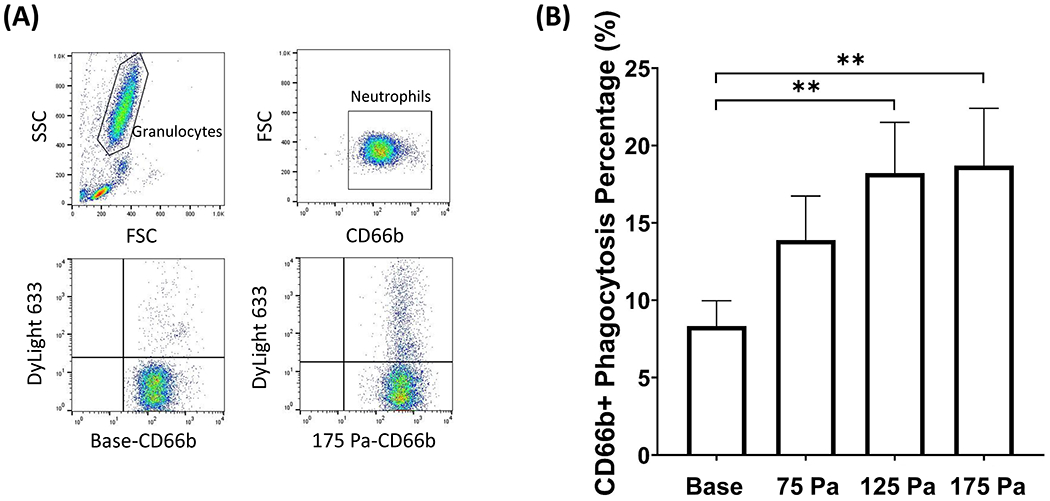
(A) Flow cytometry gating strategy for phagocytosis of DyLight 633 labeled latex beads on CD66b+ neutrophils and representative plots from one experiment for base and 175 samples. (B) Percentage of CD66b+ neutrophil that underwent phagocytosis before and after shear stress exposure of 75, 125, and 175 Pa for 1 sec (mean ± SE). **P < 0.05.
3.5. Rolling on P-selectin
In order for neutrophils to get to sites of inflammation or infection, they must first undergo the extravasation process. The first step in this process is called rolling, in which neutrophils roll on endothelial surfaces by quickly binding and unbinding to P-selectin. To determine the impact of HMSS levels on rolling, the rolling speed before and after HMSS exposure was measured. Figure 7A shows images of a representative cell track at various timepoints. The leukocyte rolling speed was tracked after exposure to HMSS, Fig. 7B. The baseline rolling speed was 13.1 μm/s. This increased to 14.7 μm/s, 18.3 μm/s, and 25.8 μm/s after 75 125, and 175 Pa exposure, respectively. However, the increase in the rolling speed became significant for 175 Pa shear exposure.
Figure 7:
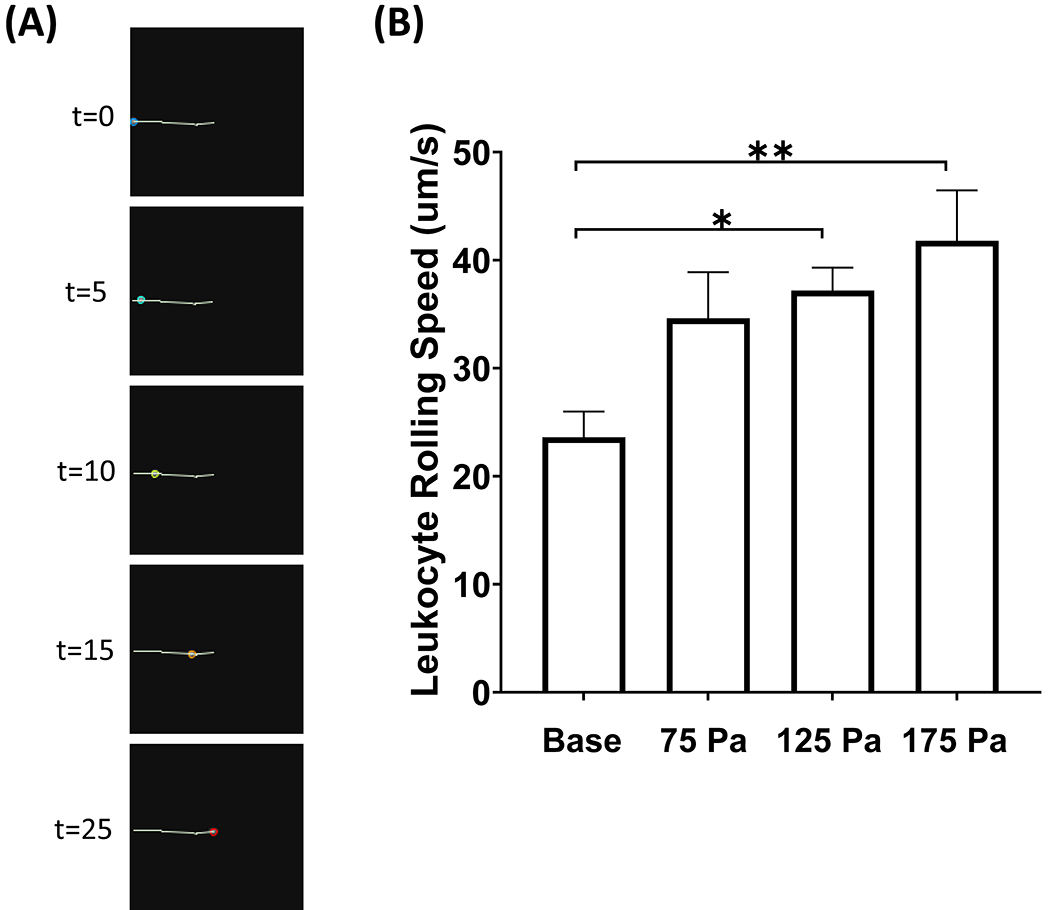
(A) Representative video frames of a leukocyte rolling track at various timepoints. (B) Leukocyte rolling speed (μm/s) on P-selectin before and after shear stress exposure of 75, 125, and 175 Pa for 1 sec (mean ± SE). **P < 0.05.
4. Discussion
This study aimed to examine the effects of HMSS with 1 second exposure on neutrophil structure and function. The results show that neutrophil structure in terms of morphology and surface receptors and functional status in activation, aggregation, and phagocytosis were all altered after exposure to HMSS. Neutrophils lost their cell membrane integrity and began to fragment, surface receptors (CD62L and CD162) got downregulated, activation and aggregation