Abstract
Patient: Male, 55-year-old
Final Diagnosis: COVID 19 infection • Guillain-Barré syndrome (GBS)
Symptoms: Dyspnea
Clinical Procedure: —
Specialty: Infectious Diseases • Neurology
Objective:
Unusual clinical course
Background:
Coronavirus disease (COVID-19) can cause various complications. We report a case of severe COVID-19 acute respiratory distress syndrome (ARDS) in a patient receiving veno-venous extracorporeal membrane oxygenation (V-V ECMO), complicated by Guillain-Barré syndrome (GBS) and cerebral infarction, as well as pulmonary embolism.
Case Report:
A 55-year-old Japanese man with a history of ulcerative colitis was admitted for COVID-19. His respiratory status worsened and progressed to ARDS, requiring intubation on hospital day (HD) 3. On HD 16, contrast computed tomography revealed PE. On HD 18, his respiratory condition worsened, and V-V ECMO was initiated. On HD 23, V-V ECMO was successfully discontinued. He regained consciousness on HD 44, but he had quad-riplegia. Deep-tendon reflexes were absent in all limbs. Cranial nerve involvement, specifically bilateral facial nerve weakness, was noted. Magnetic resonance imaging showed bilateral scattered cerebral infarctions on HD 76. Nerve conduction studies indicated severe axonal neuropathy. Cerebrospinal fluid examination showed albuminocytologic dissociation. The antibody to the ganglioside GD1a was positive. These findings were consistent with the diagnosis of GBS. He received immunoglobulin treatment on HD 89, and his neurological findings slightly improved.
Conclusions:
This study emphasized that in COVID-19, neurological complications are not rare, are difficult to diagnose, and are prone to delays in detection.
Key words: Cerebral Infarction, COVID-19, Extracorporeal Membrane Oxygenation, Guillain-Barré Syndrome, Pulmonary Embolism, Respiratory Distress Syndrome
Introduction
Coronavirus disease (COVID-19) can cause various complications. Neurological manifestations of COVID-19 have been reported in up to 36.4% of cases [1]. COVID-19 has been reported to be associated with various neurological symptoms, including dizziness, headache, confusion, myalgia, ageusia, and anosmia [1]. Several neurologic illnesses, such as encephalitis/encephalopathy, meningitis, and stroke, have reportedly occurred along with COVID-19 [1]. We report a case of severe COVID-19 acute respiratory distress syndrome (ARDS) complicated by neurological manifestations, including Guillain-Barré syndrome (GBS) and cerebral infarction, as well as pulmonary embolism (PE).
Case Report
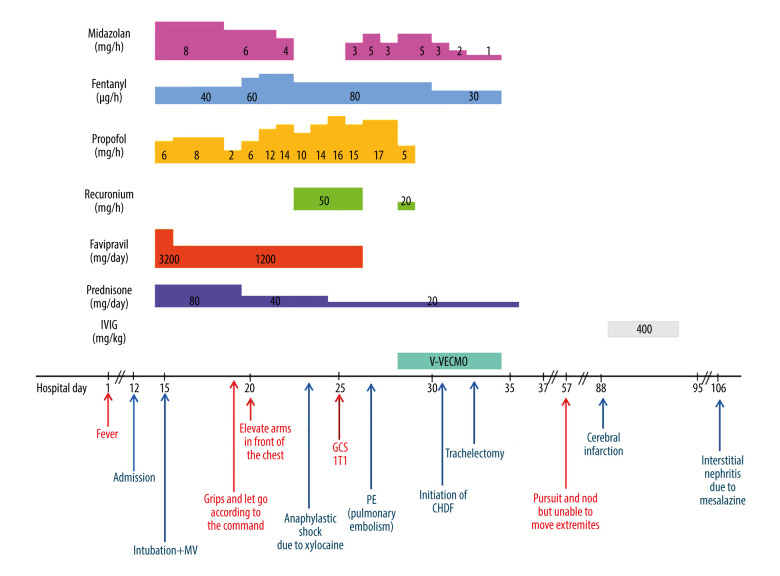
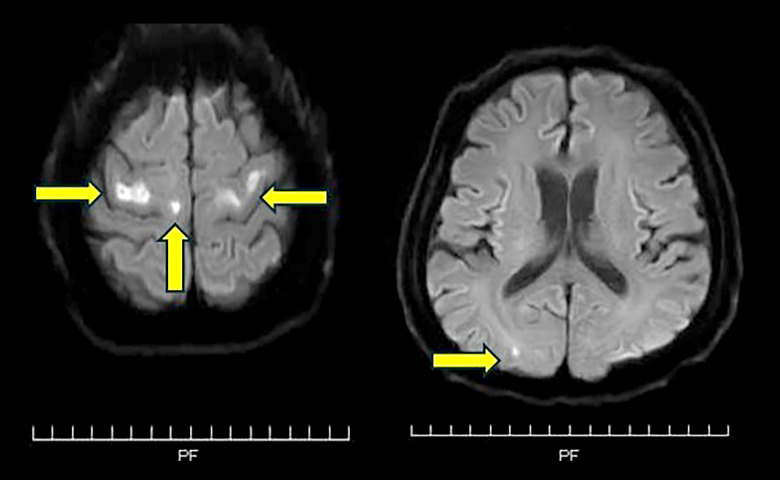
A 55-year-old Japanese man with a history of ulcerative colitis (UC), hyperlipidemia, and hypertension was admitted to our hospital with dyspnea after 11 days of fever. He tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA on reverse transcriptase polymerase chain reaction (RT-PCR) and was diagnosed with COVID-19. The patient’s C-reactive protein level was 14.14 mg/dL. He was treated with ciclesonide, favipiravir, and methylprednisolone for COVID-19. His respiratory status worsened, and he developed ARDS, requiring intubation on hospital day (HD) 3. He received midazolam, propofol, and fentanyl for analog-sedation. On