Abstract
IgA nephropathy is a kidney disease characterized by deposition of immune complexes containing abnormally O-glycosylated IgA1 in the glomeruli. Specifically, some O-glycans are missing galactose that is normally β1,3-linked to N-acetylgalactosamine of the core 1 glycans. These galactose-deficient IgA1 glycoforms are produced by IgA1-secreting cells due to a dysregulated expression and activity of several glycosyltransferases. Galactose-deficient IgA1 in the circulation of patients with IgA nephropathy is bound by IgG autoantibodies and the resultant immune complexes can contain additional proteins, such as complement C3. These complexes, if not removed from the circulation, can enter the glomerular mesangium, activate the resident mesangial cells, and induce glomerular injury. In this review, we briefly summarize clinical and pathological features of IgA nephropathy, review normal and aberrant IgA1 O-glycosylation pathways, and discuss the origins and potential significance of natural anti-glycan antibodies, namely those recognizing N-acetylgalactosamine. We also discuss the features of autoantibodies specific for galactose-deficient IgA1 and the characteristics of pathogenic immune complexes containing IgA1 and IgG. In IgA nephropathy, kidneys are injured by IgA1-containing immune complexes as innocent bystanders. Most patients with IgA nephropathy progress to kidney failure and require dialysis or transplantation. Moreover, most patients after transplantation experience a recurrent disease. Thus, a better understanding of the pathogenetic mechanisms is needed to develop new disease-specific treatments.
Keywords: autoimmune disease, IgA nephropathy, IgA1 O-glycans, immune complexes
Introduction
IgA nephropathy (IgAN), initially called Berger disease in the honor of the discoverer, was described in 1968 based on pathological analyses of kidney-tissue biopsies obtained from a group of patients with recurrent hematuria (Berger and Hinglais 1968). Specifically, the use of immunofluorescence microscopy with antibodies specific for human immunoglobulins and complement C3 proteins revealed that these patients had glomerular immunodeposits consisting of IgA, IgG, and C3. Moreover, these features were associated with glomerular mesangioproliferative pathological changes. IgA vasculitis with nephritis (IgAVN), previously called Henoch-Schönlein purpura with nephritis, is thought to be a disease related to IgAN (Davin et al. 2001; Davin 2011). Patients with IgAVN also exhibit glomerular IgA-containing immunodeposits and similar types of renal pathology (Lau et al. 2010). Thus, IgAN and IgAVN are often considered similar diseases with a different spectrum of clinical manifestations (Waldo 1988; Wyatt et al. 2006; Kiryluk et al. 2011; Kamei et al. 2016; Hastings et al. 2022). IgAN has been recognized as the most common primary glomerulonephritis in the world (Julian et al. 1988).
Patients with IgAN often exhibit a synpharyngitic hematuria at disease onset or at the time of disease activity (Wyatt and Julian 2013), thus linking upper-respiratory tract infections with IgAN. Proteinuria may be part of the initial disease manifestation. Diagnosis of IgAN requires pathological assessment of kidney biopsy specimens by routine immunofluorescence, light microscopy with histological stains (e.g. hematoxylin and eosin and periodic acid Schiff stains), and electron microscopy. IgA is detected by routine immunofluorescence as the dominant/codominant immunoglobulin, with variable amounts of IgG and/or IgM. Complement C3 is usually detected, whereas C1q is absent, suggesting a participation of the alternative complement pathway (Maillard et al. 2015). These IgA-containing immunodeposits are usually detected by electron microscopy as electron-dense deposits in the mesangial regions. Light-microscopic features usually reveal mesangioproliferative changes. Patients with IgAN have increased mortality, and life expectancy is reduced by 6–10 years (Hastings et al. 2018; Jarrick et al. 2019). A recent study showed that most IgAN patients progress to kidney failure within 10–15 years from diagnosis and that only few patients may avoid kidney failure in their lifetime (Pitcher et al. 2023). IgAN patients with kidney failure require renal replacement therapy, i.e. dialysis or transplantation (Wyatt and Julian 2013). However, more than 50% of patients with kidney transplant develop recurrent IgAN within 5 years (Berger 1988; Odum et al. 1994; Floege 2004; Chandrakantan et al. 2005). Thus, a better understanding of the disease pathogenesis is needed to inform development of disease-specific therapies to reduce IgAN-associated morbidity and mortality.
IgA in the glomerular immunodeposits of patients with IgAN is exclusively of IgA1 subclass (Conley et al. 1980). Furthermore, these glomerular immunodeposits are enriched for glycoforms of IgA1 with some O-glycans deficient in galactose, termed galactose-deficient IgA1 (Gd-IgA1) (Allen et al. 2001; Hiki et al. 2001). This finding is consistent with earlier observations of the abnormal O-glycosylation of IgA1 in the circulation of patients with IgAN [for review see (Novak et al. 2001; Julian and Novak 2004; Kiryluk et al. 2013; Kiryluk and Novak 2014; Lai et al. 2016; Novak et al. 2018; Reily et al. 2019; Hansen et al. 2021)] and the proposed role of these Gd-IgA1 glycoforms in the pathogenesis of the disease (Mestecky et al. 1993). Current understanding of the pathogenesis defines IgAN as an autoimmune disease with aberrantly O-glycosylated IgA1. These abnormal IgA1 glycoforms, i.e. Gd-IgA1, are recognized as autoantigens by autoantibodies, mainly of IgG isotype, resulting in the formation of pathogenic immune complexes in the circulation (Suzuki et al. 2011). Some of these circulating immune complexes deposit in the glomeruli and induce kidney injury. Here, we review current knowledge of IgA1 O-glycosylation pathways, immune responses to aberrantly glycosylated IgA1, formation of nephritogenic Gd-IgA1-containing immune complexes, and the subsequent processes resulting in a mesangioproliferative glomerular injury. As environmental factors are associated with IgAN, we also discuss the origin of natural anti-glycan antibodies recognizing N-acetylgalactosamine (GalNAc) and the corresponding microbial glycoconjugates.
IgA immunoglobulins in humans
IgA, together with IgM, represent the main antibody isotypes in mucosal secretions, including for example tears, saliva, colostrum, milk, nasal secretions, and intestinal fluids (de Sousa-Pereira and Woof 2019). In the circulation, IgA is the second most abundant isotype; healthy individuals have approximately 2 mg of IgA per ml of serum. IgA-producing cells secrete IgA as a monomeric or polymeric molecular form. Monomeric IgA has two heavy chains and two light chains; polymeric IgA has two or more of the IgA monomers connected by disulfide bridges between the joining chain (J-chain) and the tail segment of the IgA heavy chain (Mestecky et al. 1974; Brandtzaeg 1981; Yoo et al. 1999; Yoo and Morrison 2005). The main form of IgA in mucosal secretions is secretory IgA. This molecular form contains the secretory component, a glycoprotein derived by proteolytic cleavage from the polymeric immunoglobulin receptor during transcytosis of polymeric IgA through mucosal epithelial cells (Brandtzaeg and Prydz 1984). Mucosal surfaces of the respiratory, gastrointestinal, and genitourinary tracts contain high amounts of secretory IgA that can neutralize and eliminate pathogens to protect the host against infections (Woof and Kerr 2006).
Humans have two subclasses of IgA, IgA1 and IgA2, that share a high degree of amino-acid sequence identity (Putnam et al. 1979; Mestecky and Russell 1986; Putnam 1989). However, IgA1 and IgA2 have substantial differences in glycosylation (Baenziger and Kornfeld 1974a; Tomana et al. 1976; Mattu et al. 1998; Hansen et al. 2021). Heavy chains of IgA1 have two N-glycans and those of IgA2 have up to five N-glycans (Baenziger and Kornfeld 1974b). Moreover, IgA1 has clustered O-glycans in the hinge region of the heavy chains (Frangione and Wolfenstein-Todel 1972; Baenziger and Kornfeld 1974a; Mattu et al. 1998). These O-glycans, usually 3 to 6 per hinge region, are typically core 1 glycans, i.e. consisting of GalNAc with β1,3-linked galactose, that can be sialylated (Mattu et al. 1998; Renfrow et al. 2005; Takahashi et al. 2010; Takahashi et al. 2012). Together, these distinct glycosylation features contribute to the functional differences of human IgA subclasses (Moura et al. 2004; Moura et al. 2005; Matysiak-Budnik et al. 2008; Papista et al. 2015; Steffen et al. 2020).
