Abstract
The cytomegalovirus (CMV) basic phosphoprotein (BPP) is a component of the tegument. It remains with the nucleocapsid fraction under conditions that remove most other tegument proteins from the virion, suggesting a direct and perhaps tight interaction with the capsid. As a step toward localizing this protein within the molecular structure of the virion and understanding its function during infection, we have investigated the BPP-capsid interaction. In this report we present evidence that the BPP interacts selectively, through its amino one-third, with CMV capsids. Radiolabeled simian CMV (SCMV) BPP, synthesized in vitro, bound to SCMV B-capsids, and C-capsids to a lesser extent, following incubation with either isolated capsids or lysates of infected cells. Human CMV (HCMV) BPP (pUL32) also bound to SCMV capsids, and SCMV BPP likewise bound to HCMV capsids, indicating that the sequence(s) involved is conserved between the two proteins. Analysis of SCMV BPP truncation mutants localized the capsid-binding region to the amino one-third of the molecule—the portion of BPP showing the greatest sequence conservation between the SCMV and HCMV homologs. This general approach may have utility in studying the interactions of other proteins with conformation-dependent binding sites.
The basic phosphoprotein (BPP) is an abundant constituent of the cytomegalovirus (CMV) virion. On the basis of its presence in preparations of virions, noninfectious enveloped particles, and cytoplasmic nucleocapsids, but not immature nuclear capsids or dense bodies, BPP was classified as a tegument protein (13, 16, 21). More recent evidence from cryoelectron microscopy suggests that it contacts the capsid through the distal end of the capsomers or through the triplex subunits that interlink them (8, 43). BPP is phosphorylated in vivo (12, 24, 35), like many other herpesvirus tegument proteins (e.g., references 17, 29, and 31), and is a predominant phosphate acceptor in vitro for the virion-associated protein kinase(s) (35).
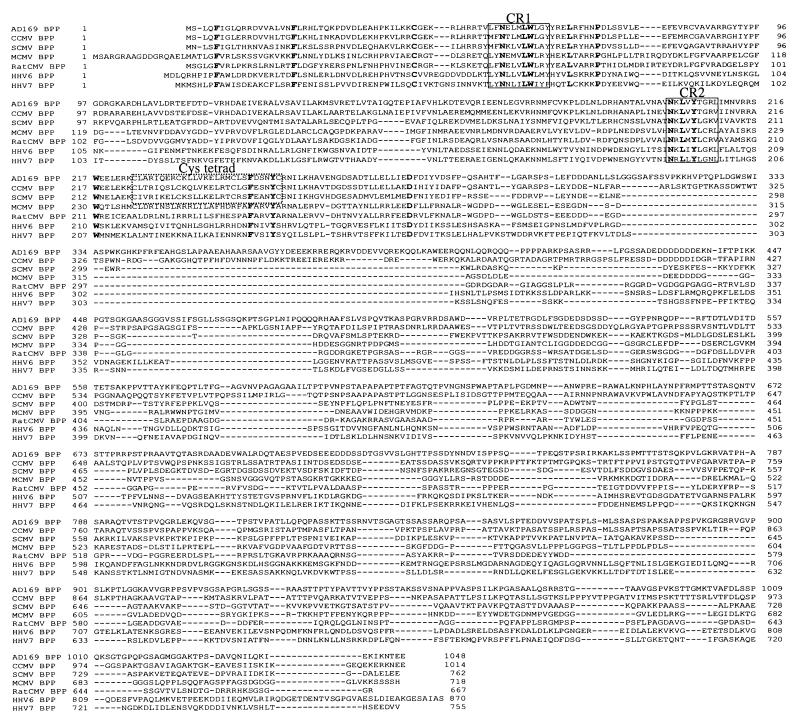
BPP homologs are recognized in other betaherpesviruses (e.g, see Fig. 2) but not in alpha- or gammaherpesviruses. The human CMV (HCMV) BPP homolog is encoded by open reading frame (ORF) UL32 (7, 23) and is predicted to have a mass of 113 kDa. However, its relative electrophoretic mobility is closer to that of the 150-kDa major capsid protein (MCP; pUL86) and is influenced by the specific conditions of electrophoresis (21, 24), potentially complicating its identification on the basis of size or relative electrophoretic mobility alone. BPP is expressed late (6, 36) and has been detected in the nuclei of infected cells (20, 33), although at later times it accumulates in the perinuclear region of the cytoplasm (20, 36, 38). Unlike its simian CMV (SCMV) counterpart, HCMV BPP is modified by the attachment of O-linked N-acetylglucosamine to two serines near its carboxyl end (2, 19). It elicits a strong humoral immune response (16, 24, 27), and there is evidence from the effects of antisense sense RNAs (30) and a spontaneous mutation in the gene (45) that BPP is important, if not essential, for the production of infectious virus.
FIG. 2.
Sequence alignments of beta-herpesvirus BPP homologs. The predicted amino acid sequence of the SCMV BPP was aligned with its homologs in HCMV (7, 23), CCMV (G. S. Hayward, personal communication), rat CMV (3), MCMV (34), HHV-6 (18), and HHV-7 (32) by using the ClustalW algorithm (40). Identical residues are in boldface; the two most highly group-conserved regions (CR1 and CR2) and a primate CMV-conserved cysteine tetrad (Cys tetrad) are boxed for reference.
Although little is known about the function of BPP, its close association with the nucleocapsid suggests potential involvements in nuclear targeting early and in nuclear egress, capsid tegumentation, and envelopment late in infection. The experiments described here were done to investigate the interaction of BPP with CMV capsids as a step toward understanding its structural and functional roles during virus assembly and infection. By monitoring in vitro binding of radiolabeled BPP to CMV capsids, we obtained evidence that this interaction can be reproduced and studied in vitro, is specific, and requires the most conserved amino one-third of the BPP molecule.
MATERIALS AND METHODS
Cells and viruses.
HCMV strain AD169 and SCMV strain Colburn were propagated in human foreskin fibroblasts (HFF) (11, 12, 21). Herpes simplex virus (HSV) type 1 strain KOS was propagated in African green monkey kidney (Vero) cells (9).
Cloning and sequencing.
Genomic DNA was purified from SCMV virions, cleaved with SalI and XbaI, and subjected to agarose gel electrophoresis and staining with ethidium bromide. The approximately 8.5-kb SalI/XbaI fragment was cut from the gel and cloned into pBluescript II SK(+) (no. 212205; Stratagene, La Jolla, Calif.) and submitted to the Johns Hopkins Medical Institutions Biosynthesis and Sequencing Facility for one round of dideoxynucleotide sequencing from the SalI end of the insert. This first round of sequence analysis revealed that, based on the colinearity of the HCMV and SCMV genomes, the ORF encoding SCMV BPP (sBPP) was close to the SalI end of the insert. The insert was therefore digested with SstI to remove the XbaI end (about 4.2 kb) and then religated and subcloned. The resulting 4.3-kb SalI/SstI insert was sequenced in its entirety on both strands.
