Abstract
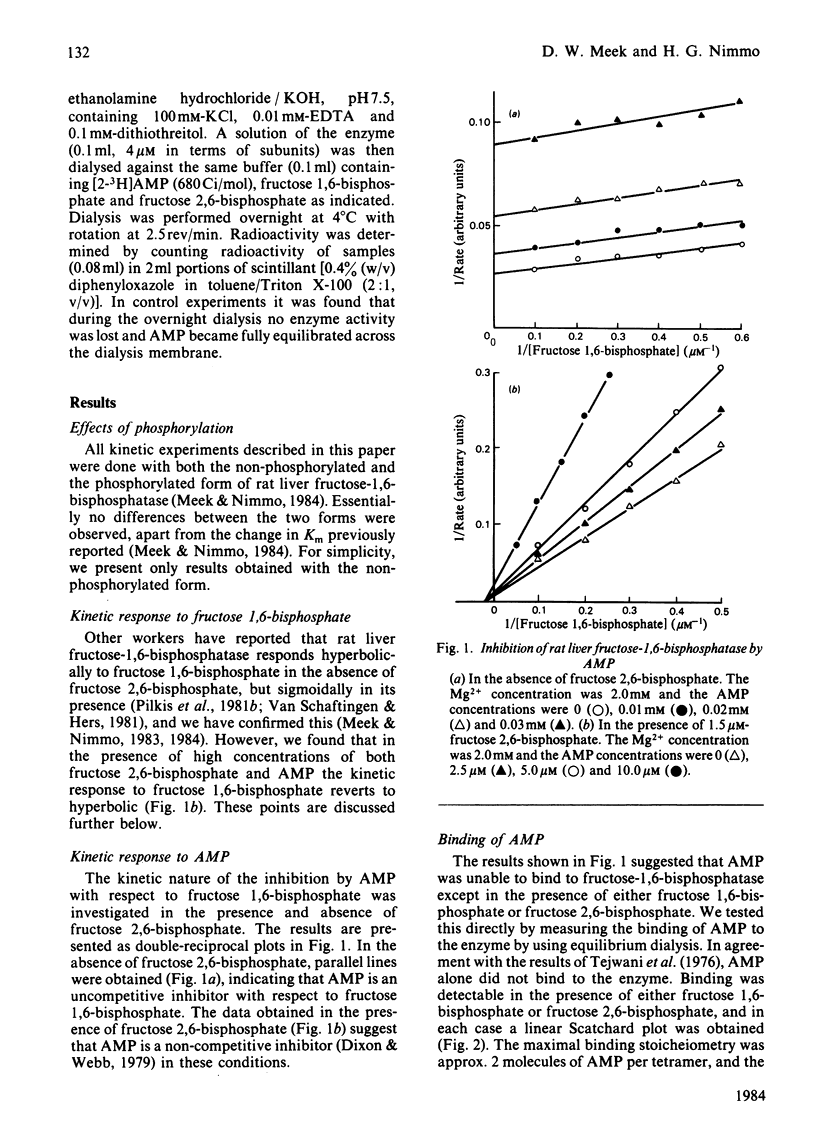
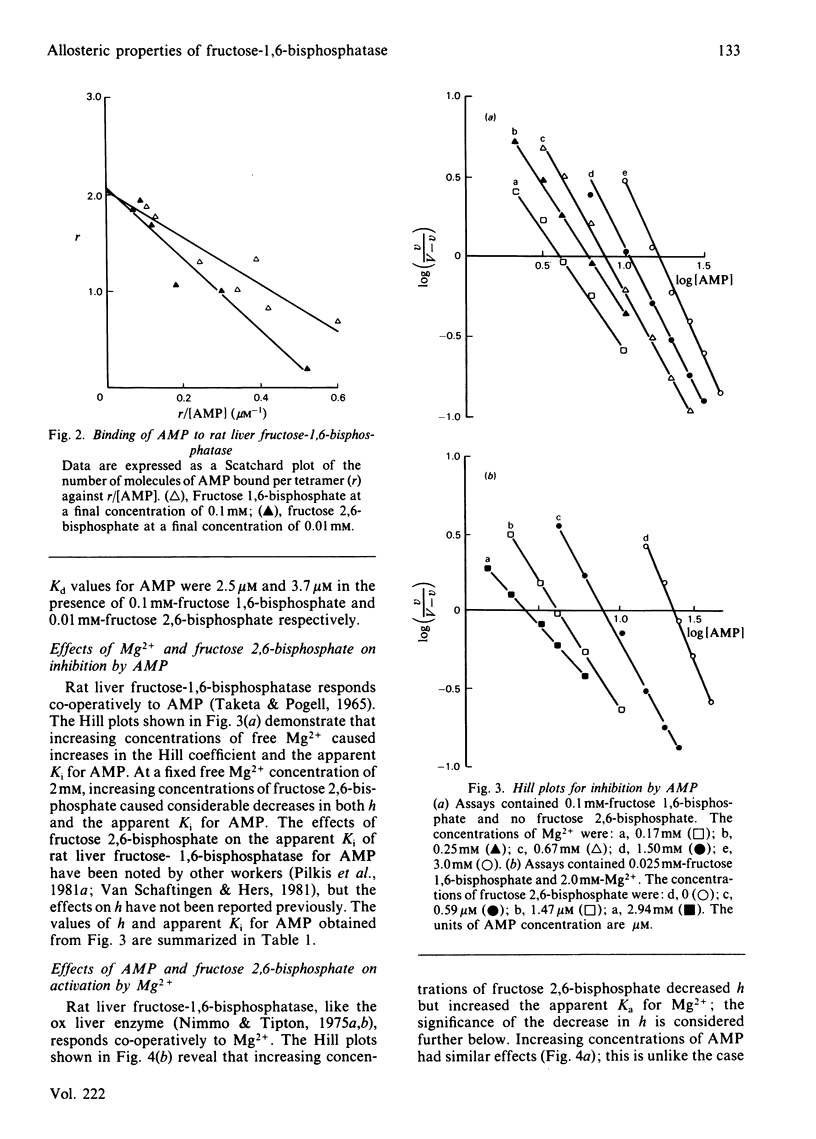
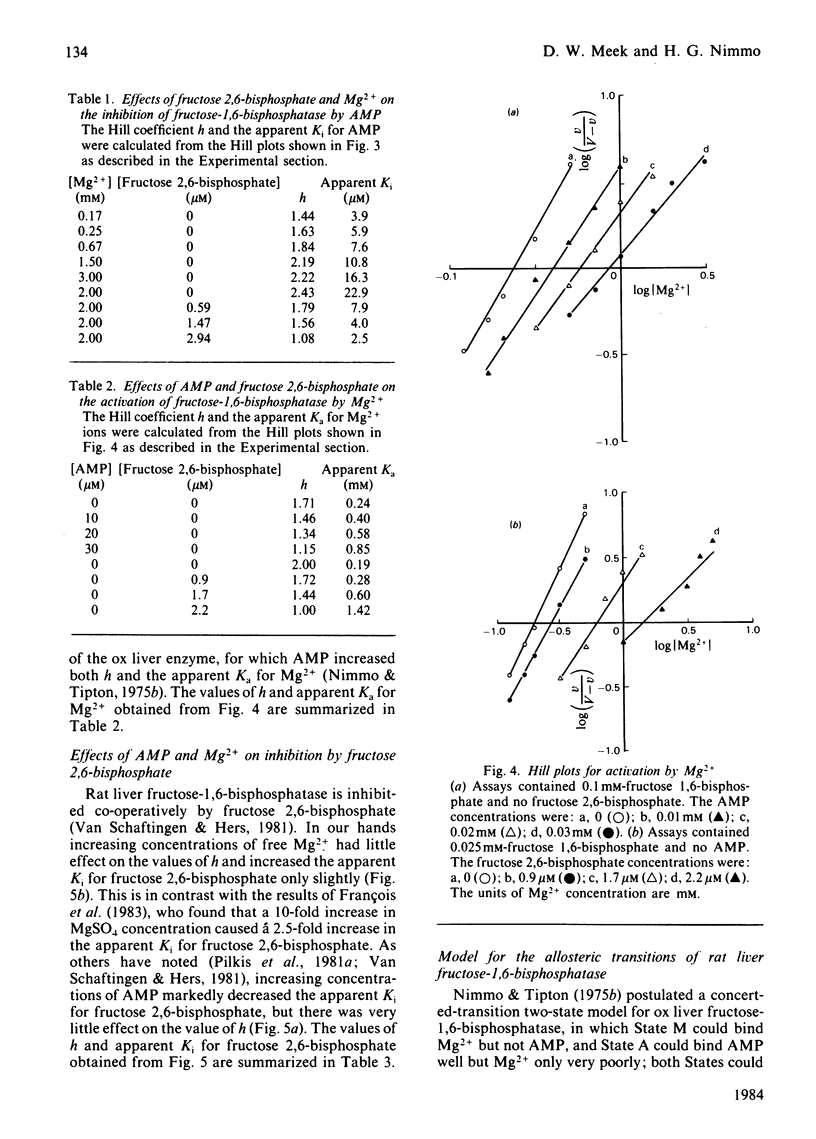
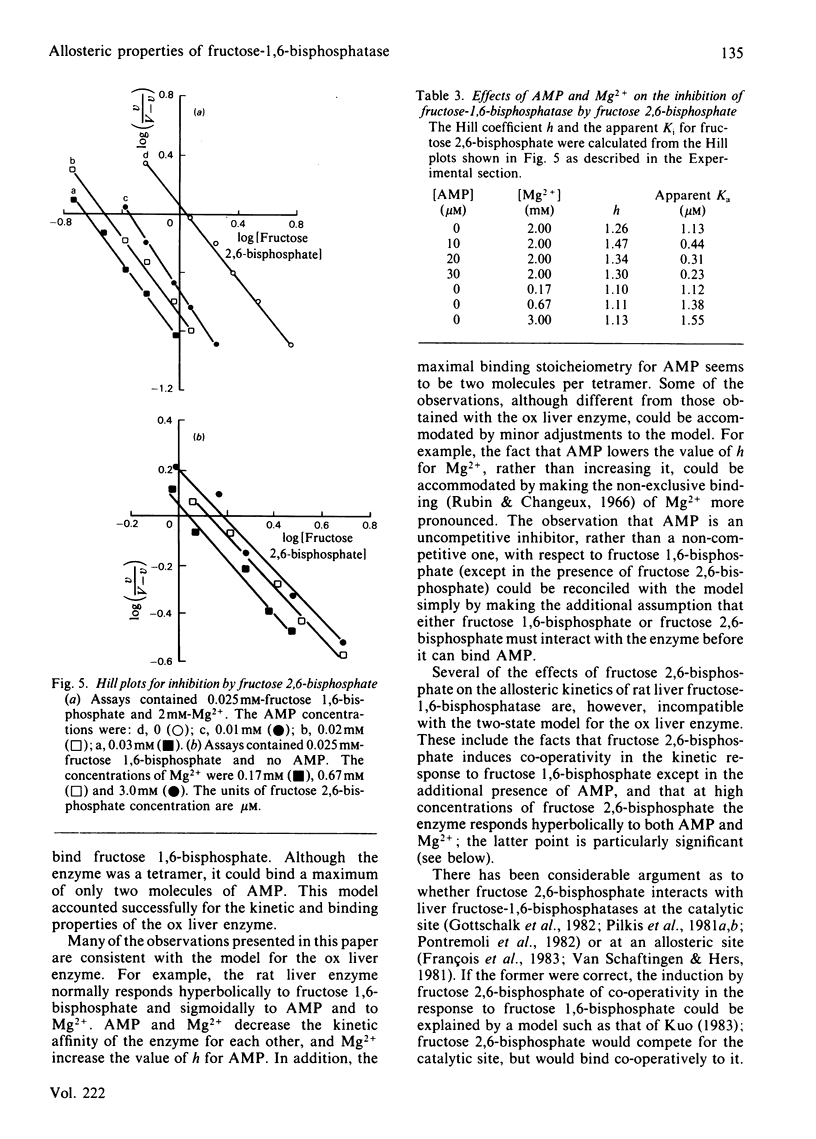
Inhibition of rat liver fructose-1,6-bisphosphatase by AMP was uncompetitive with respect to fructose 1,6-bisphosphate in the absence of fructose 2,6-bisphosphate, but non-competitive in its presence. AMP was unable to bind to the enzyme except in the presence of one of the fructose bisphosphates; the binding stoicheiometry was 2 molecules/tetramer. Increasing concentrations of Mg2+ increased the Hill coefficient h and the apparent Ki for AMP, whereas fructose 2,6-bisphosphate had the opposite effect. Increasing concentrations of both AMP and fructose 2,6-bisphosphate decreased h and increased the apparent Ka for Mg2+. AMP slightly decreased, and Mg2+ slightly increased, the apparent Ki for fructose 2,6-bisphosphate, but each had only small effects on h. These results are interpreted in terms of a new three-state model for the allosteric properties of the enzyme, in which fructose 2,6-bisphosphate can bind both to the catalytic site and to an allosteric site and AMP can bind to the enzyme only when the catalytic site is