Abstract
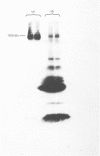
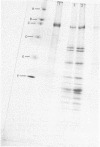
When intact maize (Zea mays) mesophyll chloroplasts were illuminated in the presence of [32P]orthophosphate and subsequently subjected to sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, a major polypeptide species of Mr 100000 was found to be heavily labelled. This polypeptide was not found in maize mesophyll thylakoid or cytoplasmic fractions, but was localized solely in the chloroplast stroma. No phosphorylation of polypeptides in the 100000-Mr region was observed in the mesophyll chloroplasts from C3 species (where the primary product of CO2 fixation is a 3-carbon compound), suggesting that this polypeptide arises from a protein associated with C4 metabolism (where the first product of CO2 fixation is a 4-carbon compound). The 100kDa polypeptide was major component of the maize mesophyll chloroplast, comprising 10-15% of the total protein, which banded in an identical position to the apoprotein of the enzyme pyruvate, orthophosphate dikinase, which catalyses a reaction of the C4 cycle [Edwards & Walker (1983) C3, C4: Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis, Blackwell Scientific Publications, Oxford and London]. Phosphorylation in the 100kDa species was prohibited by treatment of lysed chloroplasts with antibody to pyruvate, orthophosphate dikinase (EC 2.7.9.1). These data suggest that the phosphorylated polypeptide observed after sodium dodecyl sulphate/polyacrylamide-gel electrophoresis is the monomeric form of this enzyme. The 100kDa polypeptide was partially phosphorylated in darkness, but a significant increase in the degree of phosphorylation was found on illumination. This polypeptide was found to be dephosphorylated only slowly when the chloroplasts were returned to darkness. Maximum phosphorylation was observed in the presence of pyruvate or dihydroxyacetone phosphate, which also caused maximum activation of pyruvate, orthophosphate dikinase. Phosphorylation of the 100kDa polypeptide did not coincide with deactivation of pyruvate, orthophosphate dikinase, but maximum phosphorylation occurred under conditions that promoted maximum activity of the enzyme, at which time one phosphate group was associated with each enzyme molecule. Protein phosphorylation did not appear to arise from the reaction mechanism of the enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Hatch M. D. Properties and mechanism of action of pyruvate, phosphate dikinase from leaves. Biochem J. 1969 Aug;114(1):117–125. doi: 10.1042/bj1140117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Hatch M. D. Regulation of C4 photosynthesis: regulation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1983 Aug 30;115(1):53–60. doi: 10.1016/0006-291x(83)90967-1. [DOI] [PubMed] [Google Scholar]
- Bennett J. Regulation of photosynthesis by reversible phosphorylation of the light-harvesting chlorophyll a/b protein. Biochem J. 1983 Apr 15;212(1):1–13. doi: 10.1042/bj2120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Dark-light regulation of pyruvate, Pi dikinase in C4 plants: evidence that the same protein catalyses activation and inactivation. Biochem Biophys Res Commun. 1983 Feb 28;111(1):288–293. doi: 10.1016/s0006-291x(83)80149-1. [DOI] [PubMed] [Google Scholar]
- Chapman K. S., Hatch M. D. Regulation of C4 photosynthesis: mechanism of activation and inactivation of extracted pyruvate, inorganic phosphate dikinase in relation to dark/light regulation. Arch Biochem Biophys. 1981 Aug;210(1):82–89. doi: 10.1016/0003-9861(81)90166-1. [DOI] [PubMed] [Google Scholar]
- Edwards G. E. Isolation of Intact and Functional Chloroplasts from Mesophyll and Bundle Sheath Protoplasts of the C(4) Plant Panicum miliaceum. Plant Physiol. 1979 May;63(5):821–827. doi: 10.1104/pp.63.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. Regulation of enzymes in C4 photosynthesis. Curr Top Cell Regul. 1978;14:1–27. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- Nakamoto H., Edwards G. E. Influence of Oxygen and Temperature on the Dark Inactivation of Pyruvate, Orthophosphate Dikinase and NADP-Malate Dehydrogenase in Maize. Plant Physiol. 1983 Mar;71(3):568–573. doi: 10.1104/pp.71.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H., Sugiyama T. Partial characterization of the in vitro activation of inactive pyruvate, pi dikinase from darkened maize leaves. Plant Physiol. 1982 Apr;69(4):749–753. doi: 10.1104/pp.69.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk A. M., Yoshida H., Wood H. G. Isolation of 3-phosphohistidine from phosphorylated pyruvate, phosphate dikinase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4415–4419. doi: 10.1073/pnas.73.12.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]