Plasmids.
The 2.3-kb sBPP gene was subcloned into pcDNAI/Amp (no. V460-20; Invitrogen, Carlsbad, Calif.) for expression in vitro by (i) cleavage of the SstI/SalI subclone at the unique NheI site 40 bp 3′ of the BPP start codon and at a NotI site downstream of the stop codon and (ii) three-piece ligation of the resulting NheI/NotI fragment with a 50-mer EcoRI/NheI oligonucleotide (oligonucleotide no. 1 [see below]) linker into EcoRI/NotI-digested pcDNAI/Amp. Deletions of amino acid sequences 1 to 99 (MB154; oligonucleotide no. 2 [see below]) and 1 to 193 (MB155; oligonucleotide no. 3 [see below]) were made by PCR amplification of the 5′ end of the sBPP gene such that the coding sequences for the indicated amino acids were deleted and replaced by a methionine codon. Deletion of amino acids 1 to 275 (MB156) was achieved by oligonucleotide (no. 4 [see below])-mediated mutagenesis that replaced the indicated residues with a methionine codon. The double-deletion mutant expressing amino acids 194 to 275 (MB157) was generated by PCR amplification of the indicated coding region using primers that placed a start codon immediately 5′ of the codon for Ala194 and a stop codon immediately 3′ of the codon for Asp275 (oligonucleotide no. 5 [see below]).
The following pairs of synthetic oligonucleotides, numbered as indicated above, were used to make the sBPP plasmids: no. 1, sense, 5′-AATTCGGACCATGAATTTAAGCTTTATTGGACTAACGCATCGCAATGTTG-3′, and antisense, 5′-CTAGCAACATTGCGATGCGTTAGTCCAATAAAGCTTAAATTCATGGTCCG-3′; no. 2, forward, 5′-GGAAAGCTTATCATGGTACAAGCCCGTCCTCAC-3′, and reverse, 5′-ATCTTCGTAGATATTAAAATCTTCC-3′; no. 3, forward, 5′-GGAAAGCTTATCATGGCAACTAATAAACTAGTGTATCTTGGT-3′, and reverse, same as no. 2 reverse oligonucleotide; no. 4, sense, 5′-AGCTTGGTACCGAGCTCGGATCCACTATG-3′, and antisense, 5′-CATAGTGGATCCGAGCTCGGTACCA-3′; no. 5, forward, Same as no. 3 forward oligonucleotide, and reverse, 5′-TCAATCTTCGTAGATATTAAAATCTTCC-3′.
In vitro protein synthesis.
[35S]methionine (no. 51001H; ICN, Costa Mesa, Calif.)-labeled SCMV and HCMV BPPs (35S-sBPP and 35S-hBPP, respectively) were synthesized in rabbit reticulocyte lysates (TnT T7 Quick; No. L1170; Promega, Madison, Wis.) from plasmids MB150 and KG3 (19), respectively. Both proteins had approximately the same electrophoretic mobility as their infected-cell counterparts, and both were immunoprecipitated by BPP-specific antisera (see Fig. 4 and data not shown). In vitro-radiolabeled luciferase was made from the T7 control plasmid supplied with the TnT system. BPP truncation mutants lacking carboxyl sequences were made by cleaving plasmid MB150 at the StuI, EcoRV, or BsmI restriction site within the sBPP coding sequence and using the resulting DNA in the TnT system to make radiolabeled proteins. Truncation mutants lacking amino sequences were made by using plasmids MB154, MB155, MB156, and MB157 in the TnT system.
FIG. 4.
SCMV BPP binds to capsids in lysates of SCMV-infected cells. The nuclear fractions of SCMV-infected cells were incubated with 35S-sBPP, the mixtures were separated by rate-velocity centrifugation, and gradient fractions were subjected to SDS-PAGE followed by electrotransfer to Immobilon membranes. Shown are phosphorimages prepared from the membrane before (A) and after (B) it was probed with a mixture of anti-sBPP and anti-mCP. An acetate sheet and a sheet of XAR film were placed between the membrane and imaging plate to block the 35S signal for panel B. B- and C-capsids (denoted between the panels) were determined to be in fractions 6 and 9, respectively, by the pattern of CBB-stained proteins in a separate gel (not shown) and by the peak intensities of mCP following immunoassay (B). NP-40 nuclear (N) and cytoplasmic (C) fractions of nonradiolabeled SCMV-infected cells were included in the leftmost lanes as position markers for the protein bands of interest. The vertical lines between the panels indicate the first and last fractions of the gradient.
Preparation of capsids.
Capsids were recovered from virus-infected cells by rate-velocity sedimentation in sucrose gradients, essentially as described before (28). Three differences from the earlier procedure were as follows: (i) the sucrose solutions were prepared in 500 mM NaCl, 1 mM EDTA, and 20 mM Tris, pH 7.4, to increase binding stringency; (ii) the gradients were 20 to 50% sucrose; and (iii) the time of centrifugation was increased to 30 min.
Assay for BPP-capsid interaction.
Virus-infected cells were separated into nuclear and cytoplasmic fractions by treatment for 2 min on ice with Nonidet P-40 (NP-40; 0.5% in 40 mM sodium phosphate buffer [pH 7.4], 150 mM NaCl) as described before (13). The pelleted nuclei were combined with 1 ml of the same buffer lacking NP-40 and ruptured by 20 passages through a 23-gauge hypodermic needle. Particulate material was cleared from the resulting lysates by centrifugation at 16,000 × g and 4°C for 5 min.
Freshly prepared 35S-BPP-containing reticulocyte lysate was added to freshly prepared NP-40–cytoplasmic- or NP-40–nuclear-lysate fractions. The mixtures were rocked for 15 min at room temperature, placed on ice with occasional mixing for an additional 45 min, and then layered onto 20 to 50% sucrose gradients and subjected to centrifugation at 40,000 rpm at 4°C for 30 min in a Beckman SW41 rotor. The resulting gradients were inspected for light-scattering capsid bands and collected from the top using an ISCO (Lincoln, Neb.) 185 gradient fractionator. Portions of each fraction were solubilized, subjected to gel electrophoresis, and analyzed by staining for proteins, detection of [35S]methionine, and Western immunoassay.
Gel electrophoresis, Western immunoassay, and phosphorimaging.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done essentially as described by Laemmli (25); the gels were 0.75 mm thick and 10% acrylamide, and the SDS was from Bio-Rad (no. 161-0301; Melville, N.Y). Proteins were stained with Coomassie brilliant blue (CBB) (10) or silver (44).
