Abstract
Background
Bruton tyrosine kinase (BTK) inhibitors are used to treat B-cell hematologic malignancies. Ibrutinib has been associated with hepatitis B virus (HBV) reactivation. We sought to identify patients with hematologic malignancies who developed HBV reactivation after receiving first-generation (ibrutinib) or second-generation (acalabrutinib and zanubrutinib) BTK inhibitors.
Methods
We retrospectively studied all consecutive patients with hematologic malignancies with past HBV infection (HBV surface antigen [HBsAg] negative and hepatitis B core antibody [anti-HBc] positive) or chronic HBV infection (HBsAg positive and anti-HBc positive) treated with BTK inhibitors at our institution from November 1, 2015, through November 1, 2022.
Results
Of 82 patients initially identified, 53 were excluded (11 because of false-positive anti-HBc results, and 42 because they were receiving anti-HBV prophylaxis owing to recent receipt of anti-CD20 monoclonal antibodies). The 29 remaining patients were further analyzed and 3 (10%; 2 of 28 with past and 1 of 1 with chronic HBV infection) were found to have HBV reactivation. One patient received ibrutinib, and 2 received acalabrutinib. All developed HBV-associated hepatitis requiring anti-HBV therapy and survived. One patient continued receiving acalarutinib. Among the patients with past HBV infection, 13 received ibrutinib and 1 (8%) had HBV reactivation; 14 received acalabrutinib and 1 (7%) had HBV reactivation (P = 1.0).
Conclusions
HBV reactivation risk is intermediate in patients with past HBV infection who receive BTK inhibitors. For patients with past HBV infection who received BTK inhibitors, data are insufficient to recommend universal anti-HBV prophylaxis, but monitoring for HBV reactivation is warranted.
Keywords: ibrutinib, acalabrutinib, zanubrutinib, hepatitis flare, viral hepatitis
Microabstract
Bruton tyrosine kinase (BTK) inhibitors are used to treat B-cell hematologic malignancies and may cause hepatitis B virus (HBV) reactivation. We retrospectively analyzed the case of HBV reactivation in infected patients receiving BTK inhibitors. Reactivation occurred in 3 of 29 patients, including 2 of 28 with past HBV infection, suggesting that monitoring for reactivation is warranted in this scenario.
Introduction
Ibrutinib, acalabrutinib, and zanubrutinib are small molecular inhibitors of Bruton tyrosine kinase (BTK) indicated for the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL) and Waldenstrom macroglobulinemia (WM).
Hepatitis B virus (HBV) reactivation has been reported sporadically among patients treated with ibrutinib, acalabrutinib, and zanubrutinib.1-3 Previous retrospective studies focused on the risk of HBV reactivation in patients with CLL or other hematologic malignancies receiving ibrutinib4,5. However, the frequency and risk of HBV reactivation in patients with hematologic malignancies treated with different types of BTK inhibitors remain unclear. In this study, we sought to identify patients with hematologic malignancies who developed HBV reactivation after receiving first-generation (ibrutinib) or second-generation (acalabrutinib, and zanubrutinib) BTK inhibitors.
Methods
Study patients
In this retrospective study, we search electronic medical records to identify patients with past or chronic HBV infection and hematologic malignancies (CLL, MCL, MZL, and WM) treated with BTK inhibitors at The University of Texas MD Anderson Cancer Center during the period from November 1, 2015, through November 1, 2022. At our institution, patients with hematologic malignancies are routinely screened for HBV with tests for hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc). Serum samples were tested for HBV DNA using the COBAS HBV assay (Roche Molecular Systems, Branchburg, NJ), which has a quantification range of 10 to 1,000,000,000 IU/mL (1.00 to 9.00 log IU/mL). The study was approved by the Institutional Review Board.
Patients were excluded if (i) they had false-positive anti-HBc results or (ii) they were receiving anti-HBV therapy because they had received anti-CD20 monoclonal antibodies (MoAbs) within the previous 12 months. Patients with remote use of anti-HBV therapy or anti-CD20 MoAbs were included in this study.