HD 8, he was able to grip with his hands and elevate his arms. Then, his respiratory condition worsened significantly. On HD 12, he developed a right pneumothorax, and a chest drain was inserted. He experienced anaphylactic shock, presumably due to xylocaine, during the insertion of a chest drain. Despite the insertion of a chest drain, oxygenation deteriorated. Therefore, a muscle relaxant, rocuronium, was added to his regimen to reduce breathing effort. Simultaneously, the dosage of propofol was increased, and on HD 14, his level of consciousness was GCS 1T1. On HD 16, contrast computed tomography revealed PE, and by HD 18, there was an added deterioration in respiratory status, presumably due to this PE, necessitating the initiation of veno-venous extracorporeal membrane oxygenation (V-V ECMO) in conjunction with ARDS. Rocuronium was temporarily resumed during the procedure. On HD 20, he exhibited progression of metabolic acidosis, and continuous hemodiafiltration (CHDF) was initiated. His respiratory status subsequently improved, and on HD 22, tracheostomy was performed. On HD 23, V-V ECMO was successfully discontinued. The doses of sedative agents were gradually decreased, and sedatives were then discontinued on HD 24 (Figure 1). On HD 44, he was able to follow commands with visual pursuit and nodding but was unable to move his extremities. The patient regained consciousness and was able to communicate by sticking out his tongue. He was noted to be completely quadriplegic. Magnetic resonance imaging (MRI) showed bilateral scattered cerebral infarctions (small high-intensity areas in the left and right precentral gyri, right superior frontal gyrus, and right occipital lobe) on HD 76 (Figure 2). We confirmed the absence of intracardiac thrombi on echocardiography and conducted a bubble study, which also confirmed the absence of a patent foramen ovale. The heparin therapy for PE, which had been discontinued on HD 64 due to anemia, was resumed. However, this did not explain the patient’s quadriplegia. Neurological examination revealed amyotrophy of all limbs, total paralysis of the extremities and the bulbar and facial muscles, and external ophthalmoplegia. Deep-tendon reflexes were absent in all limbs. Babinski reflex was absent. These findings were symmetrical. Cranial nerve involvement, specifically bilateral facial nerve weakness, was noted. A nerve conduction study showed delayed peripheral nerve conduction velocity, disappearance of the F wave, and decreased amplitude in the left median nerve. These findings were indicative of severe axonal neuropathy. Cerebrospinal fluid showed albuminocytologic dissociation, no white blood cells (0/mm3), and elevated protein levels (124 mg/dL). Blood serum testing showed strong positivity for IgG antibodies to the ganglioside GD1a. Antibodies to the gangliosides GQ1b and GM1 were not detected. These findings were consistent with the diagnosis of GBS. He received intravenous immunoglobulin (IVIG) (0.4 g/kg) for 5 days, starting on HD 89. His neurological symptoms slightly improved after therapy, and he was able to move both shoulders and his right hand (manual muscle testing, 2/5). On HD 95, the patient developed interstitial nephritis, presumably due to mesalazine.