Western immunoassays were done essentially as described by Towbin et al. (41); Immobilon membranes (no. IPVH00010; Millipore, Bedford, Mass.) were used, the electrotransfer buffer was 50 mM Tris–10% methanol, and the time of semidry transfer was determined by the formula 2.5 × gel width (in centimeters) × gel height (in centimeters) = milliamperes per 30 min. The antisera used in the Western assays were (i) anti-sBPP, prepared by immunizing a rabbit with SDS-PAGE-purified sBPP from SCMV nuclear B-capsids (J. Lee and W. Gibson, unpublished data), and (ii) anti-minor capsid protein [mCP], anti-mCP-binding-protein [mCBP], and B-capsid-diagnostic assembly protein (AP)-specific anti-N1, all rabbit antipeptide antisera that have been described before (15, 37). 125I-protein A (no. NEX146L; NEN, Boston, Mass.) was used to detect antibodies bound to protein bands.
Detection and quantification of radioactivity was done by phosphorimaging (Fuji BAS1000 with Mac BAS version 2.5 software). [35S]methionine-labeled proteins were detected by exposing the phosphorimaging plate directly to stained and dried gels or to Immobilon membranes prior to immunoassay. 125I-protein-A was detected following Western immunoassay by exposing the phosphorimaging plate directly to the Immobilon membrane or with a piece of 0.05-mm-thick acetate and a sheet of XAR film interposed to block detection of the 35S signal when necessary.
Nucleotide sequence accession number.
The GenBank accession number of the ORF encoding the SCMV BPP is AF320757.
RESULTS
When GAL4 two-hybrid assays showed no interaction between BPP and the three individual capsid shell proteins (i.e., MCP [pUL86], mCP [pUL85], and mCBP [pUL46]), we investigated the in vitro capsid-binding assay described here as an alternative. Our rationale was that BPP interaction with the capsid may require conformational determinants available only on higher-order structures or assembly intermediates. Intracellular capsids were used as substrates because they have less BPP than virions and noninfectious enveloped particles (13, 22) and were considered more likely to have unoccupied BPP binding sites. We also used SCMV for most experiments rather than HCMV because it typically gives better yields of intracellular capsids (reference 22 and unpublished observations).
SCMV homolog of HCMV ORF UL32 identified.
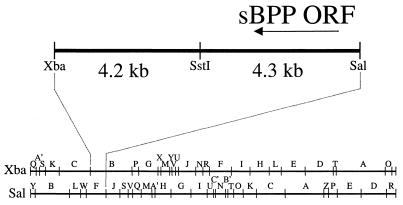
In order to do these experiments with SCMV, it was necessary to identify, clone, and express the SCMV gene encoding the homolog of HCMV BPP. Based on the genomic colinearity between SCMV and HCMV, and using the available restriction mapping data for the SCMV genome (26), the SCMV BPP coding sequence had been mapped to the XbaI B and SalI F fragments (L. Robson, J. Lee, and W. Gibson, unpublished results). The region of overlap was predicted to be an ∼8.5-kb XbaI/SalI fragment (Fig. 1). This fragment was isolated from genomic SCMV DNA, cloned into the vector pBluescript II SK(+), and subjected to nucleotide sequencing, as described in Materials and Methods.
FIG. 1.
Genomic location of the SCMV BPP homolog of HCMV UL32. The bottom two lines show the genomic location of the 8.5-kb XbaI/SalI fragment that contains the SCMV BPP gene, based on available restriction maps (26) and previous mapping data (L. Robson and W. Gibson, unpublished results). The top line shows an expanded view of this fragment. The region bounded by SstI and SalI was sequenced in its entirety on both strands. The arrow indicates the position and orientation of the coding sequence for the 2.3-kb SCMV BPP gene (sBPP ORF).
A 2.3-kb ORF was identified that is predicted to encode a 762-amino acid protein with sequence similarity to the HCMV BPP (Fig. 1). Alignment of the protein sequence with those of the HCMV, chimpanzee CMV (CCMV), rat CMV, and mouse CMV (MCMV) BPP homologs and two other beta-herpesvirus BPP homologs is presented in Fig. 2. This alignment recognized 52 (17%) similar, including 15 (5%) identical, amino acids among the seven homologs, all within the first ≈300 residues. The conservation within this region was much higher among the three primate CMV homologs (65% similar; 44% identical) and highest between the HCMV and CCMV homologs (85% similar; 72% identical). For reference, we have indicated (i) the 15 absolutely conserved residues, which include the only cysteine present in the HHV-6 BPP, (ii) the two most conserved regions (CR1 and CR2), and (iii) a cysteine tetrad (Cys-X7-Cys-X9-Cys-X7-Cys) near CR2 whose spacing is conserved among the primate CMV BPP homologs and which may have a counterpart in the MCMV BPP (i.e., Cys363-Cys389). SCMV BPP is 286 amino acids shorter than its HCMV homolog and lacks the O-GlcNAc attachment sites mapped on HCMV BPP to Ser921 and Ser952 (19). The CCMV homolog, however, which is only 34 amino acids shorter than HCMV BPP, does contain two serines that align if the scoring matrix is changed (i.e., Ser884 and Ser915 [alignment data not shown]).
BPP binds to isolated CMV capsids.
B- and C-capsids were recovered from the NP-40 nuclear fraction of SCMV-infected cells (about 0.5 ml/band), mixed, and combined with 30 μl of rabbit reticulocyte lysate containing in vitro-synthesized 35S-sBPP. The same was done with B- and C-capsids recovered from the NP-40 cytoplasmic fraction. Following incubation, the preparations were diluted 1:1 with 40 mM phosphate buffer (pH 7.4) containing 150 mM NaCl, layered onto sucrose gradients, and subjected to centrifugation. Light-scattering B- and C-capsid bands were noted, the resulting gradients were fractionated, and duplicate sets of samples were subjected to parallel SDS-PAGE and Western immunoassay, all as described in Materials and Methods.
