Abstract
Introduction
Monoclonal immunoglobulin deposition diseases (MIDDs) are a group of systemic diseases, characterized by deposition of monoclonal immunoglobulin predominantly in the kidney. In the absence of overt hematologic disease, MIDDs are classified as a part of monoclonal gammopathy of renal significance. Patients with MIDD may present with a nephrotic syndrome and kidney function impairment. Treatment usually includes anti-plasma cell therapy.
Case Presentation
We report a case of a 54-year-old female who presented with nephrotic syndrome related to light chain deposition disease of lambda type. Due to a complicated clinical course (including cardiac injury and thromboembolic stroke), plasma cell-targeted therapy was stopped. A few months later, the patient presented with severe acute kidney injury. Kidney biopsy revealed crescentic glomerulonephritis, and immunofluorescence staining was positive for lambda chain. Treatment with daratumumab was initiated resulting in stabilization of kidney function and partial nephrotic syndrome remission.
Conclusion
This case highlights an uncommon histologic manifestation in a patient diagnosed with light chain deposition disease. Furthermore, it underscores the significance of plasma cell-targeted therapy and the favorable clinical and hematological response observed with daratumumab.
Keywords: Acute kidney injury, Glomerulonephritis, Nephrotic syndrome
Introduction
Monoclonal gammopathy of renal significance (MGRS) is a group of kidney disorders involving the secretion of monoclonal protein by small plasma or B-cell clones associated with kidney impairment. By definition, MGRS is diagnosed when patients do not achieve diagnostic criteria for overt multiple myeloma or B-cell malignancies. The spectrum of kidney involvement is broad and includes glomerular, tubular, and vascular manifestations. Glomerular diseases may manifest as monoclonal immunoglobulin deposition disease (MIDD), proliferative glomerulonephritis with monoclonal immunoglobulin deposits, C3 glomerulopathy with monoclonal gammopathy, immunoglobulin-related amyloidosis, monoclonal (type I cryoglobulinemic glomerulopathy) and monotypic immunotactoid glomerulopathy. Among these, AL amyloidosis is the most prevalent subtype of MGRS [1].
LCDD is a part of MIDDs, a group of rare systemic diseases that are classified by the type of monoclonal immunoglobulin deposits. LCDD is the most frequent variant, consisting of 75–80% of MIDD cases. The predominant deposition of light chains is of the kappa type. The kidney is the most common organ to be affected, usually presented as nephrotic syndrome or impairment in kidney function [2].
Chemotherapy targeted to plasma or B-cell clones has been proposed as the mainstay therapy of MGRS [3]. This therapeutic approach has resulted in improvement in kidney function and reduction of proteinuria. Here we present a complicated case of a patient with severe nephrotic syndrome and crescentic glomerulonephritis related to MIDD, treated with daratumumab resulting in a good clinical response.
Case Presentation
A 54-year-old woman with a history of Turner’s syndrome and hypothyroidism was evaluated due to new onset of nephrotic syndrome, presenting with severe lower limb edema, hypoalbuminemia (1.8 g/dL), and proteinuria (5.2 g in a 24-h urine collection). Kidney function was normal with a creatinine level of 0.59 mg/dL. Serologic testing was notable for a high level of free lambda chains (159 mg/L) and low free kappa light chains (6 mg/L), with a free kappa/free lambda ratio of 0.04. Accordingly, immunofixation was positive for monoclonal lambda light chains in blood and urine.
Bone marrow biopsy was performed, showing increased infiltration of CD138 monoclonal plasma cells (20–25%), with positive staining for monoclonal lambda light chain. A kidney biopsy was also performed, supporting the diagnosis of monoclonal light chain deposition disease (LCDD). Congo red staining was negative in both biopsies.
Echocardiogram showed normal ventricular function, and computed tomography was negative for osteolytic bone lesions. Treatment with a protocol consisting of bortezomib (Velcade), cyclophosphamide, and dexamethasone (VCD) was initiated. The VCD regimen included subcutaneous administration of 1.3 mg/m2 bortezomib weekly, intravenous cyclophosphamide at a dosage of 300 mg/m2 every 2 weeks, and 20 mg oral dexamethasone weekly.
After 5 cycles of VCD treatment, the patient demonstrated a very good partial hematologic response. Remarkably, after a period of 5 months, there was a gradual reduction in the free lambda light chains level to 34.3 mg/L, representing a significant 78% reduction from the baseline. Concurrently, the free kappa light chains level reached 2.69 mg/L, resulting in a free kappa/free lambda ratio of 0.01.
However, despite these encouraging hematologic changes, no significant improvement was observed in proteinuria, which remained at 10.5 g, and peripheral edema persisted. Importantly, it is noteworthy that the patient did not experience any neuropathy or gastrointestinal disorders during the course of the VCD treatment.