Figure 1.
Clinical course of case related to treatment. MV – mechanical ventilation; GCS – Glasgow Coma Scale; PE – pulmonary embolism; V-V ECMO – veno-venous extracorporeal membrane oxygenation; IVIG – intravenous immunoglobulin.
Figure 2.
MRI with diffusion-weighted imaging (DWI) showed bilateral scattered cerebral infarction. A small high-intensity area was found on bilateral precentral gyrus, right superior frontal gyrus, and right occipital lobe. The areas indicated by the yellow arrows are small high-intensity areas.
Discussion
In this case, it was difficult to determine the onset of GBS because the neurological findings were difficult to evaluate in an isolated intensive care setting. Early detection of neurological complications is imperative to providing immediate intervention and achieving a better prognosis, but it is difficult to detect the initial symptoms of COVID-19-associated GBS. The initial symptoms included flaccid paralysis and facial diplegia, occurring 5–10 days after the onset of acute respiratory symptoms [2]. The initial symptoms of neurological complications in severe COVID-19 are challenging to diagnose because the patients are isolated, making neurological evaluation difficult, especially for intubated patients.
It was also difficult to diagnose the neurological complications of COVID-19, including GBS and stroke, because MRI and neurophysiological tests, including electroencephalography and nerve conduction studies, are difficult to perform in an intensive care setting. Moreover, safe nursing and adequate infection control need to be practiced.
Furthermore, it was difficult to differentiate GBS from other neurological diseases. Our patient was receiving V-V ECMO and was sedated with several drugs, including a muscle relaxant. General weakness was challenging to differentiate from ICU-acquired weakness (ICU-AW) and other neurological diseases. Muscle injury and myalgia are commonly reported neurological findings in COVID-19 (19.2%, 95% CI 15.4–23.2%) [3]. The weakness attributed to cerebral infarction on MRI was considered in the differential diagnosis. However, both pyramidal signs, including absent tendon reflexes and absent Babinski reflex, were noted, leading us to suspect a peripheral rather than central origin. Critical illness polyneuropathy (CIP) was one of the essential differential diagnoses in this patient. However, in CIP, peripheral facial paralysis is uncommon, and antiganglioside antibodies are not detected [4]. To differentiate GBS from other neurological diseases, antiganglioside antibody testing was useful in confirming the diagnosis of GBS, especially for an isolated intensive care setting because antiganglioside antibodies can be collected without moving, and are strongly associated with certain forms of GBS [5].
In our case, COVID-19 was complicated by GBS, cerebral infarction, and PE. These complications can be associated with cytokines. SARS-CoV-2 can induce an excessive immune reaction with increases production of cytokines, such as IL-6, by activated leukocytes. These stimulate the inflammatory cascade, leading to extensive tissue damage [6]. In this case, the patient developed respiratory failure, requiring V-V ECMO. In this state, a hyperinflammatory cytokine profile, often termed a “cytokine storm,” can be fatal. Cytokine storm is one of the clinical complications of SARS-CoV-2 infection [6]. In patients with GBS with mechanical ventilation, our patient required V-V ECMO (Table 1) [7–17]. In this case, the patient`s respiratory condition deteriorated to the point that V-V ECMO was required due to pulmonary embolism complicating ARDS caused by COVID-19. It is conceivable that this PE, similar to GBS and cerebral infarction, could have originated from a cytokine storm induced by COVID-19.