occupied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corredor C., Boscá L., Sols A. Biphasic effect of fructose 2,6-bisphosphate on the liver fructose-1,6-bisphosphatase: mechanistic and physiological implications. FEBS Lett. 1984 Feb 27;167(2):199–202. doi: 10.1016/0014-5793(84)80126-x. [DOI] [PubMed] [Google Scholar]
- François J., Van Schaftingen E., Hers H. G. On the mechanism of inhibition of neutral liver fructose 1,6-bisphosphatase by fructose 2,6-bisphosphate. Eur J Biochem. 1983 Aug 1;134(2):269–273. doi: 10.1111/j.1432-1033.1983.tb07561.x. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Morris R. G., Kandrach M., Rosen B. P. A multichamber equilibrium dialysis apparatus. Anal Biochem. 1972 Jun;47(2):514–526. doi: 10.1016/0003-2697(72)90146-7. [DOI] [PubMed] [Google Scholar]
- Gottschalk M. E., Chatterjee T., Edelstein I., Marcus F. Studies on the mechanism of interaction of fructose 2,6-bisphosphate with fructose-1,6-bisphosphatase. J Biol Chem. 1982 Jul 25;257(14):8016–8020. [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Kuo L. C. Allosteric cofactor-mediated enzyme cooperativity: a theoretical treatment. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5243–5247. doi: 10.1073/pnas.80.17.