were initiated, and phagocytic ability was upregulated after HMSS exposure. The changes in structure and function of neutrophils were the most drastic after exposure to the highest level of HMSS. These findings align with other published studies that showed the effects of HMSS on neutrophils. Implanted blood contacting devices that produce HMSS within the levels tested in this study can impact the patient’s immune response due to leukocyte structural and functional alterations.
Several previous studies reported morphological changes in neutrophils after exposure to low shear stresses.21–24 One common structural change is pseudopod retraction, which is usually observed at low shear stresses. Shive et al. and Moazzam et al. reported pseudopod retraction at shear levels above ~0.5 Pa.21,22 Cytoplasmic and nuclear condensations were also seen, and a reduction in cell area and F-actin concentration with increasing shear stress.21 Yet, cell membranes were intact. In this study, cell membrane integrity lost with increasing HMSS. The dramatic differences in morphological changes can be attributed to the different shear stress levels. Shear stresses above 10 Pa begin to cause major trauma to leukocytes leading to fragmentation and complete destruction.23 Dewitz et al. documented morphological alterations to leukocytes after exposure to 0-100 Pa shear stress for minutes. At shear stresses above 60 Pa, cell destruction was evident. Cell membranes were damaged, the nucleus was condensed, and cytoplasmic vacuoles were seen.24 The most profound morphological changes in our study were seen at 175 Pa; however, a significant percentage of cells were damaged at all HMSS levels. In the Dewitz study, the cells are constantly exposed to the shear stress for 10 mins, but in this study, the exposure time is 1 sec. In circulatory support devices, cells are exposed to shear stresses for a short time (> 1 sec).