For patients included in the study, data were assessed regarding demographics, cancer stage, cancer therapy, HBV characteristics, HBV treatment, and HBV-associated adverse outcomes (HBV-related hepatitis flare, liver failure, or death). HBV reactivation rates by BTK inhibitors were calculated in patients with past HBV infection; the rates were calculated in patients with past HBV infection because patients with chronic HBV infection are expected to be on antiviral therapy per US guidelines.6
Definitions
Past HBV infection was defined as negative HBsAg and positive anti-HBc test results. Chronic HBV infection was defined as positive HBsAg and positive anti-HBc test results.6,7 Liver biochemical tests (alanine transaminase, aspartate transaminase, alkaline phosphatase, and bilirubin), HBV serology, and HBV DNA were checked every 3 months as recommended by US guidelines.6,7
Positive anti-HBc test results were considered false positive if (i) the patient tested negative for anti-HBc and HBsAg before intravenous immune globin infusion and (ii) positive for anti-HBc after intravenous immune globulin infusion, and/or (iii) the patient tested negative for anti-HBc at repeat testing.5
In patients with past HBV infection, HBV reactivation was defined as one of the following: (i) HBV DNA became detectable or (ii) reverse HBsAg seroconversion occurred (reappearance of HBsAg). In patients with chronic HBV infection, HBV reactivation was defined as one of the following: (i) ≥2 log (100-fold) increase in HBV DNA compared to the baseline level, (ii) HBV DNA ≥3 log (1,000) IU/mL in a patient with previously undetectable HBV DNA, or (iii) HBV DNA ≥4 log (10,000) IU/mL if the baseline level was not available.6,7
A hepatitis flare was defined as an increase in the alanine transaminase level to ≥3 times the baseline level and >100 U/L. HBV-associated hepatitis was defined as the presence of HBV reactivation with hepatitis flare. HBV-associated liver failure was defined as one of the following: (i) impaired synthetic function (total bilirubin level >3 mg/dL or international normalized ratio >1.5), (ii) ascites, (iii) encephalopathy, or (iv) death following HBV-associated liver failure due to HBV reactivation.6,7
Our follow-up period was defined as the time from initiation of BTK inhibitors until January 31, 2023 (end of data collection), even for patients no longer on BTK inhibitors at that time.
Statistical analysis
Categorical variables were compared using Fisher’s exact test. The test was 2-sided, and P <0.05 was considered statistically significant. Statistical analyses were conducted using the software MedCalc (version 20.027; Ostend, Belgium).
Results
We identified 82 patients with hematologic malignancies who had past or chronic HBV infection and received BTK inhibitors during the study period. Fifty-three patients were excluded because of false-positive anti-HBc results after intravenous immune globulin infusion (11 patients), or concomitant anti-HBV prophylaxis (42 patients). Twenty-nine patients met our study criteria and were further analyzed (Figure 1).
Figure 1.
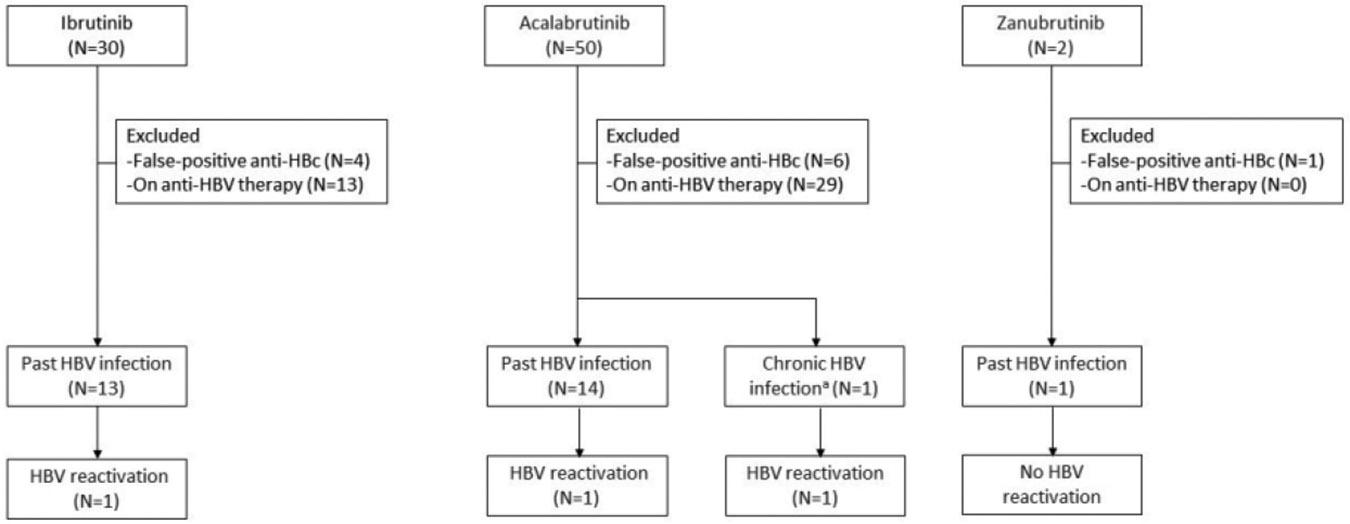
Patient selection and cases of HBV reactivation among the study patients.