Circulatory IgA is predominantly a monomer (90% of total serum IgA), with approximately 10% being contributed by polymeric forms (Mestecky et al. 1986; Novak et al. 2001). Most circulatory IgA is of IgA1 subclass (90% of serum IgA). It is thought that the monomeric form of circulatory IgA is produced mainly by plasma cells in the bone marrow. Conversely, polymeric IgA is thought to be of mucosal origin, being produced by plasma cells close to the mucosal epithelium [for review see (Novak et al. 2001; Novak et al. 2018)]. As polymeric IgA1 is the main molecular form of IgA1 in the immunodeposits in IgAN (Conley et al. 1980; Monteiro et al. 1985), it thus raises a question whether this IgA1 is produced by plasma cells in mucosal tissues and whether upper-respiratory tract infections during synpharyngitic hematuria may be involved in the process (Suzuki et al. 2014; Novak et al. 2018).
Biosynthesis of IgA1 O-glycans and aberrant O-glycosylation in IgAN
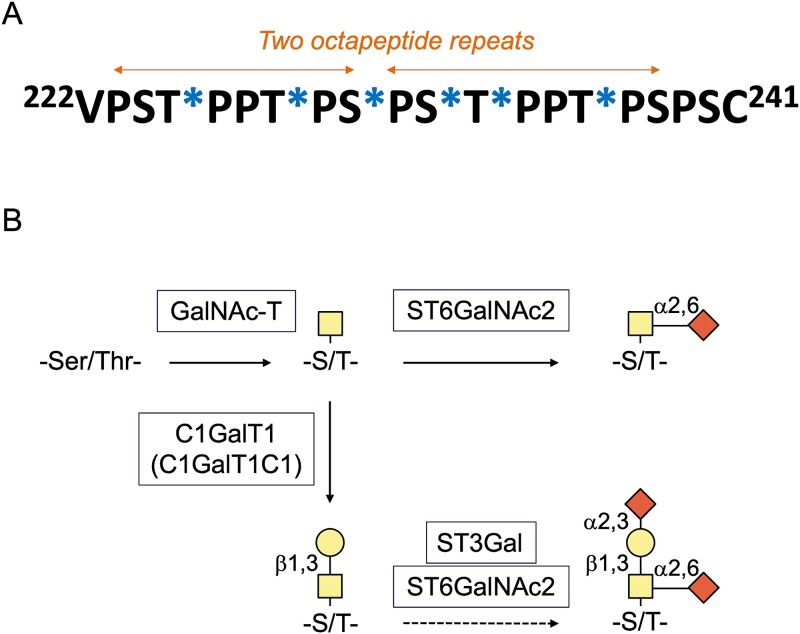
IgA1 hinge region is comprised of two octapeptide repeats (Fig. 1A) (Frangione and Wolfenstein-Todel 1972), and carries 3 to 6 O-glycans (Baenziger and Kornfeld 1974a). Circulatory IgA1 contains core 1 glycans, i.e. GalNAc with β1,3-linked galactose (Mattu et al. 1998). This disaccharide can be modified by one or two sialic acid residues, galactose can have α2,3-linked sialic acid and GalNAc can have α2,6-linked sialic acid (Takahashi et al. 2012). IgA1 O-glycans can also be without galactose, representing the so-called galactose-deficient glycans (Allen 1995; Allen et al. 1995; Tomana et al. 1997; Tomana et al. 1999; Moldoveanu et al. 2007). Those galactose-deficient glycans contain either GalNAc or GalNAc with α2,6-linked sialic acid. Notably, secretory IgA1 can contain additional O-glycan types, such as core 2 O-glycans with additional modifications not present in the O-glycans of circulatory IgA1 (Royle et al. 2003).
Fig. 1.
Hinge-region amino-acid sequence and O-glycosylation of circulatory IgA1. A) IgA1 usually has three to six sites with attached O-glycans. The six commonly used sites include T225, T228, S230, S232, T233, and T236 (marked by stars in the amino-acid sequence). B) Biosynthesis of O-glycans of circulatory IgA1. Glycosylation of IgA1 begins in the Golgi apparatus with the addition of N-acetylgalactosamine (GalNAc) to S and T residues by GalNAc-transferase (GalNAc-T). GalNAc can be then modified by core 1 synthase (C1GalT1) with a β1,3-linked galactose (Gal). This enzyme requires expression of C1GalT1-specific chaperone 1 (C1GalT1C1). In the follow-up reaction, one or both sugars can be modified by sialic acid: Galactose by α2,3-linked sialic acid and GalNAc by α2,6-linked sialic acid. These reactions are catalyzed by ST3Gal and ST6GalNAc2 enzymes, respectively. Alternatively, GalNAc can be modified before galactosylation by α2,6-linked sialic acid; this modification blocks any other glycosylation and thus yields a Gal-deficient site, also termed sialyl-Tn antigen. If GalNAc remains unmodified, it is called Tn antigen.
IgA1 hinge-region O-glycans are synthesized in a stepwise process (Fig. 1B), similarly as other mucin-type O-glycans (Bennett et al. 2012; Revoredo et al. 2016; Brockhausen et al. 2022). The initiation step, addition of GalNAc to a Ser or Thr residue, is catalyzed by a GalNAc-transferase (GalNAc-T) in the Golgi apparatus (Gerken et al. 1997; Gerken 2004; Gerken et al. 2011; Bennett et al. 2012; de Las Rivas et al. 2019; Daniel et al. 2020). Among the 20 human GalNAc-T isoenzymes, GalNAc-T2 is thought to be the main enzyme responsible for the initiation of O-glycosylation of IgA1 in IgA1-producing cells (Iwasaki et al. 2003). In addition, several other GalNAc-Ts can initiate O-glycosylation of IgA1 (Wandall et al. 2007). This conclusion is based on testing various peptide acceptors in enzymatic reactions with different GalNAc-Ts. In the next step, GalNAc can be modified by addition of β1,3-linked galactose. This step is catalyzed by core 1 synthase (glycoprotein-N-acetylgalactosamine 3-β-galactosyltransferase 1; C1GalT1) (Ju et al. 2002). Production of active C1GalT1 enzyme requires C1GalT1-specific chaperone 1 (C1GalT1C1) (Ju and Cummings 2002). After galactosylation of GalNAc, one or both sugars can be modified with sialic acid in reactions catalyzed by ST3Gal and ST6GalNAc2 enzymes (Fig. 1B) (Novak et al. 2001; Stuchlova Horynova et al. 2015; Stewart et al. 2021). If GalNAc is sialylated by ST6GalNAc2 before galactosylation, this disaccharide cannot be galactosylated (Suzuki et al. 2014). GalNAc can also remain without galactose or sialic acid; this structure is called Tn (“T nouvelle”) antigen (Dausset et al. 1959; Ju and Cummings 2005).
The above-described step-wise synthesis of IgA1 O-glycans is simplified in a sense that it considers a single site of glycosylation at a time. However, the IgA1 hinge region has a cluster of nine potential sites of O-glycosylation, thus the hinge-region amino-acid sequence serves as the initial unmodified template. The addition of a monosaccharide at one of the nine potential sites generates a new template for further glycan addition (Stewart et al. 2019; Daniel et al. 2020; Ballard et al. 2023). Thus, this process of sequential attachment of glycans at additional sites has implications for the subsequent addition of sugars at the remaining sites. As an example of the complexity of clustered O-glycosylation, we can consider the initial steps, attachment of GalNAc residues catalyzed by GalNAc-T enzymes. Most of the GalNAc-Ts, including GalNAc-T2, have two domains, a catalytic domain and a lectin domain. In general, the lectin domains exhibit glycan-binding specificity for GalNAc and are required for high-density glycosylation of GalNAc-containing glycopeptides. Moreover, lectin domains of some GalNAc-Ts exhibit an extrinsic activity that can control activity of glycosyltransferases involved in O-glycosylation pathways. This regulatory activity, described as a regulatory scaffold, involves protein–protein interactions that in some instances are substrate specific, and can exert inhibitory activity on other enzymes (Lorenz et al. 2016). The two domains of GalNAc-T2 have distinct and complementary roles in the biosynthesis of multiple sites of O-glycosylation in close proximity in the IgA1 hinge region. While the selection of the first site of addition is driven by the catalytic domain, the subsequent site-selection is influenced by the lectin domain (Stewart et al. 2019). The final glycan density, i.e. the number of GalNAc residues added to the hinge-region segment, can also be affected by the expression and activity of other GalNAc-T isoenzymes that can glycosylate IgA1 (Wandall et al. 2007). Still, there is consistent fidelity to the final distribution of IgA1 O-glycans ranging from three to six O-glycans per hinge region. Notably, selection of the initial site of GalNAc attachment impacts the utilization of sites for follow-up glycosylation (Gerken et al. 2011; de Las Rivas et al. 2019; Stewart et al. 2019; Ballard et al. 2023). In vitro studies of GalNAc-T2 enzyme reactions with IgA1 hinge-region peptide acceptor revealed that the addition of the first three sites of GalNAc is rapid and driven mostly by the catalytic domain (Stewart et al. 2019). The main initial sites utilized by GalNAc-T2 in the hinge-region peptide are Thr225 and Thr236, and the minor sites include Ser228 and Ser230. The selection of the second site is codetermined by the localization of the first site and involves both the catalytic and lectin domains of GalNAc-T2. Thus, the multiple initial sites of glycosylation and follow-up pathways determine the number of glycans added. Notably, these in vitro reactions generate glycopeptides with three to six O-glycans per hinge region, which correspond to the pattern of O-glycosylated forms of serum IgA1 (Takahashi et al. 2010; Takahashi et al. 2012; Ohyama et al. 2020b; Dotz et al. 2021; Ohyama et al. 2022). It is possible that the unique structural features of the IgA1 restrict the maximal number of O-glycans to six per hinge region. Specifically, the hinge-region segment is flanked on the N-terminal side by the globular antigen-binding fragment (Fab) and on the C-terminal side by cysteine residues involved in the formation of disulfide bonds between the component chains of IgA1 and the Fc region. Additionally, the distribution of IgA1 O-glycoforms is impacted by the presence of galactose and sialic-acid modifications on existing GalNAc residues. The addition of GalNAc moieties creates a template for addition of these monosaccharides and also generates a competition for enzyme activity and space in the hinge-region segment (Stewart et al. 2021). Ser230, Thr236, and Thr233 are the sites that commonly contain mixtures of different O-glycans, including those deficient in galactose. The process of clustered O-glycosylation and glycan density of the hinge region is likely influenced by the steric hindrances, concurrent enzyme activities, and other factors such as formation of heteromeric enzyme complexes and pH in the Golgi apparatus (Hassinen et al. 2011). Thus, many factors are likely impacting the biosynthesis of IgA1 O-glycans in IgA1-producing cells and the resultant glycan content and heterogeneity of circulatory IgA1.