Table 1.
Clinical characteristics, tests, treatment, and outcome of reported Guillain-Barré syndrome associated with SARS-CoV-2 infection with mechanical ventilation.
| No. | Country | Age | Sex | Severity of PMH |
COVID-19 Mild: 0
Severe: 1 Critical: 2 |
V-V ECMO | Hughes Functional Grade of GBS | Diagnostic method of GBS | Time from COVID-19 symptom onset to GBS symptom onset (d) | Time from neurological illness onset to nadir (d) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (7) | Italy | 55 | M | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 10 | 2 |
| 2 (7) | Italy | 61 | M | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 7 | 4 |
| 3 (7) | UK | 42 | M | NA | mild | – | 2 – able to walk 5m (across an open space) but incapable of manual work/running | Neurological findings, albuminocytologic dissociation, EDX | 13 | NA |
| 4 (7) | UK | 60 | M | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | −1 | NA |
| 5 (7) | Italy | 70 | F | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 23 | 5 |
| 6 (7) | USA | 54 | M | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings | 8 | 2 |
| 7 (7) | Italy | 71 | M | HTN, AAA, lung cancer | Critical | – | 6 – dead | Neurological findings, albuminocytologic dissociation, EDX | A few days | 4 |
| 8 (7) | France | 64 | M | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 11 | 3 |
| 9 (7) | Italy | 66 | F | HTN | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 10 | 3 |
| 10 (8) | JAPAN | 69 | M | DM | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation | 24 | 4 |
| 11 (9) | Chile | 31 | F | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, EDX | 10 | 1 |
| 12 (10) | USA | 36 | M | HTN, reanal transplants | Critical | v | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation | 22 | 2 |
| 13 (11) | USA | 62 | F | NA | mild | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation | A few days | 1 |
| 14 (12) | USA | 69 | M | Chronic myelogenous leukemia, HTN, coronary, artery disease | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 35 | 2 |
| 15 (13) | France | 53 | M | BPPV, asthma | Mild | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 22 | 3 |
| 16 (13) | France | 68 | F | Localized scleroderma | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, EDX | 32 | 22 |
| 17 (13) | France | 78 | F | Af, hypothyroidism, depressive disorder, herniated disk | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 24 | 18 |
| 18 (14) | Syria | 55 | M | NA | Critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 4 | 3 |
| 19 (15) | USA | 59 | F | HTN, hyperlipidemia, cerebral vascular accident, DM | Critical | – | 6 – dead | Neurological findings, albuminocytologic dissociation | 20 | 2 |
| 20 (16) | Iran | 38 | M | NA | critical | – | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, EDX | 11 | 3 |
| 20 (16) | UK | 57 | M | HTN, psoriasis | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation | 6 | 2 | ||
| 21 | JAPAN | 55 | M | UC, DLP, HTN | Critical | + | 5 – requiring assisted ventilation (for any part of the day or night) | Neurological findings, albuminocytologic dissociation, EDX | 57 | 2 |
| No. | MRC | GBS clinical subtype | Cranial nerve involvement | Facial palsy (unilateral or bilateral) | Brighton criteria level | Initial neurological symptoms | Protein in Key neurological signs | CSF (mg/dL) | Cell in CSF (/mm3) |
|---|---|---|---|---|---|---|---|---|---|