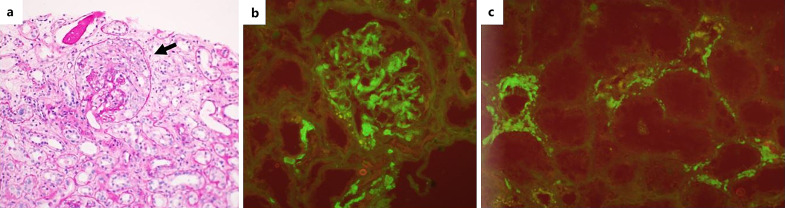
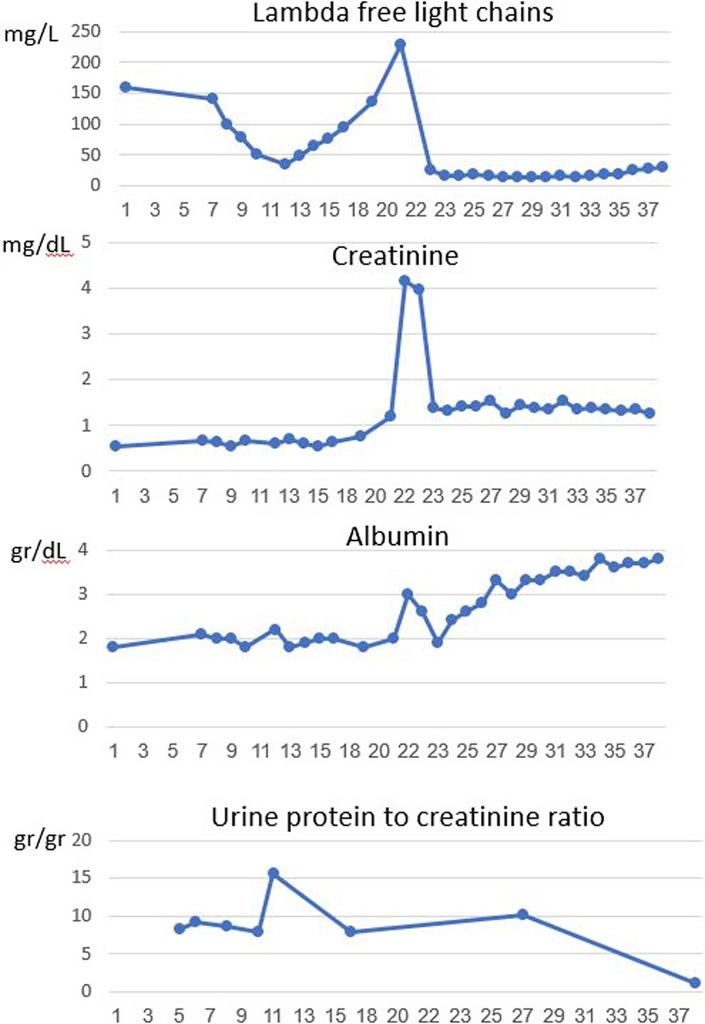
Six months following therapy (which include 6 cycles of VCD) and 10 days after the last treatment, the patient was admitted to the hospital with the onset of anterior chest pain. Electrocardiogram did not demonstrate acute ischemic changes, laboratory tests revealed elevated troponin levels, and the echocardiogram showed mild left ventricle dysfunction with mild mitral regurgitation. Coronary angiography showed non-obstructive single-vessel disease, and conservative treatment with clopidogrel was initiated. Three days later, the patient was readmitted with left-side hemiparesis and diagnosed with a cerebrovascular accident. Thrombolytic therapy (tPA) was begun with neurologic improvement. Brain magnetic resonance imaging demonstrated multiple foci of restricted diffusion scattered bilaterally, consisting of a cerebral embolic infarct. However, a thorough evaluation of the embolic source, including transesophageal echocardiogram and cardiac Holter monitoring, was unrevealing. At this point, the decision to stop treatment with VCD was made concerning bortezomib as a potential cause of myocardial injury. Other treatment options, including immunomodulatory drugs (such as lenalidomide and pomalidomide), were also unfavorable due to a high risk of thromboembolism. She was followed up closely in the hematologic clinic without treatment. Eleven months after treatment was stopped, a significant elevation was primarily observed in free lambda chains (up to 229 mg/L), with the free kappa light chain measuring 17.9 mg/L, in the blood. This was accompanied by severe acute kidney injury, as indicated by an increase in creatinine to 4.33 mg/dL and a proteinuria of 10.9 g/24 h. Urinalysis was positive for hematuria and proteinuria. Serologic tests for ANA, ANCA, C3, C4, RF, cryoglobulins, anti-GBM, HBV, HCV, and HIV were unrevealing. A repeated kidney biopsy was performed. On light microscopy, twelve glomeruli were observed, two with a cellular crescent. The other glomeruli showed swelling of the parietal epithelial cells. Signs of acute tubular injury were also noted. No signs of myeloma cast nephropathy were observed. Immunofluorescence staining was positive to lambda light chains (+3 mesangial, peripheral, and tubular basement membrane) (Fig. 1). The findings were compatible with a diagnosis of focal crescentic glomerulonephritis. Treatment with daratumumab and steroids was initiated. Six months later, a clinical and laboratory response was observed. A very good partial hematologic response was detected, with a 93% reduction from baseline in lambda free light chains (decreased to 16.9 mg/L) and kappa free light chains measuring 3.38 mg/L. The ratio of free kappa/lambda chains was 0.2. Kidney function improved (creatinine decreased to 1.27 mg/dL), a significant reduction in proteinuria was detected (1.1 g/24 h), albumin increased to 3.6 g/dL, and lower limbs edema disappeared (Table 1; Fig. 2).