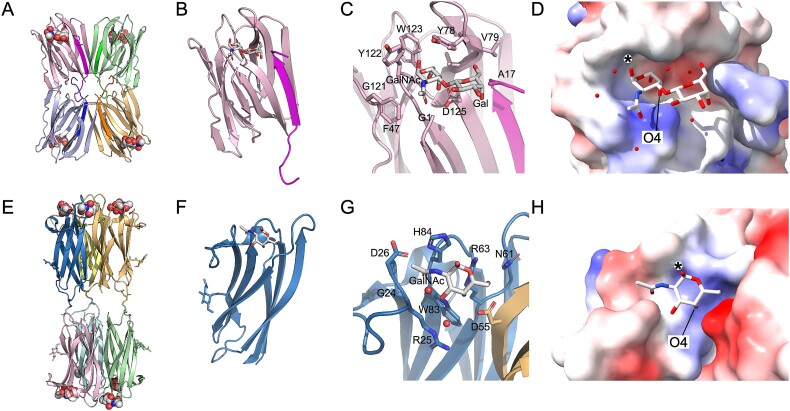
Early studies used lectins to assess O-glycosylation of IgA1 in the circulation of patients with IgAN and healthy controls (for review see (Novak et al. 2018)). Two types of lectins were especially useful: jacalin, a lectin specific for core 1 glycans (Kabir 1998; Bourne et al. 2002; Jeyaprakash et al. 2002; Wu et al. 2003; Jeyaprakash et al. 2003), and GalNAc-specific lectins from Helix aspersa and Helix pomatia. Figure 2 shows structures of jacalin and H. pomatia lectins, and illustrates the features that impart the glycan selectivity. Jacalin (Fig. 2A–D) can bind to GalNAc, galactose, and the disaccharide T antigen, although T antigen has a 36- and 100-fold greater affinity by comparison to GalNAc and galactose, respectively (Sastry et al. 1986). Crystal structures of jacalin show that the core structure is formed by a complex of the α and β chains. This heterodimer then tetramerizes to form the larger oligomer, a hetero-octamer. Structures of jacalin bound to various sugars (Sankaranarayanan et al. 1996; Jeyaprakash et al. 2002; Jeyaprakash et al. 2003) show that each monosaccharide and GalNAc of the T antigen binds to the primary glycan binding side (Fig. 2C). Figure 2A–D shows the T antigen bound to jacalin (Jeyaprakash et al. 2002). The T antigen complex reveals that GalNAc has an additional hydrogen bond mediated by the aspartic acid 125 (D125) to the O4 oxygen of GalNAc and additional interactions are contributed between the O3 oxygen of the glycosidic bond connecting the two sugars and the amine of the amino-terminal glycine in the lectin β chain (Jeyaprakash et al. 2002). Several water-mediated interactions lock the galactose residue into place. Collectively, these interactions explain why binding to the disaccharide is up to two orders of magnitude greater than binding to the monosaccharide.
Fig. 2.
Jacalin and HPA lectin structures and glycan-binding specificity. Panels (A–D) show jacalin, a lectin from Artocarpus integer, with preference for binding to O-linked Gal(β1-3) α-GalNAc. Panel (A) shows a cartoon representation of the hetero-octameric structure of jacalin composed of four chains each of the α- and β-chains [PDB ID: 1M26; (Jeyaprakash et al. 2002)] with Gal(β1-3) α-GalNAc shown in space-filling model. In (A–C), each chain is shown in alternating colors with α- and β-chains shaded in lighter and darker shades, respectively. In (B), a monomeric version of the jacalin α/β heterodimer is shown with glycan in stick model. Panel (C) shows a closeup of the glycan-binding site with amino-acid residues within five angstroms of the glycan shown as sticks and labeled. Panel (D) shows the surface rendering of jacalin with bound glycan. The surface is colored by electrostatic potential. The surface of jacalin provides an extensive pocket to accommodate the disaccharide with high specificity. Panels (E–H) show the lectin from Helix pomatia, which has a preference to bind terminal GalNAc. Panel E shows the hexameric H. pomatia complex [PDB ID: 2CVV; (Sanchez et al. 2006)], where two trimeric assemblies are covalently linked together through disulfide bonds. Each monomer harbors an N-glycan (shown as sticks) and bound GalNAc (shown in space filling model). Panel F shows the monomeric model with the GalNAc shown in stick model. Panel (G) shows a closeup of the GalNAc-binding site with amino-acid residues and water molecules within five angstroms of the glycan shown as sticks and labeled. The GalNAc binding site is generated by two adjacent lectin monomers in the trimeric assembly. Panel H features a surface rendering of the GalNAc-binding pocket colored according to electrostatic potential. For both jacalin and H. pomatia lectins, hydrogen bonding to the O4 atom of the GalNAc moiety (labeled in D and H) provides specificity to accommodate GalNAc. An (*) denotes the point of attachment of GalNAc to serine or threonine residues of a glycopeptide form of Tn antigen. Figures 2A–C and E–G were generated with PyMol (DeLano 2002). Figures 2D and H were generated with ChimeraX (Meng et al. 2023).
The lectins from H. aspersa and H. pomatia have also been studied by X-ray crystallographic methods, revealing their unique structure and specificity of glycan binding (Sanchez et al. 2006; Lescar et al. 2007; Pietrzyk et al. 2015). Figure 2E–H show the structure of the H. pomatia lectin bound to GalNAc. The active lectin is formed by six monomeric subunits assembled as two trimeric structures that are covalently bonded together through disulfide bonds. The binding site for GalNAc is comprised of amino-acid residues donated from two adjacent monomers within the trimeric substructure. Eight hydrogen bonds are formed between the GalNAc and the lectin. Three of the bonds are formed between the O4 oxygen of GalNAc and the three individual amino acids contributed from each monomer that generate the binding pocket [R63 and W83, and D55 (from the adjacent subunit)]. Atom O4 is buried deep within the pocket (Fig. 2D) and is believed to contribute to high affinity for the monosaccharide (Sanchez et al. 2006).
Studies using jacalin (Fig. 2) suggested that serum IgA1 from patients with IgAN may be differently glycosylated compared to that of healthy controls (Andre et al. 1990). However, jacalin properties may vary, depending on the origin of the lectin (Pineau et al. 1991), and additional lectins and alternative analytical techniques were explored (for review see (Novak et al. 2012; Hansen et al. 2021)). Many follow-up studies used GalNAc-specific lectins, monosaccharide compositional analysis, and mass spectrometry (Tomana et al. 1997; Allen 1999; Tomana et al. 1999; Allen et al. 2001; Hiki et al. 2001; Dotz et al. 2021). These studies determined that patients with IgAN have elevated levels of circulatory IgA1 with defects in the hinge-region O-glycans, specifically with some O-glycans missing galactose; i.e. Gd-IgA1 (Mestecky et al. 1993; Allen 1995; Tomana et al. 1997; Odani et al. 2000; Odani et al. 2010; Ohyama et al. 2020b; Ohyama et al. 2022). Furthermore, the glomerular immunodeposits of patients with IgAN are enriched for Gd-IgA1 (Allen et al. 2001; Hiki et al. 2001), thus directly linking aberrant O-glycosylation of IgA1 to the pathogenesis of IgAN.