| 1 (7) | NA | Typical | Facial weakness evolving to areflexia/diplegia | Positive | 1 | Paresthesia in the four limbs and lower limb weakness | Flaccid tetraparesis and facial weakness evolving to areflexia and respiratory failure | 193 | 0 |
| 2 (7) | NA | Typical | Facial weakness and dysphagia | Positive | 1 | Asthenia | Facial weakness, flaccid areflexic paraplegia, and respiratory failure | 40 | 3 |
| 3 (7) | NA | Typical | Dysphagia | Negative | 2 | Distal limb numbness and weakness; dysphagia | Quadriparesis; areflexia; sensory loss | 50 | 3 |
| 4 (7) | NA | Typical | Facial and bulbar weakness | Negative | 2 | Distal limb numbness and weakness | Quadriparesis; areflexia; sensory loss; dysautonomia; facial and bulbar weakness | 60 | 2 |
| 5 (7) | 4/5 UL and LL | Typical | Negative | Negative | 1 | Asthenia, hands and feet paresthesia and gait difficulties | Polyradiculoneuropathy with predominant demyelination of both motor and sensory fibers, sural sparing pattern | 48 | 1 |
| 6 (7) | 2/5 UL and LL | Typical | Negative | Negative | 3 | Numbness and weakness of his lower extremities | Absent lower extremity deep tendon reflexes along with decreased lower extremity strength compared to upper extremities. | Not performed | Not performed |
| 7 (7) | 3/5 UL, 2/5 LL | Typical | Negative | Negative | 1 | Paresthesia at limb extremities | Paresthesia at limb extremities | 54 | 9 |
| 8 (7) | 2/5 arms, 3/5 forearms, 4/5 hands, 2/5 LL | Typical | Swallowing disturbance | Negative | 1 | Paresthesia in feet and hands | Paresthesia in feet and hands and swallowing disturbance | 166 | 0 |
| 9 (7) | 4/5 distal muscle, UL | Typical | Positive | Unilateral | 1 | Weakness in LL | Proximal weakness in all limbs, dysesthesia, and unilateral facial palsy | 108 | 0 |
| 10 (8) | Mild muscle weakness | Typical | A loss of cough reflex | Negative | 1 | A loss of cough reflex | Diminished tendon reflexes, mild muscle weakness | 202 | 1 |
| 11 (9) | 1/1 UL, 1/1 LL | Typical | Facial diplegia, tongue paresis | Positive | 1 | Paresthesia at all limb extremities | Paresthesia at limb extremities | 1,8 | 5 |
| 12 (10) | 4/5 UL, 3/5 LL | Typical | Negative | Negative | 1 | Numbness tingling over his fingers, toes, and perioral region | Paresthesia in feet | 117 | 1 |
| 13 (11) | 4/4 UL, 4/4 LL | Typical | Positive | Positive | 1 | Progressive weakness and numbness in bilateral lower extremities | Flaccid tetraparesis and facial weakness evolving to areflexia and respiratory failure | 587 | 4 |
| 14 (12) | 1/1 UL, 1/1 LL | Typical | Negative | Negative | 1 | Paresthesia at all limb extremities | Flaccid tetraparesis and respiratory failure | 92,8 | 9 |
| 15 (13) | 1/1 UL, 1/1 LL | Typical | Negative | Negative | 1 | Painful tetraparesis, sensory impairment | Areflexia in lower limbs, Hyporeflexia in upper limbs | 1,7 | 2 |
| 16 (13) | 1/1 UL, 1/1 LL | Typical | Positive | Positive | 1 | Flaccid tetraplegia | Flaccid tetraplegia and external ophthalmoplegia | 0,23 | 2 |
| 17 (13) | 1/1 UL, 1/1 LL | Typical | Positive | Positive | 1 | Flaccid tetraplegia | Flaccid tetraplegia and facial diplegia | 3,31 | 2 |
| 18 (14) | 2/5 UL, 2/5 LL | Typical | Negarive | Negative | 1 | lower extremities weakness | Flaccid tetraplegia and facial diplegia | 325 | 0 |
| 19 (15) | 1/1 UL, 1/1 LL | Typical | Negative | Negative | 1 | Flaccid tetraplegia | Flaccid tetraplegia | 50 | 1 |
| 20 (16) | 4/5 UL, 2/5 LL | Typical | Positive | Positive | 1 | Parethesia of his hands and feet | Facial weakness, flaccid areflexic paraplegia, and respiratory failure | Not performed | Not performed |