The other type of structural changes that was examined in our study was the surface receptors on neutrophils. CD62L is one of the selectins that tethers to inflamed endothelial cells to initiate cell rolling. Once neutrophils firmly adhere, L-selectin is cleaved from the cell surface. L-selectin shedding is a marker for cell activation; this happens concurrently with CD11b upregulation. However, leukocyte rolling and migration are impaired when L-selectin is inhibited, as shown in the L-selectin-deficient mouse model.25 In the present study, L-selectin shedding is seen at all HMSS levels. The most significant L-selectin loss on neutrophils is seen at 175 Pa, with a 25% loss compared to that in the unsheared blood. The expression of CD162 (PSGL-1) decreased with increasing HMSS level, with a significant decrease after 175 Pa exposure. L-selectin and PSGL-1 are two of the most important receptors for neutrophil rolling and adhesion due to their interaction with E- and P-selectin on the endothelial cell surface. The binding and unbinding between these receptors allow neutrophils to roll and eventually adhere to the endothelial surface. The loss of these receptors after shear stress exposure can impact neutrophil rolling capacity due to the lack of availability for these receptors to form bonds. This decreases the likelihood of neutrophil firm adhesion and the ability of cells to transmigrate to sites of inflammation. This may increase a patient’s susceptibility to infections, similar to patients that have Leukocyte adhesion deficiency (LAD).
CD11b expression was upregulated with increasing HMSS level; there is a drastic increase after 175 Pa exposure. This pattern has been previously reported at a shorter exposure time; however, this effect is more pronounced at our exposure time of 1 second.11 The upsurge of platelet-neutrophil aggregates can also be used as a marker for neutrophil activation. However, leukocyte-platelet aggregates have been seen in patients with sepsis and multiorgan failure and should be evaluated further.26 MAC-1 (CD11b/CD18) can promote phagocytosis of complement opsonized bodies through binding to iC3b.27 Excessive stimulation of neutrophils can cause neutrophil exhaustion.28 Platelet-neutrophil interactions have also been shown to enhance neutrophil phagocytic capabilities.29 This is consistent with the increased neutrophil phagocytic capacity that was seen in our study, with increasing HMSS, in this study. Overall, it is seen that HMSS exposure causes a multitude of effects that have a severe impact on immune cells.
5. Study Limitation
We recognize that the present study has limitations. First, the experiments were performed using the blood from healthy donors, not patients with heart failure (HF). Blood from the healthy donors might be less susceptible to HMSS than patients with HF. The results may not represent real clinical scenarios for HF patients on MAC support. Secondly, the in-vitro setting does not consider the short-life span of neutrophils occurring in the human circulation. Another limitation is that we only examined a limited numbers of neutrophil functional alterations in this study. There might be other neutrophil alterations involved after exposed to HMSS. We will attempt to address these limitations in our future studies.
6. Conclusion
This study showed that HMSS with short exposure time (1 second) could induce structural and functional alternations in neutrophils. Neutrophil alterations include cell membrane damage and fragmentation, loss of surface receptors (CD62L and CD162), initiation of activation and aggregation, upregulation of phagocytic ability and increased rolling speed. This study provides new findings on the effects of circulatory support devices on neutrophils that can offer insight into a patient’s vulnerability to infections.
Acknowledgments
The study was partially supported by the National Institutes of Health (Award Numbers: R01HL118372, R01HL124170, R01HL141817, and R01HL162940).
Footnotes
Conflict of Interest:
Katherin Arias: none declared.
Wenji Sun: none declared.
Dong Han: none declared.
Bartley P Griffith: none declared.
Zhongjun J Wu: none declared.