Abbreviations: anti-HBc, hepatitis B core antibody; HBV, hepatitis B virus.
a. Patient with chronic HBV without antiviral prophylaxis.
Among the 29 patients studied, 28 (97%) had past HBV infection, and 1 (3%) had chronic HBV infection. Thirteen patients (45%) received ibrutinib, 15 (52%) received acalabrutinib, and 1 (3%) received zanubrutinib. Among the 29 patients, 21 (72%) had CLL and 20 (69%) received the BTK inhibitor as second-line or subsequent therapy (interquartile range [IQR], 2 – 4 lines) (Table 1). The median duration of BTK inhibitor use was 25 months (IQR, 8 – 47 months), and the median follow-up from BTK inhibitor therapy initiation was 25 months (IQR, 8 – 43 months). Fourteen patients received anti-CD20 MoAbs before BTK inhibitors. The median duration from the last anti-CD20 MoAb use to the initiation of BTK inhibitor therapy was 50 months (IQR, 35 – 60 months).
Table 1.
Characteristic of patients with hematologic malignancies and past or chronic HBV infection receiving Bruton tyrosine kinase inhibitor therapy without antiviral prophylaxis (N=29)
| Characteristic | Total (N=29) |
Ibrutinib (N=13) |
Acalabrutinib (N=15) |
Zanubrutinib (N=1) |
|---|---|---|---|---|
| Age, median (IQR), years | 72 (67 - 79) | 76 (71 - 82) | 71 (66 - 76) | 66 |
| Sex | ||||
| Male | 19 | 8 | 10 | 1 |
| Female | 10 | 5 | 5 | 0 |
| Race and ethnicity | ||||
| White | 21 | 10 | 10 | 1 |
| Black or African American | 4 | 3 | 1 | 0 |
| Hispanic or Latino | 4 | 0 | 4 | 0 |
| Hematologic malignancy | ||||
| Chronic lymphocytic leukemia | 22 | 12 | 9 | 1 |
| Mantle cell lymphoma | 6 | 0 | 6 | 0 |
| Marginal zone lymphoma | 1 | 1 | 0 | 0 |
| Waldenstrom macroglobulinemia | 0 | 0 | 0 | 0 |
| Type of HBV infection | ||||
| Past | 28 | 13 | 14 | 1 |
| Chronic | 1 | 0 | 1 | 0 |
| HBsAg, median (IQR), IU/L | 101 (6 - 231) | 137 (13 - 231) | 0 (0 - 214) | 204 |
| HBV reactivation | 3 | 1 | 2 | 0 |
| BTK inhibitors as first line of therapy | 9 | 7 | 2 | 0 |
| Duration of BTK inhibitors therapy, median (IQR), months | 25 (8 - 47) | 30 (3 - 50) | 14 (11 - 37) | 51 |
| Cumulative BTK inhibitors dose, median (IQR), mg | N/A | 218,400 (42,000 - 420,000) | 84,000 (66,000 -222,000) | 244,800 |
| Follow-up period, median (IQR), months | 25 (8 - 43) | 48 (5-67) | 21 (15 - 45) | 81 |
Abbreviations: BTK, Bruton tyrosine kinase; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IQR, interquartile range; N/A, not applicable.
HBV reactivation rate and characteristics
Of the 29 patients treated with BTK inhibitors, 3 (10%) developed HBV reactivation (Figure 1, Table 2). Of the 3 patients with HBV reactivation, 2 were male, 2 had past HBV infection, and all had received a BTK inhibitor as the second or subsequent line of therapy. All 3 patients developed HBV-associated hepatitis, and 2 also had HBV-associated liver failure. All received anti-HBV treatment and survived. One continued BTK inhibitor therapy (acalabrutinib) (Table 2). Among the 3 patients with HBV reactivation, the median duration of BTK inhibitor before reactivation use was 6 months, and the median follow-up from BTK inhibitor therapy initiation was 25 months.