| 20 (16) | 4/5 UL, 4/5 LL | Typical | positive | positive | 1 | Difficulty standing unaided and noticed some tingling sensation in his feet | Flaccid tetraplegia | 0,51 | 1 |
| 21 | 0/5 UL and LL | Typical | positive | bilateral | 1 | Complete quatriplegia | Bilaretal facial palsy quadplasia | 124 | 0 |
| No. | PCR assay of SARSCoV-2 on CSF | EDX subtype | Antigan-glioside antibodies | MRI (Head) | MRI (Spine) | IVIG | PEx |
|---|---|---|---|---|---|---|---|
| 1 (7) | Negative | AMAN | Negative | Normal | Enhanced of caudal nerve root | + | – |
| 2 (7) | Negative | AIDP | Negative | NP | normal | + | + |
| 3 (7) | Not performed | AIDP | NP | NP | Not performed | + | – |
| 4 (7) | Not performed | AIDP | NP | normal | Not performed | + | – |
| 5 (7) | Not performed | AIDP | NP | NP | Not performed | + | – |
| 6 (7) | Not performed | NP | NP | NP | Normal | + | – |
| 7 (7) | Negative | AIDP | NP | NP | Not performed | + | – |
| 8 (7) | Not performed | AIDP | Negative | NP | 2 | + | – |
| 9 (7) | Negative | AIDP | Negative | NP | Not performed | + | – |
| 10 (8) | Not performed | AIDP | Positive | NP | Not performed | + | v |
| 11 (9) | Not performed | AIDP | NP | NP | Not performed | + | – |
| 12 (10) | Not performed | AIDP | NP | normal | Not performed | + | + |
| 13 (11) | Not performed | NP | NP | normal | Not perfomed | + | – |
| 14 (12) | Not performed | AIDP | NP | NP | Not performed | + | – |
| 15 (13) | Not performed | AIDP | Positive | NP | Not performed | + | – |
| 16 (13) | Not performed | Miller Fisher syndrome with involvement of the peripheral nerves | Negtive | NP | Not performed | + | – |
| 17 (13) | Not performed | AIDP | Negative | Leukoencephalopathy, cortical/subcortical brain atrophy | Not performed | + | – |
| 18 (14) | Not performed | AMAN | NP | NP | Not perfomed | – | + |
| 19 (15) | Not performed | NP | Negative | NP | Not performed | + | – |
| 20 (16) | Not performed | AIDP | NP | NP | Not performed | - | + |
| 20 (16) | Not performed | AIDP | NP | NP | Not peformed | + | – |
| 21 | Negative | AMAN | Positive | Multiple cerebral infarction | Normal | + | – |
| No. | Diagnostic SARS-CoV-2 testing | Screening for other infective agents | Another reported treatment | Systemic steroid | Outcome |
|---|---|---|---|---|---|
| 1 (7) | nasopharyngeal swab was positive for SARS-Cov-2 (not mentioned RT-PCR or other) | NA | Azithromycin | – | Poor outcome, still in ICU owing to neuromuscular respiratory failure and flaccid tetraplegia. |
| 2 (7) | Positive SARS-CoV-2 IgG | Campylobacter jejuni, EBV, CMV, HSV, VZV, influenza, HIV (all were negative) | NA | – | Mechanical ventilation through tracheostomy. |
| 3 (7) | Positive nasal-pharyngeal throat SARS-CoV-2 PCR test | no | NA | – | NA |
| 4 (7) | Positive nasal-pharyngeal throat SARS-CoV-2 PCR test | no | NA | – | NA |
| 5 (7) | Positive for SARS-CoV-2-RNA on RT-PCR with a nasopharyngeal swab | Mycoplasma pneumoniae and Cytomegalovirus (CMV) serology (IgM and IgG), Legionella pneumophila and Streptococcus pneumoniae CSF: herpes simplex virus, varicella zoster virus, Epstein-Bar virus, CMV, HIV-1, Borrelia burgdorferi IgM and IgG) | NA | – | NA |
| 6 (7) | Specimen; SARS-CoV-2 test (not clearly mentioned the detail) | Espiratory viral panel testing (nasopharyngeal PCR): Rhinovirus (+) | Hydroxychloroquine | – | Clinical course showed improvement in his respiratory status with liberation from mechanical ventilation on day 4 of IVIG therapy |