Table 1 summarizes the findings from mass spectrometric profiling of IgA1 O-glycosylation. Multiple studies determined several features of O-glycans of circulatory IgA1 that are typical for patients with IgAN, including the reduced number of O-glycans in the hinge region and reduced galactosylation and sialylation (see specific references in Table 1). These characteristics and the elevated abundance of glycoforms with three O-glycans correlate with reduced kidney function. One study detailed O-glycans of polymeric molecular form of circulatory IgA1 and another study characterized IgA1 secreted in culture by tonsillar cells. These findings are consistent with the characteristics of glomerular IgA1 that is enriched for galactose-deficient asialo-glycoforms (Allen et al. 2001; Hiki et al. 2001). In terms of glycan attachment sites, the information is much more scarce, with current studies focused on IgA1 isolated from healthy individuals. Future studies will need to focus on the sites of glycan attachment and define the sites that are predominantly galactose deficient in IgA1 glycoforms in the circulating immune complexes. Nevertheless, the emerging information suggests that IgA1 in the circulating immune complexes is enriched for galactose-deficient asialo-glycoforms of polymeric IgA1 that is bound by IgG autoantibodies and associated with complement C3 (Hall et al. 2023).
Table 1.
O-glycosylation of IgA1 in IgA nephropathy: Examples of findings from mass spectrometric analyses.
| IgA1 source a | Findings for hinge-region glycopeptides b | References |
|---|---|---|
| Glomerular immunodeposits (IgAN) | Reduced number of O-glycans, decreased galactosylation, mostly not sialylated (4:0:0, 4:2:0, 4:2:1, 4:4:0, 6:2:0)b | (Hiki et al. 2001) |
| Serum, plasma (IgAN vs. HC) | Reduced number of O-glycans, decreased sialylation and galactosylation (e.g. elevated in IgAN: 5:3:3, 5:3:0, 5:2:1, 4:2:2, 3:1:1, 3:1:0)b Decreased sialylation correlated with faster decline in eGFR |
(Odani et al. 2000; Hiki et al. 2001; Odani et al. 2010; Dotz et al. 2021; Chen et al. 2022) |
| Serum (HC) | Sites with variable O-glycosylation, positional isomers: S230, T233, T236. Sites often deficient in galactose: T236 followed by S230, T233, T228, and S232. | (Takahashi et al. 2012; Ohyama et al. 2020b) |
| Serum, plasma (IgAN vs. HC) | Reduced number of O-glycans, elevated abundance of glycoforms with 3 O-glycans; both features associated with elevated blood pressure and reduced eGFR | (Ohyama et al. 2022) |
| Plasma (IgAN vs. DC vs. HC) | Reduced number of O-glycans, reduced galactosylation | (Zhang et al. 2022) |
| Plasma (IgAN vs. HC) Serum (IgAVN vs. IgAN vs. DC vs. HC) Tonsillar cells (IgAN, DC) |
Reduced number of O-glycans in polymeric IgA1, associated with a severe IgAN phenotype (crescents) Reduced number of O-glycans, elevated abundance of glycoforms with 3 O-glycans, reduced galactosylation Reduced galactosylation and sialylation |
(Yu et al. 2021) (Nakazawa et al. 2019a; Nakazawa et al. 2019b) (Horie et al. 2003) |
aAbbreviations: IgAN, IgA nephropathy; HC, healthy controls; DC, disease controls; IgAVN, IgA vasculitis with nephritis; eGFR, estimated glomerular filtration.
bx : y : z, number of GalNAc : galactose : sialic acid residues.
GalNAc-specific lectins from H. aspersa and H. pomatia (Fig. 2) have played an important role in Gd-IgA1 assays. After discovering a lectin specific for binding to GalNAc-containing IgA1 (Moore et al. 2007), the first quantitative lectin ELISA for Gd-IgA1 was developed (Moldoveanu et al. 2007). This assay revealed that most patients with IgAN have elevated serum levels of Gd-IgA1 (Moldoveanu et al. 2007; Shimozato et al. 2008). Moreover, serum levels of Gd-IgA1 predict disease progression of IgAN (Zhao et al. 2012; Maixnerová et al. 2019). Serum levels of Gd-IgA1 determined by the lectin ELISA test have also served as a quantitative phenotype for genome-wide association studies. Results of these studies identified two loci, corresponding to genes encoding the galactosyltransferase C1GalT1 and its chaperone C1GalT1C1 (Gale et al. 2017; Kiryluk et al. 2017), that are associated with serum levels of Gd-IgA1. Despite the progress of high-resolution analytical techniques (Odani et al. 2000; Renfrow et al. 2005; Renfrow et al. 2007; Takahashi et al. 2010; Wada et al. 2010; Takahashi et al. 2012; Ruhaak et al. 2013; Ohyama et al. 2020a; Ohyama et al. 2020b; Dotz et al. 2021; Ohyama et al. 2022), there are many advantages that these lectin assays provide, including quantitation, high-throughput processing, and ease of use.
Furthermore, GalNAc-specific lectins enabled detection of Gd-IgA1 in different molecular forms of IgA1 in serum samples from patients with IgAN or Gd-IgA1 secreted by IgA1-producing cell lines (Gomes et al. 2010; Reily et al. 2018). These approaches revealed that circulatory Gd-IgA1 is predominantly bound in immune complexes with IgG (Tomana et al. 1997; Tomana et al. 1999). The discovery of IgG autoantibodies specific for Gd-IgA1 (Suzuki et al. 2009) thus defined IgAN as an autoimmune disease wherein the nephritogenic IgG-IgA1 immune complexes are formed in the circulation (Suzuki et al. 2011).
Natural anti-glycan antibodies
Antibodies to a wide array of glycans and glycoconjugates, including those containing GalNAc, are abundant in human sera (Bovin et al. 2012; Schneider et al. 2015; Sterner et al. 2016; Purohit et al. 2018; Kappler and Hennet 2020; Marglous et al. 2024). These antibodies are generally considered to be part of the natural antibody repertoire, i.e. antibodies present in the sera of animals or humans in the absence of intentional vaccination or infection (Holodick et al. 2017).
Anti-glycan antibodies were first detected in connection with ABO blood-group antigens discovered by Karl Landsteiner (see e.g. (Landsteiner 1945)). The ABO blood groups are defined by the presence of a specific sugar on the surface of red blood cells, added to the trisaccharide called H antigen. If the H antigen is left unmodified, the resulting blood group is O, whereas A antigen has GalNAc added and B antigen has addition of D-galactose [biosynthetic pathways reviewed in (Green 1989)]. As the A and B antigens are immunogenic, individuals naturally develop antibodies against the ABO antigens they do not have. For example, individuals with blood group A will have anti-B antibodies, and individuals with blood group O will have both anti-A and anti-B antibodies.
The origin of natural antibodies is commonly ascribed to innate-like B cell populations, predominantly B1 cells. In mice, these antibodies are derived from germline immunoglobulin variable genes and are devoid of somatic mutations. However, these characteristics do not translate to humans, as the phenotypic markers that define this population in mice are more broadly expressed by human B cells (Griffin et al. 2011; Covens et al. 2013; Weisel et al. 2020). Furthermore, human immunoglobulins exhibiting the specificities of natural antibodies, such as for phosphorylcholine (Fiskesund et al. 2014) and carbohydrates, including αGal (Wang et al. 1995) or gangliosides (Sterner et al. 2017), underwent affinity maturation. Compared to antibodies against protein antigens, natural antibodies are commonly described as polyreactive and of low affinity, typically in the micromolar range. However, many of the characterized anti-carbohydrate antibodies often display exquisite selectivity and may achieve nanomolar affinity (Wang et al. 1995; Haji-Ghassemi et al. 2015; Gildersleeve and Wright 2016; Sterner et al. 2017).
