Abstract
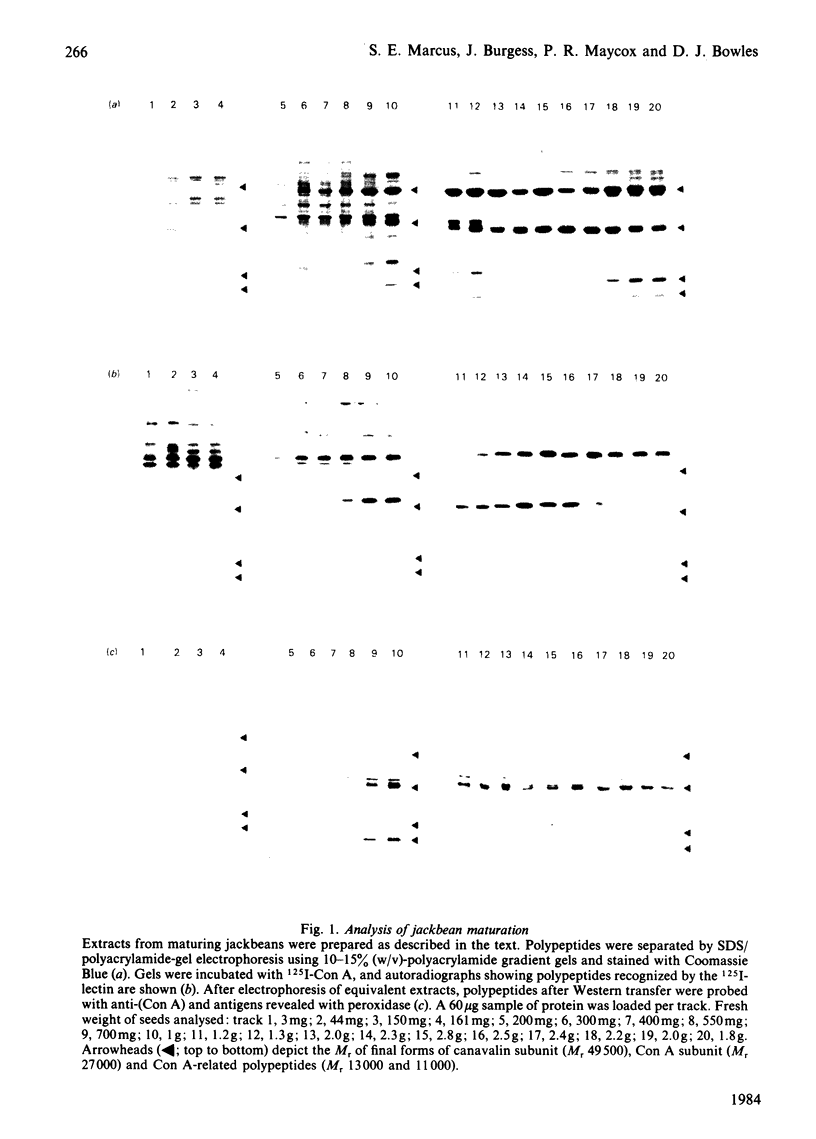
Maturation events have been studied in developing jackbean (Canavalia ensiformis) cotyledons by using a combination of analysis by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, overlays with 125I-concanavalin A (Con A) and the use of anti-(Con A) after Western transfer. The number of polypeptides recognized by 125I-Con A varies during maturation, until at maturity only one remains. Several molecular forms of the lectin occur during development; one, corresponding to Mr 33 000 and found only in immature seeds, interacts with 125I-Con A, suggesting that it is glycosylated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Chaplin M. F., Marcus S. E. Interaction of concanavalin A with native and denatured forms of jackbean alpha-D-mannosidase. Eur J Biochem. 1983 Feb 15;130(3):613–618. doi: 10.1111/j.1432-1033.1983.tb07193.x. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Craig S., Spencer D. Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J Cell Biol. 1982 Apr;93(1):5–14. doi: 10.1083/jcb.93.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Herbert E., Uhler M. Biosynthesis of polyprotein precursors to regulatory peptides. Cell. 1982 Aug;30(1):1–2. doi: 10.1016/0092-8674(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Higgins T. J., Chandler P. M., Zurawski G., Button S. C., Spencer D. The biosynthesis and primary structure of pea seed lectin. J Biol Chem. 1983 Aug 10;258(15):9544–9549. [PubMed] [Google Scholar]
- Higgins T. J., Chrispeels M. J., Chandler P. M., Spencer D. Intracellular sites of synthesis and processing of lectin in developing pea cotyledons. J Biol Chem. 1983 Aug 10;258(15):9550–9552. [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Bowles D. J. A comparative study of the localization of wheat-germ agglutinin and its potential receptors in wheat grains. Biochem J. 1982 Sep 15;206(3):571–576. doi: 10.1042/bj2060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. The synthesis of Ricinus communis agglutinin, cotranslational and posttranslational modification of agglutinin polypeptides. Eur J Biochem. 1981 Sep;119(1):31–41. doi: 10.1111/j.1432-1033.1981.tb05573.x. [DOI] [PubMed] [Google Scholar]
- Tumer N. E., Thanh V. H., Nielsen N. C. Purification and characterization of mRNA from soybean seeds. Identification of glycinin and beta-conglycinin precursors. J Biol Chem. 1981 Aug 25;256(16):8756–8760. [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Edelman G. M. Unusual fragments in the subunit structure of concanavalin A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1130–1134. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]