| 7 (7) | Nasopharyngeal swab was positive for SARS-Cov-2 (not mentioned RT-PCR or other) | NA | Hydroxychloroquine | – | Died because of progressive respiratory failure |
| 8 (7) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | Campylobacter jejuni, Mycoplasma pneumoniae, Salmonella enterica, CMV, EBV, HSV1 & 2, VZV, Influenza virus A & B, VIH, and hepatitis E (all were negative) | NA | – | NA |
| 9 (7) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | NA | Hydroxychloroquine | – | IVIg was ineffective |
| 10 (8) | Positive for SARS-CoV-2-RNA on RT-PCR with sputum sample | NA | Hydroxychloroquine | – | Clinical course showed improvement in his respiratory status with liberation from mechanical ventilation on day 5 of IVIG therapy |
| 11 (9) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | NA | NA | – | Clinical course showed improvement on day 5 of IVIG therapy |
| 12 (10) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | NA | NA | + | Clinical course showed improvement in his respiratory status with liberation from mechanical ventilation |
| 13 (11) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | NA | NA | + | Clinical course showed improvement in his respiratory status with liberation from mechanical ventilation |
| 14 (12) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | NA | NA | + | Clinical course showed improvement in his respiratory status with liberation from mechanical ventilation |
| 15 (13) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | Campylobacter jejuni, EBV, HBV, HCV, HEV, HIV (all were negative), CMV IgG positive, IgM positive | NA | – | Sensory impairment of sole, balance disorder |
| 16 (13) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | NA | Hydroxychloroquine, azithromycin, Atazanavir/ritonavvir | – | Complete recovery |
| 17 (13) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | EBV, HBV, HCV, HEV, HIV, M pneumoniae(all negative) | Dexamethasone | + | Weakness in lower limbs, balance disorder |
| 18 (14) | serum COVID-19 antibodies | NA | NA | – | Clinical course showed improvement in his respiratory status with liberation from mechanical ventilation and improvement in his muscles weakness |
| 19 (15) | Positive for SARS-CoV-2 on RT-PCR with nasopharyngeal swab | NA | Dexamethasone | + | Died |
| 20 (16) | Negative | NA | Dexamethasone | + | Complete recovery |
| 20 (16) | Negative | NA | NA | – | Clinical course showed improvement in his respiratory status with liberation from mechanical ventilation |
| 21 | Positive nasal-pharyngeal throat SARS-CoV-2 PCR test | Campylobacter jejuni, Legionella pneumophila and Streptococcus pneumoniae,HIV, EBV, Syphilis, Tuberculosis CSF: bacteria, Cryptococcus, CVM (all negative) | Ciclesonide, Favipiravir and methylprednisolone | + | Mechanical ventilation through tracheostomy |
AAA – abdominal aortic aneurysm; AIDP – acute inflammatory demyelinating polyneuropathy; AMAN – acute motor axonal neuropathy; COVID-19 – coronavirus disease 2019; d – days; DLP – dyslipidemia; DM – diabetes mellitus; EDX – electrophysiological; F – female; GBS – Guillain-Barré syndrome; HTN – hypertension; ICU – Intensive Care Unit; IVIg – intravenous immunoglobulin; LL – lower limbs; M – male; MRC – Medical Research Council scale; MRI – magnetic resonance imaging; NA – not applicable; NP – not performed; PEx – plasma exchange; PMH – past medical history; UC – ulcerative colitis; UL – upper limbs; V-V ECMO – veno-venous extracorporeal membrane oxygenation.