There is substantial evidence that anti-carbohydrate antibodies, also called anti-glycan antibodies, arise from immune responses to the commensal microbiota. In both humans and mice, the emergence of serum anti-carbohydrate antibodies occurs postnatally, and the levels of these antibodies increase with age (Hamanova et al. 2015; Muthana and Gildersleeve 2016; Bello-Gil et al. 2019). Germ-free mice exhibit reduced levels of anti-carbohydrate antibodies which are induced following colonization. Moreover, the reactivity profiles of serum anti-carbohydrate antibodies differ between animals depending on bacteria present in gnotobiotic systems (Khasbiullina et al. 2018). Development of B cells producing antibodies reacting with a single anti-carbohydrate specificity, such as N-acetylglucosamine (GlcNAc), depends on the commensal microbiota (New et al. 2020). This conclusion was based on the analyses of murine B cells producing antibodies recognizing GlcNAc-containing carbohydrates of Streptococcus pyogenes. Compared to specific-pathogen free (SPF) mice, germ-free animals did not have serum GlcNAc-reactive antibodies; this finding was consistent with the significant reduction in the number of GlcNAc-binding B cells. Single-cell-based sequencing of these antibodies revealed a near lack of canonical gene rearrangements that are associated with GlcNAc-reactive B cells in SPF mice. Conversely, colonization of germ-free mice with a conventional microbiota restored both the GlcNAc-reactive B cells and antibody production, including IgA-producing plasma cells in the small intestine (New et al. 2020).
In humans, sera of individuals exhibiting differences in both the carbohydrates targeted and the levels of anti-carbohydrate antibodies likely reflect differences in the composition or timing of colonization of members of the microbiota (Bello-Gil et al. 2017; Luetscher et al. 2020; New et al. 2020). Carbohydrate-reactive antibody specificity appears to be directed to the terminal sugars of complex carbohydrates (Schneider et al. 2015), although glycans that share similar structures are differentially targeted. This is indicative of restricted fine specificities within anti-carbohydrate natural antibodies (Schneider et al. 2015; Luetscher et al. 2020), while precise specificities of some anti-carbohydrate antibodies, such as those recognizing ABO blood-group antigens, are genetically determined. The enzymes that generate these antigens are differentially expressed among individuals thereby defining the ABO blood-group types. As ABO blood-group structures expressed within an individual represent autoantigens, ABO-reactive B cells are differentially censored by negative selection during B-cell development. Thus, ABO blood-group types, ethnicity, and age, contribute to variations in levels of anti-carbohydrate antibodies (Muthana and Gildersleeve 2016; Luetscher et al. 2020). These factors that influence anti-glycan antibody diversity should be considered in studies of disease-specific biomarkers, such as anti-carbohydrate antibodies.
Human anti-carbohydrate antibodies are present as IgM as well as IgG and IgA isotypes with overlapping specificities (Muthana et al. 2015). The mechanisms that direct carbohydrate-reactive B cells to undergo affinity maturation and class switching are not clear. In part due to the association with mucosal responses cited above, anti-carbohydrate antibodies are commonly attributed to extra-follicular responses, and several models have been proposed to explain these observations [reviewed in (Bemark et al. 2024)]. One model postulates a participation of glycan-reactive B cells in the germinal-center reactions. Glycans covalently linked to proteinaceous antigens function as haptens; this property has been exploited as a vaccine strategy designed to induce protective immunity against the capsular polysaccharides of pathogenic bacteria, such as Streptococcus pneumoniae, where conjugate vaccines as well as non-conjugate polysaccharides are used (Davies et al. 2022).
Tumor-associated Tn antigens occur on glycoproteins, such as mucin MUC-1, and the abnormal glycosylation induces humoral immune responses (Tarp et al. 2007; Storr et al. 2008; Pinheiro et al. 2010; Stuchlova Horynova et al. 2013; Taylor-Papadimitriou et al. 2018; Bermejo et al. 2024). Tumor-associated Tn-antigen-induced antibodies are considered protective and their epitopes include GalNAc, GalNAc-glycopeptide, or GalNAc-induced glycopeptide conformations (Martinez-Saez et al. 2015; Sanz-Martinez et al. 2023). In IgAN, the precise epitopes of Gd-IgA1-specific autoantibodies are not known, but these autoantibodies are required for the formation of pathogenic immune complexes.
Microbial glycoconjugates containing GalNAc
GalNAc-containing glycoconjugates occur in some viruses and bacteria. For example, some enveloped viruses can have one or more glycoproteins with O-glycans that can contain GalNAc (Dausset et al. 1959) or sialylated GalNAc (also termed sialyl Tn antigen) (Brockhausen et al. 2022). Some of these glycoconjugates may induce corresponding antibodies. In early studies of HIV-neutralizing antibodies targeting these epitopes, it was proposed that these glycan-containing epitopes may be of clinical relevance for vaccine development (Hansen et al. 1990; Hansen et al. 1991; Hansen et al. 1996). Other enveloped viruses with O-glycans on their envelope glycoproteins include herpes simplex virus, varicella zoster virus, human cytomegalovirus, Epstein–Barr virus, as well as influenza virus and SARS-Co-V2 (Bagdonaite et al. 2016; Olofsson et al. 2016; Mayr et al. 2018; Bagdonaite et al. 2021).
Bacteria can express multiple different GalNAc-containing glycoconjugates. These structures include glycoproteins from both Gram-negative and Gram-positive bacteria, such as those found in Campylobacter jejuni (multiple proteins) (Bernatchez et al. 2005), Streptococcus mutans (Chia et al. 2001), and Streptococcus parasanguinis (fimbrial adhesin) (Stephenson et al. 2002). In Gram-negative bacteria, GalNAc is frequently found in various serotypes of the O-antigens of lipopolysaccharides, including those from Escherichia coli (Stenutz et al. 2006), Salmonella (Liu et al. 2014), and Haemophilus influenzae (Shao et al. 2002), and in the shorter lipooligosaccharides from C. jejuni (Bernatchez et al. 2005) and Neisseria gonorrhoeae (Gotschlich 1994). In Gram-positive bacteria, GalNAc is an important component of the wall teichoic and lipoteichoic acids of S. pneumoniae (Fischer et al. 1993; Seo et al. 2008), the wall teichoic acids (group antigens) of the group C, D, F, and G streptococci (Pritchard et al. 1981), and the wall teichoic acids of some Staphylococcus aureus strains (Mnich et al. 2019). In both Gram-positive and Gram-negative bacteria, GalNAc can be found in capsular polysaccharides [e.g. multiple serotypes of S. pneumoniae (Bentley et al. 2006), group 4 capsules of E. coli (Sande and Whitfield 2021)], other surface polysaccharides [e.g. those of the oral streptococci S. mitis, Streptococcus gordonii, Streptococcus oralis, S. sanguis (Koga et al. 1983; Abeygunawardana et al. 1991; Reddy et al. 1994; Cisar et al. 1997), S. aureus (Lei et al. 2024)], and in exopolysaccharides (e.g. Pel in Pseudomonas aeruginosa (Le Mauff et al. 2022) and possibly numerous other Gram-negative and Gram-positive bacteria (Bundalovic-Torma et al. 2020).
Autoantibodies specific for Gd-IgA1 in IgAN
Serologic studies showed that IgA-deficient individuals develop anti-IgA antibodies (Sennhauser et al. 1988; Koskinen et al. 1995). Furthermore, healthy individuals also produce anti-IgA antibodies and these antibodies bind to the Fab portion of IgA (Jackson et al. 1987b). These antibodies are of IgM and IgG isotypes. Additional studies performed with IgAN patients revealed that their sera have anti-IgA antibodies of IgG isotype and circulating immune complexes consisting of IgA and IgG (Jackson et al. 1987a; Jackson 1988). These results were further extended by the finding that these immune complexes in sera of IgAN patients contain IgA1 with O-glycans deficient in galactose (Tomana et al. 1997), thus linking abnormal glycosylation of IgA1 with formation of Gd-IgA1-IgG immune complexes in IgAN. Follow-up mechanistic studies determined that these immune complexes in sera of IgAN patients consist of polymeric Gd-IgA1 with IgG antibodies that recognize Gd-IgA1 in a glycan-specific context (Tomana et al. 1999), i.e. bind Gd-IgA1 with exposed GalNAc. Smaller quantities of such antibodies were found also in patients with non-IgA proliferative glomerulonephritis and in healthy controls (Tomana et al. 1999). Fab fragment that contained part of the O-glycosylated hinge-region segment with GalNAc was recognized in ELISA by antibodies of IgG isotype and, to a lesser degree, also by antibodies of IgA and IgM isotypes (Tomana et al. 1999). Removal of O-glycans from this Fab fragment by O-glycanase reduced binding of IgG and IgA1 but not of IgM. These findings suggest that abnormal O-glycosylation of IgA1 results in the generation of GalNAc-containing antigenic determinants that are recognized by IgG and IgA1 antibodies. The role and specificity of IgM antibodies in IgAN are not well understood. It is possible that IgM antibodies bind to Gd-IgA1 due to their anti-glycan activity (Matsumoto et al. 2022).