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- McGrane M. M., El-Maghrabi M. R., Pilkis S. J. The interaction of fructose 2,6-bisphosphate and AMP with rat hepatic fructose 1,6-bisphosphatase. J Biol Chem. 1983 Sep 10;258(17):10445–10454. [PubMed] [Google Scholar]
- Meek D. W., Nimmo H. G. Effects of phosphorylation on the kinetic properties of rat liver fructose-1,6-bisphosphatase. Biochem J. 1984 Aug 15;222(1):125–130. doi: 10.1042/bj2220125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek D. W., Nimmo H. G. The interaction of fructose 2,6-bisphosphate with an allosteric site of rat liver fructose 1,6-bisphosphatase. FEBS Lett. 1983 Aug 22;160(1-2):105–109. doi: 10.1016/0014-5793(83)80946-6. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Tipton K. F. The allosteric properties of beef-liver fructose bisphosphatase. Eur J Biochem. 1975 Oct 15;58(2):575–585. doi: 10.1111/j.1432-1033.1975.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Tipton K. F. The effect of pH on the kinetics of beef-liver fructose bisphosphatase. Eur J Biochem. 1975 Oct 15;58(2):567–574. doi: 10.1111/j.1432-1033.1975.tb02407.x. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., McGrane M. M., Pilkis J., Claus T. H. The role of fructose 2,6-bisphosphate in regulation of fructose-1,6-bisphosphatase. J Biol Chem. 1981 Nov 25;256(22):11489–11495. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. J Biol Chem. 1981 Apr 25;256(8):3619–3622. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Michetti M., Salamino F., Sparatore B., Horecker B. L. On the mechanism of inhibition of fructose 1,6-bisphosphatase by fructose 2,6-bisphosphate. Arch Biochem Biophys. 1982 Oct 15;218(2):609–613. doi: 10.1016/0003-9861(82)90386-1. [DOI] [PubMed] [Google Scholar]
- Rubin M. M., Changeux J. P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol. 1966 Nov 14;21(2):265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- TAKETA K., POGELL B. M. ALLOSTERIC INHIBITION OF RAT LIVER FRUCTOSE 1,6-DIPHOSPHATASE BY ADENOSINE 5'-MONOPHOSPHATE. J Biol Chem. 1965 Feb;240:651–662. [PubMed] [Google Scholar]
- Tejwani G. A., Pedrosa F. O., Pontremoli S., Horecker B. L. The purification of properties of rat liver fructose 1,6-bisphosphatase. Arch Biochem Biophys. 1976 Nov;177(1):253–264. doi: 10.1016/0003-9861(76)90435-5. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981 May;78(5):2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]