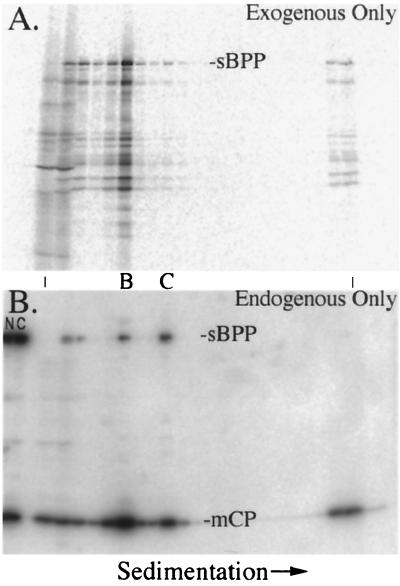
Phosphorimages were prepared from the stained and dried gels (Fig. 3) and from the membranes after probing them with a mixture of antisera to three capsid proteins (i.e., mCP, mCBP, and AP) (Fig. 3, insets). Measurements from these data showed the following. (i) Most of the 35S-sBPP in the gradient containing nuclear capsids moved from the loading volume into the capsid-resolving portion of the gradient (i.e., fractions 8 to 12). Eighty percent of the total BPP cosedimented with B-capsids in fractions 9 and 10 (distinguished by the AP band), where it represented 32% of the radioactivity in those lanes, and ≤1% was present with C-capsids in fraction 12 (Fig. 3A). (ii) Less 35S-sBPP moved from the loading volume into the capsid-resolving region of the gradient containing cytoplasmic capsids. Twenty-six percent of the total cosedimented with B-capsids in fractions 8 and 9, and 6% cosedimented with C-capsids in fraction 11 (Fig. 3B). The lower percentage of capsid-bound BPP in the cytoplasmic preparation is attributed primarily to the ∼6-fold-reduced amount of B- plus C-capsids in that gradient, as estimated from the amount of triplex proteins (Table 1). (iii) B-capsids bound at least 10-fold more 35S-sBPP than C-capsids in the nuclear preparation and about 5-fold more in the cytoplasmic preparation (Table 1). We attach greater significance to the general finding that B-capsids bind more 35S-sBPP than C-capsids than to the specific magnitude of this difference. (iv) When capsids were omitted from the starting sample, 99% of the 35S-sBPP (and smaller radiolabeled species) remained in the loading volume (the first two fractions), and the small amount that entered the gradient trailed off quickly, with no evidence of oligomerization or aggregation (data submitted at review but not shown here).
FIG. 3.
SCMV BPP binds to isolated SCMV capsids in vitro. Nuclear and cytoplasmic capsids were combined with 35S-sBPP and subjected to rate-velocity centrifugation; the resulting gradients were fractionated, and duplicate sets of samples were subjected to SDS-PAGE (separate gels for each gradient) and Western immunoassay (a single gel and membrane containing all cytoplasmic and nuclear gradient fractions). Phosphorimages of the nuclear (A) and cytoplasmic (B) gradient fractions following SDS-PAGE are shown. The insets show a portion of the Western immunoassay after the membrane was probed to detect mCP, mCBP, and AP. B and C denote fractions containing B- and C-capsids. The B-capsid peak was split between two fractions in both gradients. The nuclear capsid preparation (3.5 ml) and the cytoplasmic capsid preparation (2.5 ml) were loaded onto the gradients; that material is in the first 7 (A) or 5 (B) fractions, labeled Load. Pel denotes the pellet. The arrow in panel B indicates the location of the SCMV BPP band. The asterisks denote proteins in the BPP translation mixture (the leftmost lane in both panels) that did not bind well to the capsids. The numbers at the bottoms of the panels and insets indicate corresponding fraction numbers.
TABLE 1.
Relative amounts of 35S-sBPP bound to B- and C-capsids isolated from the nuclear and cytoplasmic fractions of SCMV-infected cells
| Virus particle | 35S-sBPPa (A) | Triplexb (B) | 35S-sBPP/triplex (A/B) | Ratioc |
|---|---|---|---|---|
| Nuclear | ||||
| B-capsid | 12,402 | 24,540 | 0.50 | ≥10:1 |
| C-capsid | ≤150 | 3,130 | ≤0.05 | |
| Cytoplasmic | ||||
| B-capsid | 1,553 | 2,190 | 0.71 | 4.7:1 |
| C-capsid | 355 | 2,344 | 0.15 |
Phosphorimaging measurements were made from the CBB-stained dried gels shown in Fig. 3. The sBPP bands quantified are from lanes 9, 10 (nuclear B-capsids), and 12 (nuclear C-capsids) of Fig. 3A and lanes 8, 9 (cytoplasmic B-capsids), and 11 (cytoplasmic C-capsids) of Fig. 3B. The numbers are in units of photostimulated luminescence (PSL; proportional to the amount of radioactivity present) and are corrected for background.
Phosphorimaging measurements were made from the bands shown in the Fig. 3 insets. Triplex protein bands (i.e., mCP and mCBP, in the lanes specified in footnote a) were quantified separately and corrected for background, and the PSL values were combined to give the numbers shown. The triplex proteins are in a fixed copy number in capsids (15) and were used to normalize the 35S-sBPP measurements. Triplex values for nuclear and cytoplasmic capsids were obtained from the same coprocessed membrane and are directly comparable.
Calculated as (A/B for B-capsids) divided by (A/B for C-capsids).
The shorter-than-full-length species synthesized in vitro (e.g., Fig. 3, leftmost lanes) are taken to be fragments of sBPP, based on the findings that (i) many were immunoprecipitated by a rabbit antiserum to full-length sBPP (data submitted at review but not shown here) and (ii) all are larger than 6.1 kDa, the largest protein predicted to be encoded by ORFs out of phase with and unrelated to the cloned sBPP sequence. Considering that more than half of these fragments bind to capsids (Fig. 3 to 6) and that capsid binding by BPP is mediated through its amino end (see below), it is likely that many of the fragments result from premature termination of translation in vitro. Several smaller proteins in the starting preparation did not bind to capsids (e.g., Fig. 3A), showing that binding among the translation products is selective.
FIG. 6.
Amino one-third of BPP is required for its capsid interaction. Lysates of nuclei from SCMV-infected cells were combined with 35S-sBPP or with [35S]methionine-labeled truncation mutants of sBPP and assayed by centrifugation, SDS-PAGE, and phosphorimaging. Shown here are the relevant portions of the resulting phosphorimages. The proteins tested were SCMV BPP (amino acids 1 to 762) (A); the carboxyl truncations δC321 (amino acids 1 to 441) (B), δC487 (amino acids 1 to 275) (C), and δC568 (amino acids 1 to 194) (D); the amino truncations δN275 (amino acids 276 to 762) (E), δN193 (amino acids 194 to 762) (F), and δN99 (amino acids 100 to 762) (G); and a mutant lacking both amino and carboxyl sequences, δN193/δC487 (amino acids 194 to 275) (H). The data are from two separate experiments, the first shown in panels A to D and the second shown in panels E to H. Because of its small size, the gradient samples for the 9.5-kDa double-deletion mutant, 194 to 275, were subjected to SDS-PAGE in two parallel Tricine 10 to 20% gradient gels (no. EC66255; Novex, San Diego, Calif.). The fractions containing B-capsids were identified by protein staining and are indicated by the letter B. The asterisks denote the full-length test protein. The amino acid sequence represented by the test protein is indicated in the lower right-hand corner of each panel. Samples of the starting reticulocyte preparations are shown in the leftmost lane of each panel. The mutant proteins are depicted schematically in Fig. 7.