Circulatory IgG autoantibodies specific for Gd-IgA1 are found in an uncomplexed form as monomeric IgG or in immune complexes bound to Gd-IgA1 [for review see (Knoppova et al. 2016; Knoppova et al. 2021)]. These IgG autoantibodies are produced by B cells and can be detected in blood. More information about these antibodies emerged from studies using immortalized B cells derived from peripheral blood of patients with IgAN and healthy controls. These cells were used for single-cell-based cloning and sequencing of the variable segments of the heavy and light chains (VH and VL) of IgG antibodies specific for Gd-IgA1. The expanded cultures of the single-cell clones enabled characterization of the secreted IgG antibodies (Suzuki et al. 2009). Moreover, some of the VH-VL paired sequences were cloned and expressed as recombinant IgG. The IgG autoantibodies from patients with IgAN are specific for Gd-IgA1 with GalNAc residues in the hinge-region O-glycans (Suzuki et al. 2009). In vitro modification of these GalNAc residues with galactose or sialic acid blocks IgG binding. These IgG autoantibodies from IgAN patients exhibit an unusual feature: substitution of Ala97 for Ser97. This residue is located in the framework 3, near the third complementarity determining region 3 (CDR3) of the heavy chain and is a co-determinant for binding to Gd-IgA1 (Suzuki et al. 2009). This alteration originates from somatic mutations of the germline sequence rather than from rare variants of VH genes in IgAN patients (Huang et al. 2016). Notably, IgG derived from healthy controls can also bind Gd-IgA1, albeit weakly, and these clones do not contain the Ser97 substitution found in IgAN-derived IgG autoantibodies. However, when a change of Ala97 to Ser97 was introduced to recombinant IgG derived from a healthy control, this modified IgG bound Gd-IgA1 more strongly (Suzuki et al. 2009), showing 80% binding compared to the recombinant IgG derived from the IgAN patient. Follow-up studies identified additional regions of IgG autoantibodies that are essential for the recognition of Gd-IgA1. These regions include CDR1, CDR3, and framework regions of the heavy chains and framework region 3 of the light chain (Green et al. 2023). A more detailed structure–function characterization of the IgG autoantibodies will define features critical for the formation of pathogenic Gd-IgA1-containing immune complexes.
IgG autoantibodies specific for Gd-IgA1 play an important role in the clinical expression of IgAN. In sera collected at the time of kidney biopsy, serum IgG autoantibody levels correlated with proteinuria (Suzuki et al. 2009), a commonly accepted surrogate outcome biomarker in clinical trials (Thompson et al. 2019). Elevated serum levels of IgG autoantibodies are predictive of disease progression (Berthoux et al. 2012; Maixnerová et al. 2019). Furthermore, serum levels of Gd-IgA1 and the corresponding IgG autoantibodies correlate in IgAN patients, but not in disease or healthy controls (Yanagawa et al. 2014; Placzek et al. 2018).
Immune complexes consisting of IgG autoantibodies and Gd-IgA1 in IgAN
Multiple lines of evidence support the hypothesis that immune complexes consisting of Gd-IgA1 and IgG autoantibodies play a key role in IgAN (Fig. 3) (Suzuki et al. 2011; Novak et al. 2012; Wyatt and Julian 2013; Kiryluk and Novak 2014; Knoppova et al. 2016; Lai et al. 2016). This conclusion is further supported by the finding that IgG is in the immunodeposits in all biopsies of patients with IgAN, even those in kidney biopsies without detection of IgG by routine immunofluorescence (Rizk et al. 2019; Rizk et al. 2023). Furthermore, this IgG, but not IgG extracted from immunodeposits of patients with membranous nephropathy or lupus nephritis, is specific for Gd-IgA1 (Rizk et al. 2019).
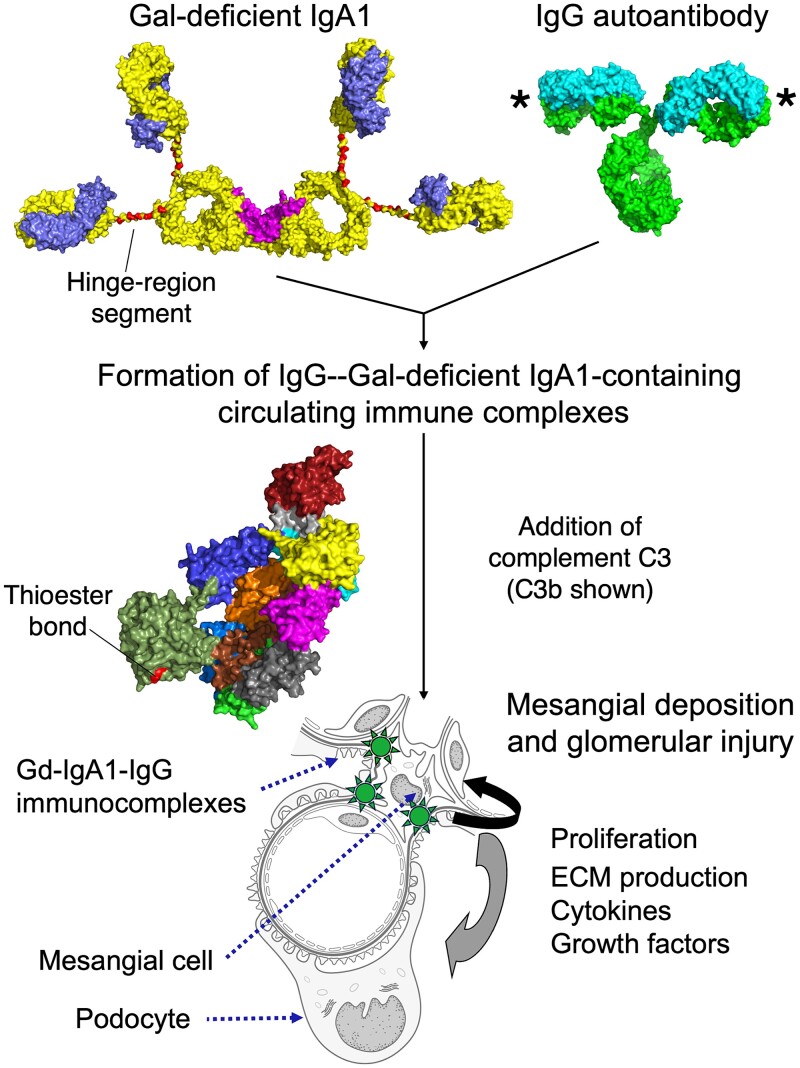
Fig. 3.
Pathogenesis model of IgA nephropathy. Pathogenesis of the autoimmune disease IgA nephropathy (IgAN) has been described as a multi-step process of IgA1 and IgG antibody generation, immune-complex formation, and deposition in the glomeruli, leading to kidney injury (Suzuki et al. 2011). Polymeric IgA1 (dimeric in this figure) harboring O-glycans in the hinge region, with an incomplete repertoire of galactose moieties [galactose (Gal)-deficient IgA1 (Gd-IgA1)], is found elevated in the circulation of most IgAN patients and some of these glycoforms are recognized as an autoantigen. IgG autoantibodies with a specificity for the glycosylated hinge region of Gd-IgA1 bind Gd-IgA1 (antigen-binding sites of IgG marked by *), and pathogenic immune complexes are formed. Larger complexes are formed as additional proteins are added, including complement C3. C3 or activated C3b covalently binds to IgA1 or IgG through the thioester bond found in the thioester-containing domain. Some of these immune complexes pass through the fenestration of glomerular endothelial cells, enter glomerular mesangial space, and activate mesangial cells. A cascade of effects is associated with mesangial-cell activation, including cellular proliferation and overproduction of extracellular matrix (ECM), cytokines, chemokines, some of which and can activate podocytes. These events can lead to glomerular injury. Glycan content at up to six Ser/Thr residues (225, 228, 230, 232, 233, 236; shaded red) and the IgA1 hinge-region conformation distinguishes whether the IgA1 molecule is viewed as normal IgA1 or is identified as an autoantigen. Space filling models of IgA1, IgG, and complement C3 were generated from coordinates published in (Person et al. 2022), (PDB ID: 1IGT; (Harris et al. 1997)), and (PDB ID: 2I07; (Janssen et al. 2006)), respectively. Component chains of Gd-IgA1 and IgG are shown in different colors. Complement C3b is colored by domain as in (Rajasekaran et al. 2023).