Table 2.
HBV reactivation in patients who received BTK inhibitors
| Patient 1 (current study) |
Patient 2 (current study) |
Patient 3 (previous report)8 |
|
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 92 | 66 | 68 |
| Sex | Female | Male | Male |
| Cancer | CLL | MCL | MZL |
| History of anti-CD20 MoAb therapy | Yes | Yes | Yes |
| HBsAg / Anti-HBs results | + / − | − / − | − / − |
| HBV DNA, log IU/mL | NA | Undetectable | Undetectable |
| BTK inhibitor administration before HBV reactivation | |||
| BTK inhibitor | Acalabrutinib | Acalabrutinib | Ibrutinib |
| Concomitant cancer treatment | No | Venetoclax | No |
| Treatment duration, months | 2 | 21 | 6 |
| Cumulative dose, mg | 12,000 | 126,000 | 100,800 |
| Laboratory data at time of HBV reactivation | |||
| Lymphocyte count, cells/mL | 38,270 | 800 | 1,670 |
| Bilirubin, mg/dL | 0.5 | 10.6 | 5.3 |
| AST / ALT, U/L | 103/135 | 1,057/1,160 | 872/1293 |
| INR | 1.06 | 1.01 | 1.09 |
| HBsAg | + | − | + |
| HBV DNA, IU/mL | 7.11 | 8.62 | 5.83 |
| HBV-associated adverse outcomes | |||
| HBV-associated hepatitisa | Yes | Yes | Yes |
| HBV-associated liver failurea | No | Yes | Yes |
| Antiviral therapy and outcome | |||
| Antiviral therapy | Entecavir | Entecavir | Entecavir |
| Survival at last follow-up | Alive | Alive | Alive |
| BTK inhibitor continued | Yes | No | No |
| Follow-up time, months | 25 | 4 | 47 |
Abbreviations: +, positive; −, negative; ALT, alanine transaminase; anti-HBc: hepatitis B core antibody; AST, aspartate transaminase; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; INR, international normalized ratio; MCL, mantle cell lymphoma; MoAb, monoclonal antibody; MZL, marginal zone lymphoma; NA, not available.
Defined in the Methods section.
Case presentations
Details of the patient who developed HBV reactivation after ibrutinib were previously published by our group in this journal8 Details of the other 2 patients with HBV reactivation are as follows.
Patient 1 was a 92-year-old woman with chronic HBV infection and CLL diagnosed in 1998. She had been treated with an obinutuzumab (anti-CD20 MoAb)-based regimen at another institution. The last dose of obinutuzumab was administrated in June 2017. Entecavir was stopped in June 2018, which was 12 months after the last obinutuzumab dose. The patient received no cancer treatment from June 2018 through September 2020. In October 2020, acalabrutinib monotherapy was initiated because of relapsed CLL. The patient was not receiving anti-HBV prophylaxis. In December 2020, two months after starting acalabrutinib, the patient has an alanine transaminase level of 135 IU/L and a total bilirubin level of 0.5 mg/dL. Further testing revealed an HBV DNA level of 13,000,000 IU/mL (7.11 log10 IU/mL). Other infectious and noninfectious causes of liver failure were excluded, and HBV reactivation was diagnosed. Entecavir 0.5 mg orally daily was initiated, resulting in the normalization of liver function tests within 60 days after the start of therapy. The patient’s HBV DNA viral load decreased to low-grade HBV viremia (<10 IU/mL) in 14 months (Figure 2A). Acalabrutinib was continued.
Figure 2.