Cytokine storm was also associated with increased IL-6 levels, resulting in hyperviscosity and increased risk of stroke [6]. Hypercoagulability was also a possible cause of PE. In our case, the patient had COVID-19 complicated by stroke and PE. This case emphasized that multiple coagulation complications can occur sequentially in COVID-19.
Conclusions
Neurological complications of severe COVID-19 ARDS are challenging to diagnose because patients are deeply sedated, and neurological symptoms need to be distinguished from ICU-AW. Neurologic examinations in COVID-19 patients are also limited by isolation guidelines. Early detection and treatment of neurological complications are essential to achieve a good prognosis. This case emphasizes that neurological complications are not uncommon and are prone to delays in detection. When evaluating patients with COVID-19, neurological complications, including GBS and stroke, should be strongly suspected.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020;77(6):683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–76. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinzon RT, Wijaya VO, Buana RB, et al. Neurologic characteristics in coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Front Neurol. 2020;11:565. doi: 10.3389/fneur.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou C, Wu L, Ni F, et al. Critical illness polyneuropathy and myopathy: A systematic review. Neural Regen Res. 2014;9(1):101–10. doi: 10.4103/1673-5374.125337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregson NA, Koblar S, Hughes RA. Antibodies to gangliosides in Guillain-Barré syndrome: Specificity and relationship to clinical features. Q J Med. 1993;86(2):111–17. [PubMed] [Google Scholar]
- 6.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–29. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Sanctis P, Doneddu PE, Viganò L, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. A systematic review. Eur J Neurol. 2020;27(11):2361–70. doi: 10.1111/ene.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada S, Nagasaki Y, Arimizu Y, et al. Neurological disorders identified during treatment of a SARS-CoV-2 infection. Intern Med. 2020;59(17):2187–89. doi: 10.2169/internalmedicine.5447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cea G, Romero C, Aguilar S, Salinas R. Guillain-Barré syndrome in patients with SARS-CoV-2 infection. Report of three cases. Rev Med Chile. 2021;149(12):1812–16. doi: 10.4067/s0034-98872021001201812. [DOI] [PubMed] [Google Scholar]
- 10.Rajdev K, Victor N, Buckholtz ES, et al. A case of Guillain-Barré syndrome associated with COVID-19. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620961198. 2324709620961198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rane RP, Jain A, Hussain KM, et al. A rare case of Guillain-Barré syndrome associated with SARS-CoV-2 infection requiring mechanical ventilation. Cureus. 2022;14(6):e25810. doi: 10.7759/cureus.25810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yiu AC, Hussain A, Okonkwo UA, et al. Guillain-Barré syndrome associated with COVID-19 pneumonia – the first documented case in a U.S. Military Intensive Care Unit. Military Medicine. 2023;188(3–4):e852–e56. doi: 10.1093/milmed/usab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz P, Leveque M, Hautecloque G, et al. The challenge of diagnosing Guillain-Barré syndrome in patients with COVID-19 in the intensive care unit. J Neuroimmunol. 2022;366:577842. doi: 10.1016/j.jneuroim.2022.577842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labbad I, Shammas I, Abbas A, et al. Guillan-Barré syndrome during COVID-19 pandemic: A case series from Syria. Ann Med Surgery (Lond) 2023;85(6):3166–70. doi: 10.1097/MS9.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidhom F, Sandhu H. Guillain-Barré syndrome in a patient with COVID-19 infection. Cureus. 2021;13(8):e17052. doi: 10.7759/cureus.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okhovat AA, Ansari B, Hemasian H, et al. Guillain-Barré syndrome in patients with coronavirus disease-2019: Report of six cases and review of literature. Curr J Neurol. 2020;19(3):122–30. doi: 10.18502/cjn.v19i3.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb S, Wallace VC, Martin-Lopez D, Yogarajah M. Guillain-Barré syndrome following COVID-19: A newly emerging post-infectious complication. BMJ Case Rep. 2020;13(6):e236182. doi: 10.1136/bcr-2020-236182. [DOI] [PMC free article] [PubMed] [Google Scholar]