Experimental proof of the pathogenic potential of IgG autoantibodies in vivo was obtained by using a passive mouse model of IgAN. In this model, immunodeficient mice are injected with immune complexes formed in vitro from human Gd-IgA1 and human IgG autoantibodies. These i.v.-injected immune complexes induce mesangioproliferative glomerular injury, whereas individual components alone (i.e. Gd-IgA1 or IgG) do not (Moldoveanu et al. 2021). Notably, this animal model of IgAN can be used for pre-clinical testing and assessment of mechanisms of drugs for IgAN treatments, such as those that are kidney-targeting (Reily et al. 2024).
Analyses of serum samples from patients with IgAN indicated that the amounts of IgA-IgG immune complexes are elevated at the time of disease activity as compared to those from the time of disease quiescence (Coppo et al. 1982; Novak et al. 2011b). Using a model of cultured primary human mesangial cells enables additional testing, such as assessment of the influence of various factors on cellular proliferation and production of cytokines, chemokines, and components of extracellular matrix (Chen et al. 1994; Novak et al. 2002; Novak et al. 2005; Novak et al. 2008; Novak et al. 2011a; Novak et al. 2011b; Ebefors et al. 2016; Liu et al. 2017; Bian et al. 2021). Based on the use of size-exclusion chromatography for isolation of immune complexes, it was determined that large-molecular-mass IgA-IgG complexes (Mr >700 kDa) stimulate cellular proliferation of quiescent primary human mesangial cells in culture (Hall et al. 2023). These immune complexes contain IgA1, IgG, and complement C3. IgA1 in these complexes is enriched for galactose-deficient and minimally sialylated O-glycoforms. Stimulatory activity of these immune complexes is removed by depleting the serum of total IgA1 by jacalin affinity chromatography. This process removes IgA1-containing immune complexes with IgG, and C3, suggesting that these components are associated in the stimulatory IgA1-containing immune complexes. Furthermore, C3 in these complexes is covalently associated with IgG as well as IgA1, presumably through the thioester bond of C3 (Fig. 3). Molecular forms of C3 in these complexes include C3 as well as its activated and inactivated forms, i.e. C3b and iC3b, respectively (Hall et al. 2023).
These results together indicate that the pathogenic immune complexes in IgAN contain Gd-IgA1 that is bound by IgG autoantibodies, and that complement C3 is associated with these complexes, with at least some C3 covalently bound to the immunoglobulins. Future studies will determine the function and stoichiometry of individual components and the mechanism by which these complexes activate mesangial cells.
Emerging and future treatment approaches for IgAN
The basic approach in IgAN therapy has been based on the control of blood pressure. With the improved understanding of the pathogenetic pathways in IgAN, new opportunities for treatment emerged and current therapies and clinical trials have been summarized in a recent review (Lim et al. 2024). These approaches include gut-released budesonide to reduce gut-produced IgA, B-cell targeting therapies to reduce production of IgA and IgG, and thus Gd-IgA1 and IgG autoantibodies, complement-targeting reagents to reduce complement activation, and kidney-specific treatments to mitigate responses of resident glomerular cells. While all these approaches have a good rationale, the issue is which of the treatments can sufficiently reduce the eGFR slope to prevent the progressive loss of kidney function. With the new and emerging knowledge on complement C3, IgA1 O-glycoforms, and IgG autoantibodies that are all needed to form nephritogenic complexes, it is hoped that additional potential targets emerge that may enable removal of Gd-IgA1-containing immune complexes from the circulation or mitigate complement C3 attachment to the complexes. Other approaches that can prevent formation of nephritogenic immune complexes may utilize blockade or removal of autoantibodies or removal of the cells that produce them. A better understanding of the pathophysiology of this complex disease may inform development of such new approaches in the future.
Concluding remarks
The last two decades witnessed significant advances in the understanding of the pathogenesis of IgAN. IgAN is now recognized as an autoimmune disease wherein Gd-IgA1 is the main autoantigen bound by IgG autoantibodies. These Gd-IgA1-IgG complexes formed in the circulation can then associate with other proteins, such as complement C3. Some of these complexes that are not cleared from the blood may enter the mesangial space in the glomeruli, activate the resident mesangial cells, and induce glomerular injury (Fig. 3). This hypothesis provides a framework for developing preclinical tests, designing clinical trials, and selecting biomarkers (Lim et al. 2024). However, despite the progress, a recent study revealed that most IgAN patients progress to kidney failure within 10–15 years from diagnosis and that only few patients may avoid kidney failure in their lifetime (Pitcher et al. 2023). It is therefore important to determine the key biochemical, immunological, and genetic factors involved in pathogenesis of IgAN to develop a knowledge base for new disease-specific therapeutic approaches. Defining molecular mechanisms of aberrant IgA1 O-glycosylation leading to the production of autoantigen and the recognition by IgG autoantibodies are among the key issues. New mechanistic studies may provide insights to inform development of therapeutic approaches that target abnormal IgA1 O-glycosylation, production of IgG autoantibodies, or immune-complex formation and catabolism (Knoppova et al. 2021). Furthermore, determining the role of C3 in the pathogenicity of IgA1 immune complexes will inform the utility of complement modulation in IgAN (Rajasekaran et al. 2023).
Contributor Information
Jan Novak, Department of Microbiology, University of Alabama at Birmingham, 845 19th Street South, Birmingham, AL 35294, United States.
R Glenn King, Department of Microbiology, University of Alabama at Birmingham, 845 19th Street South, Birmingham, AL 35294, United States.
Janet Yother, Department of Microbiology, University of Alabama at Birmingham, 845 19th Street South, Birmingham, AL 35294, United States.
Matthew B Renfrow, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, 720 20th Street South, Birmingham, AL 35294, United States.
Todd J Green, Department of Microbiology, University of Alabama at Birmingham, 845 19th Street South, Birmingham, AL 35294, United States.
Author contributions
Jan Novak (Conceptualization [equal], Funding acquisition [equal], Resources [lead], Writing—original draft [lead], Writing—review & editing [lead]), R. Glenn King (Conceptualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Janet Yother (Conceptualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Matthew B. Renfrow (Conceptualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), and Todd J. Green (Conceptualization [equal], Funding acquisition [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal])
Funding
This work was supported in part by grants from the National Institutes of Health DK078244, AI149431, DK082753, and DK105124; a gift from IGA Nephropathy Foundation; and by UAB Research Acceleration Funds. The authors apologize to their colleagues in the field whose work is not adequately discussed or cited in this article due to space limitations.
Conflict of interest statement. JN is a co-founder and co-owner of and consultant for Reliant Glycosciences, LLC. MBR is a co-founder and co-owner of Reliant Glycosciences, LLC. JN and MBR are co-inventors on US patent application 14/318,082 (assigned to the UAB Research Foundation [UABRF] and licensed by UABRF to Reliant Glycosciences, LLC).