Fig. 1.
Kidney biopsy. a Glomerulus with a crescent. Immunofluorescence staining positive for lambda light chain – glomerular mesangial and capillary loop (b) and tubular basement membrane (c).
Table 1.
Blood and urine laboratory tests during disease course
| At diagnosis | Maximum depth of response to VCD | Disease progression | Maximum depth of response to daratumumab | |
|---|---|---|---|---|
| Kappa free light chains – serum, mg/L | 6.02 | 2.69 | 17.9 | 3.38 |
| Lambda free light chains – serum, mg/L | 159 | 34.3 | 229 | 16.9 |
| Free kappa/free lambda ratio – serum | 0.04 | 0.1 | 0.1 | 0.2 |
| Immunofixation (serum) | ||||
| IgA | Polyclonal | |||
| IgG | Monoclonal | |||
| IgM | Negative | |||
| Kappa | Polyclonal | |||
| Lambda | Monoclonal | |||
| Immunofixation (urine) | ||||
| Bence Jones | Lambda positive | |||
| Kappa free light chains – urine, mg/L | 5.4 | NA | 167 | NA |
| Lambda free light chains – urine, mg/L | 132 | NA | 140 | NA |
| Free kappa/free lambda ratio – urine | 0.04 | NA | 1.19 | NA |
| Creatinine, mg/dL | 0.59 | 0.6 | 4.33 | 1.27 |
| BUN, mg/dL | 14 | 22 | 43 | 28 |
| 24-h urine collection, g/24 h | 5.2 | 10.5 | 10.9 | 1.1 |
NA, not available.
Fig. 2.
Evolution of free lambda light chains level, serum creatinine, albumin, and proteinuria during 3 years from diagnosis.
Discussion
This case presents a presentation of kidney involvement related to lambda LCDD, demonstrating a complicated clinical course associated with challenging hematologic management and treatment resulting in an improvement in both kidney function and proteinuria. The patient initially presented with nephrotic syndrome secondary to LCDD. The common histologic finding of LCDD in kidney biopsy is nodular glomerulosclerosis, characterized by positive periodic acid-Schiff staining and negative Congo red staining [4]. However, other pathologic lesions have been also described, ranging from minimal glomerular changes to membranoproliferative glomerulonephritis [5–7].
Regarding treatment, chemotherapy targeting plasma or B-cell clones is the recommended approach for treating MIDDs. Common protocol include bortezomib-based regimens and thalidomide-/lenalidomide-based regimens. High-dose melphalan with autologous stem cell transplant has also been reported to be associated with high rate of hematologic and kidney responses [8–10].
In our patient, the presence of a monoclonal light chain in plasma and urine, detectable plasma cell clones in the bone marrow, and significant kidney involvement required anti-plasma cell therapy treatment. Indeed, soon after diagnosis, treatment with VCD protocol was implemented. However, the decision to temporarily stop the therapy was made due to a complicated clinical course, including concerning bortezomib related to cardiac injury. Use of an alternative therapeutic option, such as thalidomide-based therapy was also precluded due to the high thrombotic risk, indicated by the clinical appearance of a thromboembolic stroke. Our patient demonstrates this challenging decision, leading to severe kidney injury paralleled by an increase in free serum lambda chains (Table 1).
Treatment with daratumumab (an anti-CD38 monoclonal antibody) resulted in a favorable clinical response. Recent studies have highlighted positive outcomes with daratumumab in patients with MGRS. In individuals with AL amyloidosis, daratumumab-based regimens demonstrated a positive hematologic and organ response, even in advanced stages of the disease [11, 12]. A randomized study involving 388 patients with a new diagnosis of systemic AL amyloidosis showed that adding daratumumab to the VCD regimen was associated with a higher frequency of hematologic complete response and survival free from major organ deterioration. However, overall survival did not differ significantly between the groups in this study [13].