BPP binds to capsids when added to lysates of infected cells.
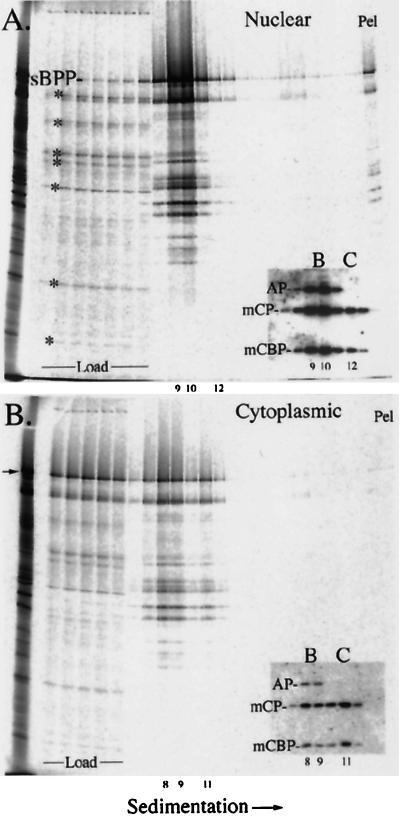
The binding of sBPP to capsids in cruder preparations was tested by adding in vitro-synthesized 35S-sBPP to a lysate prepared from the NP-40 nuclear fraction of infected cells. After the mixture was incubated for 60 min, it was subjected to rate-velocity centrifugation and gradient fractionation. Portions of the resulting gradient fractions were solubilized and subjected to SDS-PAGE, and the proteins were electrotransferred to an Immobilon membrane.
Phosphorimage analysis of the membrane before and after Western immunoassay was done such that the exogenous 35S-sBPP added to the reaction mixture (Fig. 4A; there was insufficient protein mass to be detected in Western assay) and the endogenous BPP already present in the lysates and on capsids (detected only by Western immunoassay; Fig. 4B) could be measured independently and compared. We found that 70% of the full-length 35S-sBPP in the gradient was in fraction 6 and that 1% was in fraction 9 (Fig. 4A). These were the fractions containing B- and C-capsids, respectively, as determined by CBB staining (separate gel not shown) and by the relative intensity peaks of mCP in the Western immunoassay (Fig. 4B). The Western assay also showed that endogenous BPP was more abundant on C-capsids than on B-capsids (Fig. 4B; see the BPP bands at B- and C-capsid positions), in contrast to the added 35S-sBPP. Calculations made from these data indicate that nuclear B-capsids contain ≈6-fold less endogenous BPP than C-capsids from the same preparation and bind ≈12-fold more exogenous 35S-sBPP (Table 2)—close to the ≈10-fold difference in 35S-sBPP binding calculated for isolated nuclear B- versus C-capsids (Table 1). When the experiment was repeated with a lysate prepared from noninfected HFF cells, 96% of all 35S-sBPP remained in the loading volume (the first two 0.5-ml fractions) with no evidence of aggregated material in the gradient or pellet fractions (data submitted at review but not shown here).
TABLE 2.
Relative amounts of exogenous 35S-sBPP and endogenous BPP bound to B- and C-capsids in nuclear preparations of SCMV-infected cells
| Virus particle | BPP
|
Triplex
|
BPP/triplex
|
||||
|---|---|---|---|---|---|---|---|
| 35Sa (A) | Westernb (B) | Westernc (C) | 35S (A/C) | Ratiod | Western (B/C) | Ratioe | |
| B-capsid | 2,710 | 1,177 | 22,791 | 0.12 | 12:1 | 0.05 | 1:6 |
| C-capsid | 51 | 1,920 | 6,042 | 0.01 | 0.32 | ||
Phosphorimaging measurements were made from CBB-stained dried gel. The samples in the gel were the same as those shown after electrotransfer to Immobilon membranes in Fig. 4A; measurements were from corresponding lanes 6 (B-capsids) and 9 (C-capsids). The numbers are in units of photostimulated luminescence (PSL; proportional to the amount of radioactivity present) and were corrected for background.
PSL of BPP bands on Immobilon membrane shown in Fig. 4B, lanes 6 (B-capsids) and 9 (C-capsids).
PSL of mCP bands on Immobilon membrane shown in Fig. 4B, lanes 6 (B-capsids) and 9 (C-capsids). mCP is one of the two triplex proteins and is present in a fixed copy number in capsids (15); it was used to normalize the 35S-sBPP measurements to the relative amounts of B- and C-capsids.
Calculated as (A/C for B-capsids) divided by (A/C for C-capsids).
Calculated as (B/C for B-capsids) divided by (B/C for C-capsids).
Evidence that BPP binding is specific.
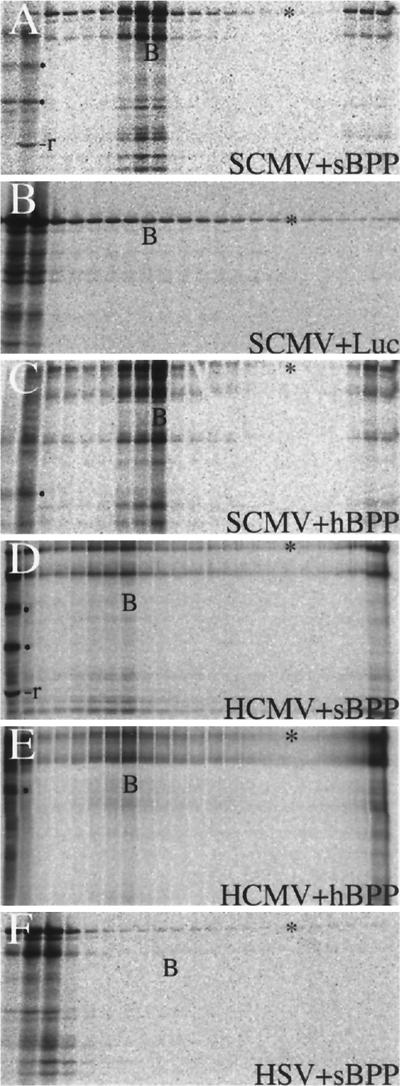
To increase confidence that BPP binding to CMV capsids is specific, we did the following controls. First, we repeated the binding assay with [35S]methionine-labeled luciferase, an unrelated protein that would not be expected to interact with BPP-specific sites on the capsid. In the positive control, 66% of the 35S-sBPP bound to B-capsids (Fig. 5A, fractions 8 and 9), but there was no evidence for association of 35S-luciferase with B-capsids (Fig. 5B; i.e., no luciferase peak and <0.4% of the total at the B-capsid position).
