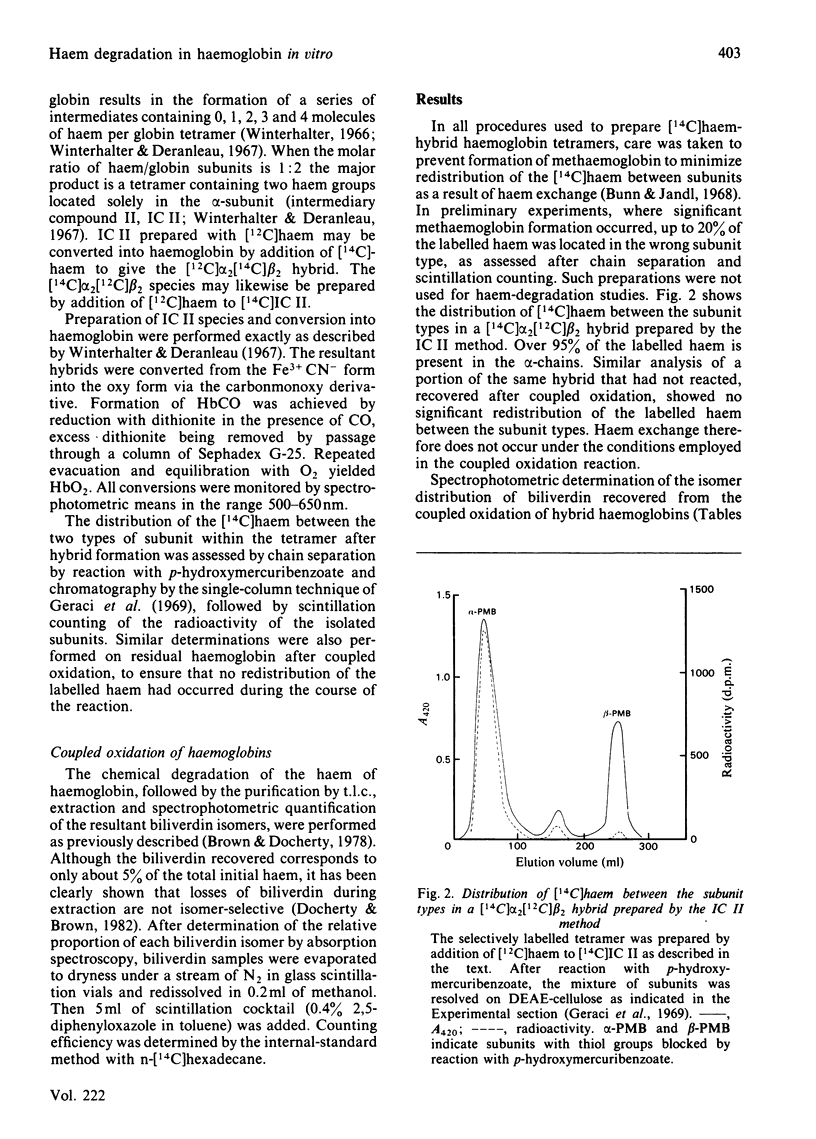
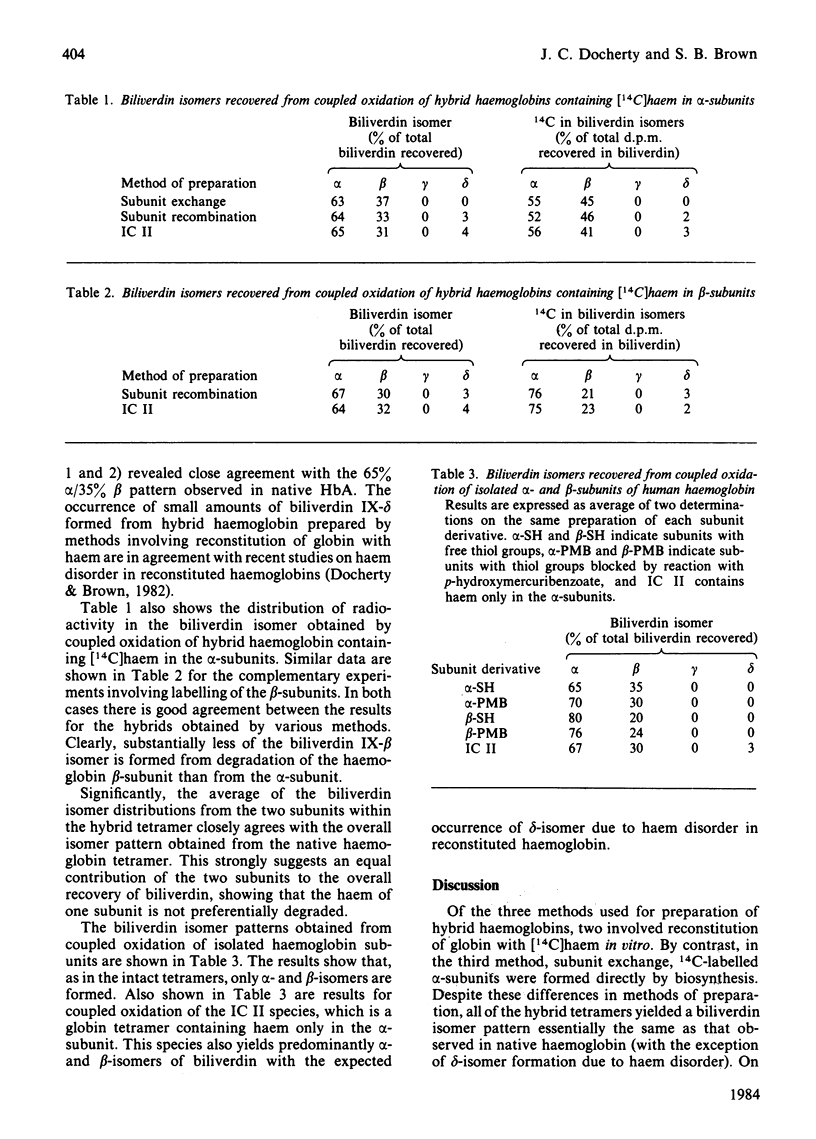
Abstract
Human haemoglobin was prepared containing [14C]haem in either the alpha- or the beta-subunits. Coupled oxidation of such hybrid haemoglobins with ascorbate and O2 showed that the biliverdin produced by the alpha-subunits contained approx. 55% alpha-isomer and 45% beta-isomer, whereas that produced by the beta-subunits contained approx. 75% alpha-isomer and 25% beta-isomer. Coupled oxidation of isolated alpha- and beta-subunits gave approx. 70% alpha-isomer, 30% beta-isomer and 78% alpha-isomer, 22% beta-isomer respectively. These results are consistent with calculations of differences in the haem environment in the two subunit types.
Full text
PDF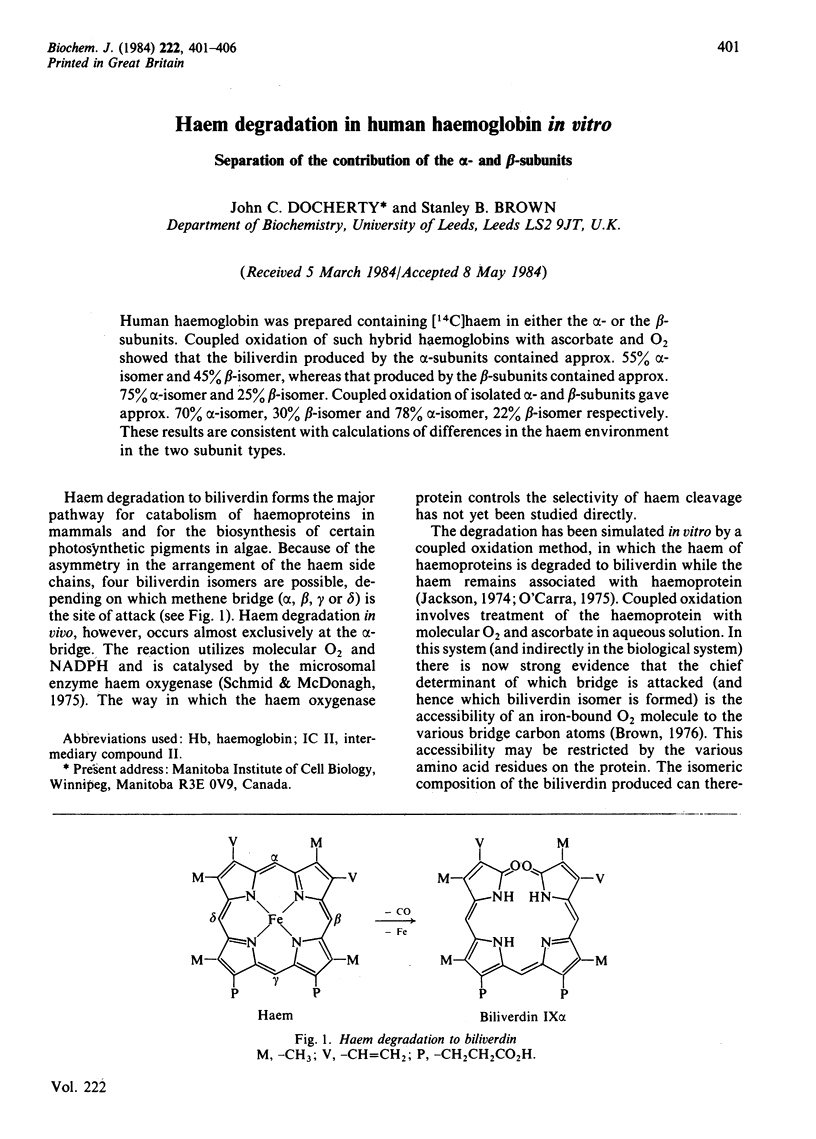



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benesch R. E., Benesch R. The mechanism of interaction of red cell organic phosphates with hemoglobin. Adv Protein Chem. 1974;28:211–237. doi: 10.1016/s0065-3233(08)60231-4. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Chabot A. A., Enderby E. A., North A. C. Orientation of oxygen in oxyhaemoproteins and its implications for haem catabolism. Nature. 