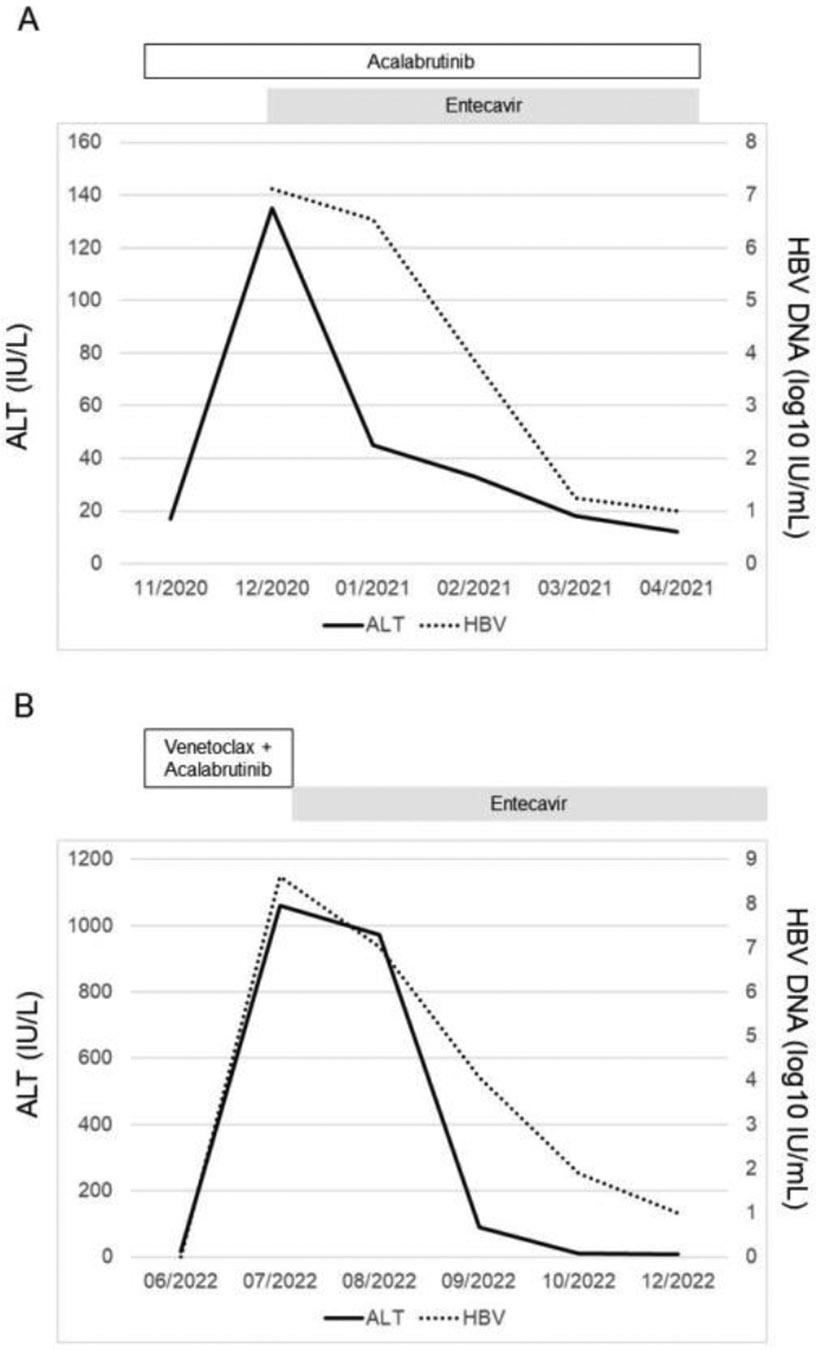
HBV reactivation in patients who received acalabrutinib.
A. Patient with chronic HBV infection.
B. Patient with past HBV infection.
Abbreviations: ALT, alanine transaminase; HBV, hepatitis B virus.
Patient 2 was a 66-year-old man with past HBV infection and MCL diagnosed in 2017. He had been treated with a rituximab (anti-CD20 MoAb)-based regimen. The last dose of rituximab was administrated in October 2017. Entecavir was stopped in October 2018, which was 12 months after the last rituximab dose. The patient received no cancer treatment from October 2017 through August 2020. HBV DNA was undetectable during that period. In September 2020, acalabrutinib and venetoclax were initiated because of relapsed MCL. The patient was not receiving anti-HBV prophylaxis, and HBV DNA remained undetectable. In July 2022, 21 months after starting acalabrutinib, the patient was hospitalized for fatigue, anorexia, and jaundice. Laboratory values included an alanine transaminase 1,160 IU/mL and a total bilirubin level of 10.6 mg/dL. Further testing revealed the reappearance of HBsAg and an HBV DNA level 421,000,000 IU/mL (8.62 log10 IU/mL). Other infectious and noninfectious causes of liver failure were excluded, and the patient was diagnosed with HBV reactivation presenting as reverse seroconversion (reappearance of HBsAg; HBV DNA became detectable), and HBV-associated liver failure (total bilirubin level 10.6 mg/dL). Entecavir 0.5 mg daily orally was initiated, resulting in improvement of the liver function tests in 5 days and normalization of liver function tests within 30 days after the start of therapy. The patient’s HBV viral load decreased to low-grade HBV viremia (<10 IU/mL) in 5 months (Figure 2B). Acalabrutinib and venetoclax were permanently discontinued.