FIG. 5.
SCMV BPP binding to capsids shows specificity. Equal amounts of nuclear lysate from SCMV-infected cells (SCMV) were combined with 35S-sBPP (A), [35S]methionine-labeled luciferase (Luc) (B), or 35S-hBPP (C). In a separate experiment, an equal amount of nuclear lysate from HCMV-infected cells (HCMV) was combined with 35S-sBPP (D) or 35S-hBPP (E). The pellet fractions of these two gradients were approximately 10-fold more concentrated than those of the other gradients. (F) In a third experiment, the nuclear lysate from HSV-infected cells (HSV) was combined with 35S-sBPP. Each preparation was incubated, subjected to centrifugation and gradient fractionation, and then analyzed by SDS-PAGE of the gradient fractions, followed by protein staining with CBB (SCMV and HSV preparations) or silver (HCMV preparations) and phosphorimaging of the dried gels. Shown here are the resulting phosphorimages. The leftmost two or three lanes represent the loading volume. The fractions containing B-capsids were identified by protein staining in all gradients and are indicated in the corresponding lanes by the letter B. In Panels A, D, E, and F, B-capsids were split between two fractions. The asterisks denote the protein band in each gel corresponding to the full-length in vitro translation product.
We next tested whether the closely related HCMV BPP would bind to SCMV capsids. In vitro-synthesized, 35S-hBPP was added to nuclear and cytoplasmic lysates of SCMV-infected cells and assayed as described above for binding to capsids. Its distribution in the gradient was similar to that of 35S-sBPP, and 39% of the total was present with SCMV B-capsids in fraction 9 (Fig. 5, compare panels A and C; similar data not shown for the cytoplasmic fraction). The reciprocal experiment was also done to determine whether SCMV BPP (and HCMV BPP) binds to HCMV capsids. The HCMV intracellular B-capsid bands were much weaker than those of SCMV, as expected (22), and were determined (from a silver-stained gel not shown) to be most concentrated in fraction 8 of each gradient, which contained ≈10% of the total 35S-sBPP (Fig. 5D) and 35S-hBPP (Fig. 5E). The lower percentage of bound 35S-BPP in these HCMV gradients is thought to reflect the lesser amount of capsids present in the starting lysates.
An additional test for specificity was to determine whether the CMV BPP would bind to HSV B-capsids, which are grossly similar to CMV B-capsids (e.g., in size, subunit structure, and protein counterparts) (43) and could conceivably bind CMV BPP through such shared general features. The possibility of a sequence-specific interaction between the two, however, seemed unlikely because the HSV B-capsid proteins themselves have little sequence homology with their CMV counterparts and HSV has no recognized homolog of CMV BPP (7, 18, 32).
HSV capsids were recovered from monkey kidney (Vero) cells, because yields from HFF are low, and protease inhibitors (no. 1836153; Roche, Indianapolis, Ind.) were included in the lysis buffer from this point on to overcome BPP proteolysis observed in an initial experiment with Vero cell lysates (data not shown). The results of the experiment showed no evidence of BPP binding to either HSV B- or C-capsids (Fig. 5F, <0.3% of total BPP was in gradient with the B-capsid fraction), even though the capsid amounts were equal to or greater than those of SCMV (data not shown). Thus, BPP binding to CMV capsids is not due to some general feature of the capsid shared by all herpesviruses.
Sequences within amino one-third of SCMV BPP required for capsid binding.
The finding that HCMV BPP binds to SCMV capsids and vice versa (Fig. 5C and D), and the observation that the strongest amino acid similarities between sBPP and hBPP are at their amino ends (Fig. 2), suggested that BPP may interact with the capsid through these sequences. This was tested by using a set of amino and carboxyl deletion mutants, as summarized in Fig. 6 and 7. SCMV BPP (Fig. 6A) and a deletion mutant lacking the carboxyl 321 amino acids bound well to B-capsids (δC321 [Fig. 6B]). The small amounts of full-length BPP in the preparations of all three carboxyl truncation mutants (e.g., Fig. 6B and C, top left) resulted from incomplete cleavage of plasmid MB150 prior to its use in the reticulocyte lysate. A second carboxyl deletion mutant, constructed to retain CR2 and the Cys tetrad, also bound well to B-capsids (δC487 [Fig 6C]), but the third carboxyl truncation mutant, constructed to remove that same CR2-Cys tetrad sequence, showed no binding (δC568 [Fig. 6D]). These results implicate the amino 275 residues of sBPP as the capsid-binding region (Fig. 7).
FIG. 7.
Summary of BPP truncation mutants. The results of capsid-binding experiments with truncation mutants of SCMV BPP (Fig. 6) are summarized. A schematic of each protein tested is shown, indicating landmarks present or absent. The boxes represent CR1 and CR2, and the gray ovals represent the cysteine tetrad, both described in the legend to Fig. 2. The solid circles represent Cys41, which is absolutely conserved among all beta-herpesvirus BPP homologs. +, strong capsid binding; −, no binding; −∗, no binding to B-capsids but perceptibly increased radioactivity in the C-capsid fraction. The data panels indicate the panel in Fig. 6 showing the test protein.
This was tested more directly by deleting the first 275 residues of sBPP to create mutant δN275 (Fig. 7), which showed no capsid binding (Fig. 6E). Two shorter amino-end deletions were also tested. One (δN193) removed residues 1 to 193, leaving CR2 and the Cys tetrad; the other (δN99) removed residues 1 to 99, leaving CR2 and the Cys tetrad but eliminating Cys41 and CR1 (Fig. 7). In addition, we tested a double deletion containing only the sequence Ala194 to Asp275 (δN193/δC487 [Fig. 7]), which made the difference between binding (δC487) and not binding (δC568) to capsids (Fig. 6C and D). None of these amino deletions showed significant binding to B-capsids (Fig. 6E to G), indicating that the first 99 amino acids of sBPP also contain B-capsid-binding determinants. We have not investigated the indication that all of the amino-deletion mutants containing residues 194 to 275 showed weak binding to C-capsids (i.e., two to three-fold above flanking lanes [Fig. 6F, G, and H]). These data are summarized in Fig. 7 and indicate that binding of sBPP to B-capsids involves (i) the sequence that includes CR2 and the Cys tetrad (i.e., its deletion eliminated binding [Fig. 6D and E]) and (ii) the amino-terminal sequence containing the absolutely conserved Cys and CR1 (i.e., its deletion eliminated binding [Fig. 6G]).