1981 Jan 1;289(5793):93–95. doi: 10.1038/289093a0. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Docherty J. C. Haem degradation in abnormal haemoglobins. Biochem J. 1978 Sep 1;173(3):985–987. doi: 10.1042/bj1730985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Holroyd J. A., Troxler R. F., Offner G. D. Bile pigment synthesis in plants. Incorporation of haem into phycocyanobilin and phycobiliproteins in Cyanidium caldarium. Biochem J. 1981 Jan 15;194(1):137–147. doi: 10.1042/bj1940137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B. Stereospecific haem cleavage. A model for the formation of bile-pigment isomers in vivo and in vitro. Biochem J. 1976 Oct 1;159(1):23–27. doi: 10.1042/bj1590023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- De Renzo E. C., Ioppolo C., Amiconi G., Antonini E., Wyman J. Properties of the alpha and beta chains of hemoglobin prepared from their mercuribenzoate derivatives by treatment with 1-dodecanethiol. J Biol Chem. 1967 Nov 10;242(21):4850–4853. [PubMed] [Google Scholar]
- Docherty J. C., Brown S. B. Haem disorder in reconstituted human haemoglobin. Biochem J. 1982 Dec 1;207(3):583–587. doi: 10.1042/bj2070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- HUISMAN T. H., DOZY A. M. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med. 1962 Aug;60:302–319. [PubMed] [Google Scholar]
- Huehns E. R., Shooter E. M., Beaven G. H. The time course of the recombination of human adult and canine haemoglobins. Biochem J. 1964 May;91(2):331–334. doi: 10.1042/bj0910331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P. W., Asakura T. Use of heme spin-labeling to probe heme environments of alpha and beta chains of hemoglobin. J Biol Chem. 1979 Apr 25;254(8):2595–2599. [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- WINTERHALTER K. H., HUEHNS E. R. PREPARATIONS, PROPERTIES, AND SPECIFIC RECOMBINATION OF ALPHA-BETA-GLOBIN SUBUNITS. J Biol Chem. 1964 Nov;239:3699–3705. [PubMed] [Google Scholar]
- Winterhalter K. H., Deranleau D. A. The structure of a hemoglobin carrying only two hemes. Biochemistry. 1967 Oct;6(10):3136–3143. doi: 10.1021/bi00862a022. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H. Sequence of linkage between the prosthetic groups and the polypeptide chains of haemoglobin. Nature. 1966 Aug 27;211(5052):932–934. doi: 10.1038/211932a0. [DOI] [PubMed] [Google Scholar]