HBV reactivation rates by BTK inhibitors in patients with past HBV infection
Of the 28 patients with past HBV infection who received BTK inhibitors, 13 received ibrutinib, of whom 1 (8%) developed HBV reactivation, and 14 received acalabrutinib, of whom 1 (7%) developed HBV. The rate of HBV reactivation was similar between ibrutinib (first-generation BTK inhibitor) and acalabrutinib (second-generation BTK inhibitor) (P = 1.0). Only one patient received zanubrutinib, and that patient did not develop HBV reactivation.
Discussion
To our knowledge, this is the first study describing HBV reactivation in patients with different types of hematologic malignancies receiving first-generation or second-generation BTK inhibitors. All of the patients with HBV reactivation developed HBV-associated hepatitis and required antiviral therapy, and all experienced resolution of the HBV reactivation and survived.
The risk of HBV reactivation can be categorized as low (<1%), intermediate (1% - 10%), or high (>10%).9 In our study, 8% of the patients with past HBV infection receiving ibrutinib developed HBV reactivation. This finding is consistent with previous studies,1,4,10 which showed an intermediate risk (1% - 10%) of HBV reactivation in patients receiving ibrutinib (first-generation BTK inhibitor). Our study is the first to report the risk of HBV reactivation in patients receiving acalabrutinib (second-generation BTK inhibitor), which was 7%.
The HBV reactivation rate associated with BTK inhibitor therapy is impacted by previous or concomitant treatments for hematologic malignancies, including anti-CD20 MoAbs, hematopoietic stem cell transplant, and intravenous immune globulin.6,11 Hypogammaglobulinemia is the predominant inherent immune defect in patients with hematologic malignancies.12 Although BTK inhibitors result in partial reconstitution of humoral immunity, serum immunoglobulin G levels still decrease over time,13 which may cause the loss of protective immunity against HBV, leading to viral reactivation.
Several questions remain about the impact of BTK inhibitors on HBV replication. First, is there a dose-dependent risk of HBV reactivation? Second, can HBV reactivation occur months after completion of BTK inhibitor therapy, as reported after treatment with anti-CD20 MoAbs?6 Given the unanswered questions, the appropriate duration of follow-up for HBV reactivation in patients treated with BTK inhibitors remains unknown.
This study has several limitations. First, this is a single-institution retrospective study with a small sample size. Second, only 1 patient received a zanubrutinib-containing regimen, so we could not ascertain the impact of zanubrutinib on HBV viremia. Third, venetoclax (a BCL-2 inhibitor) could have played a role in the HBV reactivation in patient 2, but the contribution of venetoclax to HBV reactivation is unknown.
Conclusion
BTK inhibitor therapy appears to be associated with an intermediate risk of HBV reactivation in patients with hematologic malignancies. These cases of HBV reactivation can be severe, but they respond well to anti-HBV therapy. Our findings support that in patients with past HBV infection receiving BTK inhibitors, close monitoring with on-demand anti-HBV treatment at the first sign of significant reactivation is warranted. Prospective studies, including studies with a larger sample size of HBV-infected patients receiving BTK inhibitors, are needed to validate our results.
Clinical Practice Points.
Bruton tyrosine kinase (BTK) inhibitors are used to treat B-cell hematologic malignancies.
Hepatitis B virus (HBV) reactivation has been reported among patients treated with a first-generation BTK inhibitor (ibrutinib) and second-generation BTK inhibitors (acalabrutinib and zanubrutinib).
We found an intermediate risk of HBV reactivation in patients with past HBV infection treated with ibrutinib (8%) or acalabrutinib (7%).
At this time, data are insufficient to recommend universal anti-HBV prophylaxis for patients with past HBV infection who receive BTK inhibitors, but monitoring such patients for HBV reactivation is warranted.
Acknowledgments
We thank Stephanie Deming, Research Medical Library, MD Anderson Cancer Center, for editing the manuscript.