DISCUSSION
There is evidence that the CMV BPP interacts directly and strongly with the capsid (14, 43). When our attempts to investigate this interaction through pairwise GAL4 two-hybrid screens of the capsid proteins were unsuccessful, we tested an assay intended to detect BPP interactions with either individual proteins or higher-order assemblages. By combining in vitro-synthesized 35S-BPP with isolated capsids or with infected-cell lysates, we were able to demonstrate that BPP binds selectively to CMV capsids and that the interaction requires sequences within its amino end.
Most of our experiments were done with SCMV, which required first identifying and cloning the SCMV BPP ORF. Comparison of its sequence with those of the other beta-herpesvirus BPP homologs (Fig. 2) showed that it is most similar to the HCMV and CCMV counterparts, but shorter, and that all have sequence conservation through their first ≈300 amino acids but much less similarity after that. The amino end of SCMV BPP contains (i) two conserved regions, CR1 and CR2, that are represented among all beta-herpesvirus BPP homologs; (ii) an absolutely conserved cysteine (e.g., SCMV Cys41), the only one present in the human herpesvirus 6 (HHV-6) homolog; and (iii) a cluster of four other cysteines in a Cys-X7-Cys-X9-Cys-X7-Cys tetrad that is present in the three primate CMV BPPs, possibly with a counterpart in MCMV beginning at Cys363. The less conserved carboxyl end of SCMV BPP is 286 amino acids shorter than HCMV BPP, in line with its smaller size as estimated by SDS-PAGE. It is unexplained, however, why SDS-PAGE size estimates for both proteins (i.e., ≈115 and ≈150 kDa, respectively) are so much larger than predicted (i.e., 85 and 113 kDa, respectively). Among the HCMV sequences missing from SCMV BPP are those that contain the two serines modified by O-linked N-acetylglucosamine (19), explaining why this sugar was not detected on SCMV BPP (2). Considering that these serines do not appear to be conserved in any of the other BPP homologs, with the possible exception of CCMV (see Results), it will be interesting to determine whether they are conserved and modified in all strains of HCMV and, if so, whether they may represent a functionally significant evolutionary change.
Our main conclusion that BPP binds with specificity to CMV capsids was based initially on the finding that, when in vitro-synthesized BPP was combined with isolated capsids or infected-cell lysates, it cosedimented with B- and, to a lesser extent, C-capsids. The finding that this interaction occurred in crude lysates of infected cells containing a broad range and comparatively high concentrations of potentially competing molecules suggested some degree of specificity, which was substantiated in several ways. First, the closely related HCMV BPP bound approximately as well to SCMV capsids, but an unrelated protein, luciferase, did not bind. Second, SCMV BPP bound well to HCMV capsids, whose proteins are closely related to those of SCMV capsids, but not to HSV capsids, whose proteins have much less sequence similarity to their SCMV counterparts. Third, BPP bound B-capsids preferentially to C-capsids, which have more endogenous BPP (Fig. 3 and Tables 1 and 2) (13). The last observation is consistent with a specific inhibition by endogenous BPP on the binding of exogenous radiolabeled BPP and is compatible with a saturable number of capsid-binding sites for BPP.
The interchangeability of the SCMV and HCMV BPPs in binding to capsids, and the higher degree of sequence conservation at their amino ends, suggested that residues toward the amino end of BPP might mediate capsid binding. This was supported by evidence from BPP deletion mutants, which showed that the sequence Met1 to Asp275 was both necessary (e.g., δC568 and δN275) and sufficient (e.g., δC487) for binding to B-capsids (Fig. 6D, G, and C, respectively). Additionally, we have recently determined that this amino 275-amino-acid fragment of BPP will direct green fluorescent protein (as a BPP-δC487 GFP fusion protein) to bind B-capsids (data not shown). Deletions in this 275-amino-acid fragment from either end profoundly reduced or eliminated its capsid binding. These results could be accounted for by two separate interactions being required for BPP binding, one mediated by the CR1-containing sequence 1 to 99 and the other mediated by the CR2-containing sequence 194 to 275. This would be compatible with the two separate contacts observed by cryoelectron microscopy for the tegument protein extending outward from the triplexes and bridging to the capsomer-capping protein (43). Alternatively, the capsid-binding domain of BPP may be composed of multiple nonlinear elements that collectively form a conformational binding interface. Although our data do not distinguish between these possibilities, or necessarily eliminate other less straightforward interpretations, they do suggest targets for site-directed mutagenesis (e.g., Cys41, CR1, CR2, and the Cys tetrad) that are expected to refine understanding of the BPP-capsid interaction.
Our efforts to demonstrate pairwise interactions of BPP with the individual capsid proteins by using GAL4 two-hybrid assays have not yet been productive. One explanation for this is that our GAL4 test constructs may be sterically hindered by their fusion domains and unable to interact. Another is that the capsid-binding sites for BPP may become available only after the protein(s) involved has been incorporated into the capsid and has possibly also undergone maturational rearrangements. Such conformational changes occur in both bacteriophage (1, 4, 5, 39) and eukaryotic viruses, including HSV (42), and may help drive the assembly process forward. If BPP or other proteins do associate with nascent particles through sites formed sequentially during the maturation process, assays such as the one described here may be necessary to study their interactions.
ACKNOWLEDGMENTS
We thank Prashant Desai and Stan Person for providing HSV-infected Vero cells for the experiment shown in Fig. 5F and for discussions of similar experiments with HSV VP26 (unpublished data). We also thank Jenny Borchelt and Dustin Rush for technical support and acknowledge the JHMI Biosynthesis and Sequencing Facility for sequence analysis of the SCMV BPP gene and validation of all BPP mutant constructs.
M.K.B. was a student in the Biochemistry, Cellular, and Molecular Biology graduate program. This work was aided by USPHS research grant AI13718 to W.G. from the Allergy and Infectious Diseases branch of NIH.