References:
- 1.Jezovnik MK, Gregoric ID, Poredos P. Medical complications in patients with LVAD devices. European Society of Cardiology 2017;14:37. [Google Scholar]
- 2.Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections. Circulation 2013;127:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilic A. The future of left ventricular assist devices. Journal of thoracic disease 2015;7:2188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esquer Garrigos Z, Castillo Almeida NE, Gurram P, et al. Management and Outcome of Left Ventricular Assist Device Infections in Patients Undergoing Cardiac Transplantation. Open forum infectious diseases 2020;7:ofaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Jena SK, Giridharan GA, et al. Flow features and device-induced blood trauma in CF-VADs under a pulsatile blood flow condition: A CFD comparative study. International journal for numerical methods in biomedical engineering 2018;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. Journal of biomechanical engineering 2012;134:081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Sun A, Wang H, Fan Y, Deng X. Non-physiological shear stress-induced blood damage in ventricular assist device. Medicine in Novel Technology and Devices 2019;3:100024. [Google Scholar]
- 8.Papanastasiou CA, Kyriakoulis KG, Theochari CA, Kokkinidis DG, Karamitsos TD, Palaiodimos L. Comprehensive review of hemolysis in ventricular assist devices. World Journal of Cardiology 2020;12:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaeske K, Dieterlen MT, Eifert S, et al. Device-induced platelet dysfunction in patients after left ventricular assist device implantation. J Thromb Haemost 2021; 19:1331–1341. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch M, Boval B, Damy T, et al. Importance of monocyte deactivation in determining early outcome after ventricular assist device implantation. Int J Artif Organs 2012;35:169–76. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, Wang S, Zhang J, Arias K, Griffith BP, Wu ZJ. Neutrophil injury and function alterations induced by high mechanical shear stress with short exposure time. Artif Organs 2021;45:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun W, Zhang J, Shah A, et al. Neutrophil dysfunction due to continuous mechanical shear exposure in mechanically assisted circulation in vitro. Artif Organs 2022;46:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X, Larsen B, Rutledge J, et al. The profile of the systemic inflammatory response in children undergoing ventricular assist device support. Interact Cardiovasc Thorac Surg 2012;15:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimball PM, Flattery M, McDougan F, Kasirajan V. Cellular immunity impaired among patients on left ventricular assist device for 6 months. Ann Thorac Surg 2008;85:1656–61. [DOI] [PubMed] [Google Scholar]
- 15.Radovancevic R, Matijevic N, Bracey AW, et al. Increased leukocyte-platelet interactions during circulatory support with left ventricular assist devices. ASAIO J 2009;55:459–64. [DOI] [PubMed] [Google Scholar]
- 16.Diehl P, Aleker M, Helbing T, et al. Enhanced microparticles in ventricular assist device patients predict platelet, leukocyte and endothelial cell activation. Interact Cardiovasc Thorac Surg 2010;11:133–7. [DOI] [PubMed] [Google Scholar]
- 17.Mondal NK, Sorensen E, Hiivala N, Feller E, Griffith B, Wu ZJ. Oxidative stress, DNA damage and repair in heart failure patients after implantation of continuous flow left ventricular assist devices. International journal of medical sciences 2013;10:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondal NK, Sorensen EN, Pham SM, et al. Systemic Inflammatory Response Syndrome in End-Stage Heart Failure Patients Following Continuous-Flow Left Ventricular Assist Device Implantation: Differences in Plasma Redox Status and Leukocyte Activation. Artif Organs 2016;40:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsiaris AG, Tachmitzi SV, Batis N, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 2007;44:375–86. [PubMed] [Google Scholar]
- 20.Weirather J, Frantz S. Chapter 2 - Role of the Innate Immune System in Ischemic Heart Failure. In: Blankesteijn WM, Altara R, eds. Inflammation in Heart Failure. Boston: Academic Press; 2015:19–38. [Google Scholar]
- 21.Shive MS, Salloum ML, Anderson JM. Shear stress-induced apoptosis of adherent neutrophils: A mechanism for persistence of cardiovascular device infections. Proc Natl Acad Sci 2000;97:6710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moazzam F, DeLano FA, Zweifach BW, Schmid-Schonbein GW. The leukocyte response to fluid shear stress. Proc Natl Acad Sci 1997;94:5338–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radley G, Ali S, Pieper IL, Thornton CA. Mechanical shear stress and leukocyte phenotype and function: Implications for ventricular assist device development and use. International Journal of Artificial Organs 2019;42:133–42. [DOI] [PubMed] [Google Scholar]
- 24.Dewitz TS, McIntire LV, Martin RR, Sybers HD. Enzyme release and morphological changes in leukocytes induced by mechanical trauma. Blood Cells 1979;5:499–512. [PubMed] [Google Scholar]
- 25.Arbonés ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1994;1:247–60. [DOI] [PubMed] [Google Scholar]
- 26.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest 1995;25:843–51. [DOI] [PubMed] [Google Scholar]
- 27.MacPherson M, Lek HS, Morrison V, Fagerholm S (2013) Leukocyte Beta2-Integrins; Genes and Disease. J Genet Syndr Gene Ther 2013;4:154. [Google Scholar]
- 28.Hong C-W. Current Understanding in Neutrophil Differentiation and Heterogeneity. Immune Network 2017;17(5):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res 2018;371(3):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]