Support
Supported by the NIH/NCI under award number P30CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure
Dr. Ahmed has research support paid to MD Anderson Cancer Center for clinical trials from Seattle Genetics, Merck, Xencor, Chimagen, and Tessa Therapeutics. Dr. Ahmed is a member of the Tessa Therapeutics and Chimagen scientific advisory committees, serves on the Data Safety Monitoring Board for Myeloid Therapeutics, and is a consultant for ADC Therapeutics, and Gilead Sciences. Dr. Thomas has research support from BMS, Cellectar Biosciences, Acerta Pharma, X4 Pharma, and Genentec, and has been a paid scientific advisor for Cellectar Biosciences. Dr. Khawaja has received an honorarium from MEDSCAPE for educational activities. Dr. Torres is or has been the principal investigator for research grants from the National Cancer Institute, Gilead Sciences, and Merck & Co., Inc., with all funds paid to MD Anderson Cancer Center. Dr. Torres is or has been a paid scientific advisor for AbbVie, Inc., Gilead Sciences, Janssen Pharmaceuticals, Inc., Merck & Co., Inc., and Dynavax Technologies; MD Anderson Cancer Center is managing the terms of these arrangements in accordance with its conflict-of-interest policies. All other authors report no conflicts of interest.
References
- 1.Ni Y, Gao L, Lu Y, et al. Risk of HBV reactivation in relapsed or refractory diffuse large B-cell lymphoma patients receiving Bruton tyrosine kinase inhibitors therapy. Front Immunol. 2022;13:982346. doi: 10.3389/fimmu.2022.982346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. May 11 2020;13(1):48. doi: 10.1186/s13045-020-00884-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustafayev K, Torres H. Hepatitis B virus and hepatitis C virus reactivation in cancer patients receiving novel anticancer therapies. Clin Microbiol Infect. Oct 2022;28(10):1321–1327. doi: 10.1016/j.cmi.2022.02.042 [DOI] [PubMed] [Google Scholar]
- 4.Innocenti I, Reda G, Visentin A, et al. Risk of hepatitis B virus reactivation in chronic lymphocytic leukemia patients receiving ibrutinib with or without antiviral prophylaxis. A retrospective multicentric GIMEMA study. Haematologica. Jun 1 2022;107(6):1470–1473. doi: 10.3324/haematol.2021.280325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu H, Lok AS, Warneke CL, et al. Passive transfer of anti-HBc after intravenous immunoglobulin administration in patients with cancer: a retrospective chart review. Lancet Haematol. Oct 2018;5(10):e474–e478. doi: 10.1016/s2352-3026(18)30152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang JP, Feld JJ, Hammond SP, et al. Hepatitis B Virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol. Nov 1 2020;38(31):3698–3715. doi: 10.1200/jco.20.01757 [DOI] [PubMed] [Google Scholar]
- 7.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. Apr 2018;67(4):1560–1599. doi: 10.1002/hep.29800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malek AE, Nieto Y, Szvalb AD, et al. Hepatitis B virus-associated liver failure in a patient with B-cell non-Hodgkin lymphoma after anti-cancer therapy including ibrutinib. Clin Lymphoma Myeloma Leuk. Mar 2020;20(3):e124–e127. doi: 10.1016/j.clml.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papatheodoridis GV, Lekakis V, Voulgaris T, et al. Hepatitis B virus reactivation associated with new classes of immunosuppressants and immunomodulators: a systematic review, meta-analysis, and expert opinion. J Hepatol. Dec 2022;77(6):1670–1689. doi: 10.1016/j.jhep.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 10.Hammond SP, Chen K, Pandit A, Davids MS, Issa NC, Marty FM. Risk of hepatitis B virus reactivation in patients treated with ibrutinib. Blood. Apr 26 2018;131(17):1987–1989. doi: 10.1182/blood-2018-01-826495 [DOI] [PubMed] [Google Scholar]
- 11.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. Apr 2013;13(2):106–11. doi: 10.1016/j.clml.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sim B, Ng JY, Teh BW, Talaulikar D. Immunoglobulin replacement in hematological malignancies: a focus on evidence, alternatives, dosing strategy, and cessation rule. Leuk Lymphoma. Oct 11 2022:1–12. doi: 10.1080/10428194.2022.2131424 [DOI] [PubMed] [Google Scholar]
- 13.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. Nov 5 2015;126(19):2213–9. doi: 10.1182/blood-2015-04-639203 [DOI] [PMC free article] [PubMed] [Google Scholar]