A meta-analysis, including 30 studies and 997 patients, evaluated the efficacy and safety of daratumumab-based treatment for AL amyloidosis. The overall hematologic response was observed in 77% of patients, with cardiac and renal response rates of 41% and 43%, respectively [14]. Although data regarding treatment with daratumumab in LCDD are limited, encouraging results have also been reported. In 2020, Milani et al. [15] presented the first case series of patients with previously treated LCDD who were treated with daratumumab, resulting in a rapid and favorable hematologic response in 7 of 8 patients with refractory disease. Another study on 25 patients with MGRS (80% with LCDD and 4% with proliferative glomerulonephritis with monoclonal IgG deposits) demonstrated a positive hematologic and renal response to daratumumab-based therapy [16].
In summary, our patient presents severe nephrotic syndrome complicated by crescentic glomerulonephritis, related to lambda chain deposition. This case also underscores the importance of plasma cell-targeted therapy, the unfavorable outcome when ceasing treatment, and finally, a positive kidney response to plasma cell-targeted therapy with daratumumab.
Acknowledgment
The authors thank Professor Juri Kopolovich for his interpretation of the kidney biopsy.
Statement of Ethics
This study protocol was reviewed and the need for approval was waived by the Ethics Committee of Shaare Zedek Medical Center. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funds, grants, or other support was received.
Author Contributions
Dr. Alon Bnaya wrote the manuscript. Dr. Chezi Ganzel and Prof. Linda Shavit critically revised the manuscript. All authors followed the patient.
Funding Statement
No funds, grants, or other support was received.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.
References
- 1. Leung N, Bridoux F, Nasr SH. Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384(20):1931–41. [DOI] [PubMed] [Google Scholar]
- 2. Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, D’Agati VD, et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7(2):231–9. [DOI] [PubMed] [Google Scholar]
- 3. Fermand JP, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, et al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood. 2013;122(22):3583–90. [DOI] [PubMed] [Google Scholar]
- 4. Gokden N, Barlogie B, Liapis H. Morphologic heterogeneity of renal light-chain deposition disease. Ultrastruct Pathol. 2008;32(1):17–24. [DOI] [PubMed] [Google Scholar]
- 5. Sanders PW, Herrera GA, Kirk KA, Old CW, Galla JH. Spectrum of glomerular and tubulointerstitial renal lesions associated with monotypical immunoglobulin light chain deposition. Lab Invest. 1991;64(4):527–37. [PubMed] [Google Scholar]
- 6. Ergen P, Yuyucu Karabulut Y, Kıykım AA, Ballı E, Köse EÇ. Light chain deposition disease diagnosed with renal biopsy: a case report. Turk J Nephrol. 2019;28(4):331–4. [Google Scholar]
- 7. Venkataseshan VS, Faraggiana T, Hughson MD, Buchwald D, Olesnicky L, Goldstein MH. Morphologic variants of light-chain deposition disease in the kidney. Am J Nephrol. 1988;8(4):272–9. [DOI] [PubMed] [Google Scholar]
- 8. Royer B, Arnulf B, Martinez F, Roy L, Flageul B, Etienne I, et al. High dose chemotherapy in light chain or light and heavy chain deposition disease. Kidney Int. 2004;65(2):642–8. [DOI] [PubMed] [Google Scholar]
- 9. Cohen C, Royer B, Javaugue V, Szalat R, El Karoui K, Caulier A, et al. Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int. 2015;88(5):1135–43. [DOI] [PubMed] [Google Scholar]
- 10. Masood A, Ehsan H, Iqbal Q, Salman A, Hashmi H. Treatment of light chain deposition disease: a systematic review. J Hematol. 2022;11(4):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wechalekar AD, Sanchorawala V. Daratumumab in AL amyloidosis. Blood. 2022;140(22):2317–22. [DOI] [PubMed] [Google Scholar]
- 12. Jeryczynski G, Antlanger M, Duca F, Binder-Rodriguez C, Reiter T, Simonitsch-Klupp I, et al. First-line daratumumab shows high efficacy and tolerability even in advanced AL amyloidosis: the real-world experience. ESMO Open. 2021;6(2):100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. [DOI] [PubMed] [Google Scholar]
- 14. Sun C, Wang X, Zhang R, Xu L, Wang B, Li J. Efficacy and safety of intravenous daratumumab-based treatments for AL amyloidosis: a systematic review and meta-analysis. Cancer Cell Int. 2022;22(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milani P, Basset M, Curci P, Foli A, Rizzi R, Nuvolone M, et al. Daratumumab in light chain deposition disease: rapid and profound hematologic response preserves kidney function. Blood Adv. 2020;4(7):1321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kastritis E, Theodorakakou F, Roussou M, Psimenou E, Gakiopoulou C, Marinaki S, et al. Daratumumab-based therapy for patients with monoclonal gammopathy of renal significance. Br J Haematol. 2021;193(1):113–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.