REFERENCES
- 1.Aebi U, van Driel R, Bijlenga R K L, ten Heggeler B, van der Broek R, Steven A C, Smith P R. Capsid fine structure of T-even bacteriophages. Binding and localization of two dispensable capsid proteins into the p23* surface lattice. J Mol Biol. 1977;110:687–698. doi: 10.1016/s0022-2836(77)80084-3. [DOI] [PubMed] [Google Scholar]
- 2.Benko D M, Haltiwanger R S, Hart G W, Gibson W. Virion basic phosphoprotein from human cytomegalovirus contains O-linked N-acetylglucosamine. Proc Natl Acad Sci USA. 1988;85:2573–2577. doi: 10.1073/pnas.85.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuken E, Grauls G, Bruggeman C A, Vink C. The rat cytomegalovirus R32 gene encodes a virion-associated protein that elicits a strong humoral immune response in infected rats. J Gen Virol. 1999;80:2719–2728. doi: 10.1099/0022-1317-80-10-2719. [DOI] [PubMed] [Google Scholar]
- 4.Black L W, Showe M K. Morphogenesis of the T4 head. Washington, D.C.: American Society for Microbiology; 1984. [Google Scholar]
- 5.Casjens S, King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- 6.Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan J S, Bittner A, Frueh K, Jackson M R, Peterson P A, Erlander M G, Ghazal P. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J Virol. 1999;73:5757–5766. doi: 10.1128/jvi.73.7.5757-5766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 8.Chen D H, Jiang H, Lee M, Liu F, Zhou Z H. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology. 1999;260:10–16. doi: 10.1006/viro.1999.9791. [DOI] [PubMed] [Google Scholar]
- 9.Desai P, DeLuca N A, Person S. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology. 1998;247:115–124. doi: 10.1006/viro.1998.9230. [DOI] [PubMed] [Google Scholar]
- 10.Fairbanks G, Steck T L, Wallach D F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 11.Gibson W. Protease-facilitated transfer of high-molecular-weight proteins during electrotransfer to nitrocellulose. Anal Biochem. 1981;118:1–3. doi: 10.1016/0003-2697(81)90147-0. [DOI] [PubMed] [Google Scholar]
- 12.Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983;128:391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- 13.Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981;111:516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- 14.Gibson W. Structure and assembly of the virion. Intervirology. 1996;39:389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- 15.Gibson W, Baxter M K, Clopper K S. Cytomegalovirus “missing” capsid protein identified as heat-aggregable product of human cytomegalovirus UL46. J Virol. 1996;70:7454–7461. doi: 10.1128/jvi.70.11.7454-7461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson W, Irmiere A. Selection of particles and proteins for use as human cytomegalovirus subunit vaccines. Birth Defects. 1984;20:305–324. [PubMed] [Google Scholar]
- 17.Gibson W, Roizman B. Proteins specified by herpes simplex virus. X. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974;13:155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E D, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 19.Greis K D, Gibson W, Hart G W. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J Virol. 1994;68:8339–8349. doi: 10.1128/jvi.68.12.8339-8349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hensel G, Meyer H, Gartner S, Brand G, Kern H F. Nuclear localization of the human cytomegalovirus tegument protein pp150 (ppUL32) J Gen Virol. 1995;76:1591–1601. doi: 10.1099/0022-1317-76-7-1591. [DOI] [PubMed] [Google Scholar]
- 21.Irmiere A, Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983;130:118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- 22.Irmiere A, Gibson W. Isolation of human cytomegalovirus intranuclear capsids, characterization of their protein constituents, and demonstration that the B-capsid assembly protein is also abundant in noninfectious enveloped particles. J Virol. 1985;56:277–283. doi: 10.1128/jvi.56.1.277-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahn G, Kouzarides T, Mach M, Scholl B C, Plachter B, Traupe B, Preddie E, Satchwell S C, Fleckenstein B, Barrell B G. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J Virol. 1987;61:1358–1367. doi: 10.1128/jvi.61.5.1358-1367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahn G, Scholl B-C, Traupee B, Fleckenstein B. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J Gen Virol. 1987;68:1358–1367. doi: 10.1099/0022-1317-68-5-1327. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.LaFemina R L, Hayward G S. Structural organization of the DNA molecules from human cytomegalovirus. In: Fields B N, Jaenisch R, editors. Animal virus genetics. New York, N.Y: Academic Press; 1980. pp. 39–55. [Google Scholar]
- 27.Landini M-P, Re M C, Mirolo G, Galdassarri B, La Placa M. Human immune response to cytomegalovirus structural polypeptides studied by immunoblotting. J Med Virol. 1985;17:303–311. doi: 10.1002/jmv.1890170403. [DOI] [PubMed] [Google Scholar]
- 28.Lee J Y, Irmiere A, Gibson W. Primate cytomegalovirus assembly: evidence that DNA packaging occurs subsequent to B capsid assembly. Virology. 1988;167:87–96. doi: 10.1016/0042-6822(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 29.Lemaster S, Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of polypeptides phosphorylated in the virion. J Virol. 1980;35:798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer H H, Ripalti A, Landini M P, Radsak K, Kern H F, Hensel G M. Human cytomegalovirus late-phase maturation is blocked by stably expressed UL32 antisense mRNA in astrocytoma cells. J Gen Virol. 1997;78:2621–2631. doi: 10.1099/0022-1317-78-10-2621. [DOI] [PubMed] [Google Scholar]
- 31.Morrison E E, Wang Y F, Meredith D M. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol. 1998;72:7108–7114. doi: 10.1128/jvi.72.9.7108-7114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nii S, Uno F, Yoshida M, Akatsuka K. Structure and assembly of human beta herpesviruses. Nippon Rinsho. 1998;56:22–28. . (In Japanese.) ***** [PubMed] [Google Scholar]
- 34.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986;59:714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez V, Greis K D, Sztul E, Britt W J. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol. 2000;74:975–986. doi: 10.1128/jvi.74.2.975-986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenk P, Woods A S, Gibson W. The 45-kDa protein of cytomegalovirus (Colburn) B-capsids is an amino-terminal extension form of the assembly protein. J Virol. 1991;65:1525–1529. doi: 10.1128/jvi.65.3.1525-1529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scholl B-C, Von Hintzenstern J, Borisch B, Traupe B, Broker M, Jahn G. Prokaryotic expression of immunogenic polypeptides of the large phosphoprotein (pp150) of human cytomegalovirus. J Gen Virol. 1988;69:1195–1204. doi: 10.1099/0022-1317-69-6-1195. [DOI] [PubMed] [Google Scholar]
- 39.Steven A C, Couture E, Aebi U, Showe M K. Structure of T4 polyheads. II. A pathway of polyhead transformations as a model for T4 capsid maturation. J Mol Biol. 1976;106:187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- 40.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trus B L, Booy F P, Newcomb W W, Brown J C, Homa F L, Thomsen D R, Steven A C. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 43.Trus B L, Gibson W, Cheng N, Steven A C. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J Virol. 1999;73:2181–2192. doi: 10.1128/jvi.73.3.2181-2192.1999. . (Erratum, 73:4530.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray W, Boulakis T, Wray V P, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 45.Zipeto D, Baldanti F, Percivalle E, Gerna G, Milanesi G. Identification of a human cytomegalovirus mutant in the pp150 matrix phosphoprotein gene with a growth-defective phenotype. J Gen Virol. 1993;74:1645–1648. doi: 10.1099/0022-1317-74-8-1645. [DOI] [PubMed] [Google Scholar]