References
- Abeygunawardana C, Bush CA, Cisar JO. Complete structure of the cell surface polysaccharide of Streptococcus oralis C104: a 600-MHz NMR study. Biochemistry. 1991:30(35):8568–8577. [DOI] [PubMed] [Google Scholar]
- Allen AC. Abnormal glycosylation of IgA: is it related to the pathogenesis of IgA nephropathy? Nephrol Dial Transplant. 1995:10(7):1121–1124. [PubMed] [Google Scholar]
- Allen AC. Methodological approaches to the analysis of IgA1 O-glycosylation in IgA nephropathy. J Nephrol. 1999:12(2):76–84. [PubMed] [Google Scholar]
- Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995:100(3):470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AC, Bailey EM, Brenchley PEC, Buck KS, Barratt J, Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001:60(3):969–973. [DOI] [PubMed] [Google Scholar]
- Andre PM, Le Pogamp P, Chevet D. Impairment of jacalin binding to serum IgA in IgA nephropathy. J Clin Lab Anal. 1990:4(2):115–119. [DOI] [PubMed] [Google Scholar]
- Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974a:249(22):7270–7281. [PubMed] [Google Scholar]
- Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974b:249(22):7260–7269. [PubMed] [Google Scholar]
- Bagdonaite I, Norden R, Joshi HJ, King SL, Vakhrushev SY, Olofsson S, Wandall HH. Global mapping of O-glycosylation of varicella zoster virus, human cytomegalovirus, and Epstein-Barr virus. J Biol Chem. 2016:291(23):12014–12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdonaite I, Thompson AJ, Wang X, Sogaard M, Fougeroux C, Frank M, Diedrich JK, Yates JR 3rd, Salanti A, Vakhrushev SY, et al. Site-specific O-glycosylation analysis of SARS-CoV-2 spike protein produced in insect and human cells. Viruses. 2021:13(4):551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard CJ, Paserba MR, Paul Daniel EJ, Hurtado-Guerrero R, Gerken TA. Polypeptide N-acetylgalactosaminyltransferase (GalNAc-T) isozyme surface charge governs charge substrate preferences to modulate mucin type O-glycosylation. Glycobiology. 2023:33(10):817–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Gil D, Khasbiullina N, Shilova N, Bovin N, Manez R. Repertoire of BALB/c mice natural anti-carbohydrate antibodies: mice vs. humans difference, and otherness of individual animals. Front Immunol. 2017:8:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Gil D, Audebert C, Olivera-Ardid S, Perez-Cruz M, Even G, Khasbiullina N, Gantois N, Shilova N, Merlin S, Costa C, et al. The formation of glycan-specific natural antibodies repertoire in GalT-KO mice is determined by gut microbiota. Front Immunol. 2019:10:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemark M, Pitcher MJ, Dionisi C, Spencer J. Gut-associated lymphoid tissue: a microbiota-driven hub of B cell immunity. Trends Immunol. 2024:45(3):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012:22(6):736–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006:2(3):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J. Recurrence of IgA nephropathy in renal allografts. Am J Kidney Dis. 1988:12(5):371–372. [DOI] [PubMed] [Google Scholar]
- Berger J, Hinglais N. Les dépôts intercapillaires d'IgA-IgG (Intercapillary deposits of IgA-IgG). J Urol Nephrol. 1968:74(9):694–695. [PubMed] [Google Scholar]
- Bermejo IA, Guerreiro A, Eguskiza A, Martinez-Saez N, Lazaris FS, Asin A, Somovilla VJ, Companon I, Raju TK, Tadic S, et al. Structure-guided approach for the development of MUC1-glycopeptide-based cancer vaccines with predictable responses. JACS Au. 2024:4(1):150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatchez S, Szymanski CM, Ishiyama N, Li J, Jarrell HC, Lau PC, Berghuis AM, Young NM, Wakarchuk WW. A single bifunctional UDP-GlcNAc/Glc 4-epimerase supports the synthesis of three cell surface glycoconjugates in Campylobacter jejuni. J Biol Chem. 2005:280(6):4792–4802. [DOI] [PubMed] [Google Scholar]
- Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol. 2012:23(9):1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Q, Anderson JC, Zhang XW, Huang ZQ, Ebefors K, Nystrom J, Hall S, Novak L, Julian BA, Willey CD, et al. Mesangioproliferative kidney diseases and platelet-derived growth factor-mediated AXL phosphorylation. Kidney Med. 2021:3(6):1003–1013.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Astoul CH, Zamboni V, Peumans WJ, Menu-Bouaouiche L, Van Damme EJ, Barre A, Rouge P. Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem J. 2002:364(1):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovin N, Obukhova P, Shilova N, Rapoport E, Popova I, Navakouski M, Unverzagt C, Vuskovic M, Huflejt M. Repertoire of human natural anti-glycan immunoglobulins. Do we have auto-antibodies? Biochim Biophys Acta. 2012:1820(9):1373–1382. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin Exp Immunol. 1981:44(2):221–232. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P, Prydz M. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of polymeric immunoglobulins. Nature. 1984:311(5981):71–73. [DOI] [PubMed] [Google Scholar]
- Brockhausen I, Wandall HH, Hagen KGT, Stanley P. O-GalNAc glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Mohnen D, Kinoshita T, Packer NH, Prestegard JH, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2022. pp. 117–128 [Google Scholar]
- Bundalovic-Torma C, Whitfield GB, Marmont LS, Howell PL, Parkinson J. A systematic pipeline for classifying bacterial operons reveals the evolutionary landscape of biofilm machineries. PLoS Comput Biol. 2020:16(4):e1007721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakantan A, Ratanapanichkich P, Said M, Barker CV, Julian BA. Recurrent IgA nephropathy after renal transplantation despite immunosuppressive regimens with mycophenolate mofetil. Nephrol Dial Transplant. 2005:20(6):1214–1221. [DOI] [PubMed] [Google Scholar]
- Chen A, Chen WP, Sheu LF, Lin CY. Pathogenesis of IgA nephropathy: in vitro activation of human mesangial cells by IgA immune complex leads to cytokine secretion. J Pathol. 1994:173(2):119–126. [DOI] [PubMed] [Google Scholar]
- Chen HF, Kao CC, Ka SM, Wang SY, Chen MX, Chen GY, Weng TI, Lai RY, Yeh SC, Lin YC, et al. Development of an enrichment-free one-pot sample preparation and ultra-high performance liquid chromatography-tandem mass spectrometry method to identify immunoglobulin A1 hinge region O-glycoforms for immunoglobulin A nephropathy. J Chromatogr A. 2022:1685:463589. [DOI] [PubMed] [Google Scholar]
- Chia JS, Chang LY, Shun CT, Chang YY, Tsay YG, Chen JY. A 60-kilodalton immunodominant glycoprotein is essential for cell wall integrity and the maintenance of cell shape in Streptococcus mutans. Infect Immun. 2001:69(11):6987–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar JO, Sandberg AL, Reddy GP, Abeygunawardana C, Bush CA. Structural and antigenic types of cell wall polysaccharides from viridans group streptococci with receptors for oral actinomyces and streptococcal lectins. Infec Immun. 1997:65(12):5035–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley ME, Cooper MD, Michael AF. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J Clin Invest. 1980:66(6):1432–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppo R, Basolo B, Martina G, Rollino C, De Marchi M, Giacchino F, Mazzucco G, Messina M, Piccoli G. Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgA nephropathy and with Henoch-Schönlein nephritis. Correlation with clinical and histologic signs of activity. Clin Nephrol. 1982:18(5):230–239. [PubMed] [Google Scholar]
- Covens K, Verbinnen B, Geukens N, Meyts I, Schuit F, Van Lommel L, Jacquemin M, Bossuyt X. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013:121(26):5176–5183. [DOI] [PubMed] [Google Scholar]
- Daniel EJP, Las Rivas M, Lira-Navarrete E, Garcia-Garcia A, Hurtado-Guerrero R, Clausen H, Gerken TA. Ser and Thr acceptor preferences of the GalNAc-Ts vary among isoenzymes to modulate mucin-type O-glycosylation. Glycobiology. 2020:30(11):910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J, Moullec J, Bernard J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn). Blood. 1959:14(10):1079–1093. [PubMed] [Google Scholar]
- Davies LRL, Cizmeci D, Guo W, Luedemann C, Alexander-Parrish R, Grant L, Isturiz R, Theilacker C, Jodar L, Gessner BD, et al. Polysaccharide and conjugate vaccines to Streptococcus pneumoniae generate distinct humoral responses. Sci Transl Med. 2022:14(656):eabm4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin JC. Henoch-Schönlein purpura nephritis: pathophysiology, treatment, and future strategy. Clin J Am Soc Nephrol. 2011:6(3):679–689. [DOI] [PubMed] [Google Scholar]
- Davin JC, Ten Berge IJ, Weening JJ. What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis? Kidney Int. 2001:59(3):823–834. [DOI] [PubMed] [Google Scholar]
- de Sousa-Pereira P, Woof JM. IgA: structure, function, and developability. Antibodies (Basel). 2019:8(4):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL molecular graphics system. New York, NY, USA: Schrödinger, LLC; 2002. Available online: http://www.pymol.org [Google Scholar]
- Dotz V, Visconti A, Lomax-Browne HJ, Clerc F, Hipgrave Ederveen AL, Medjeral-Thomas NR, Cook HT, Pickering MC, Wuhrer M, Falchi M. O- and N-glycosylation of serum immunoglobulin A is associated with IgA nephropathy and glomerular function. J Am Soc Nephrol. 2021:32(10):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebefors K, Liu P, Lassen E, Elvin J, Candemark E, Levan K, Haraldsson B, Nystrom J. Mesangial cells from patients with IgA nephropathy have increased susceptibility to galactose-deficient IgA1. BMC Nephrol. 2016:17(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Behr T, Hartmann R, Peter-Katalinic J, Egge H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide). Eur J Biochem. 1993:215(3):851–857. [DOI] [PubMed] [Google Scholar]
- Fiskesund R, Steen J, Amara K, Murray F, Szwajda A, Liu A, Douagi I, Malmstrom V, Frostegard J. Naturally occurring human phosphorylcholine antibodies are predominantly products of affinity-matured B cells in the adult. J Immunol. 2014:192(10):4551–4559. [DOI] [PubMed] [Google Scholar]
- Floege J. Recurrent IgA nephropathy after renal transplantation. Semin Nephrol. 2004:24(3):287–291. [DOI] [PubMed] [Google Scholar]
- Frangione B, Wolfenstein-Todel C. Partial duplication in the "hinge" region of IgA1 myeloma proteins. Proc Natl Acad Sci USA. 1972:69(12):3673–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale DP, Molyneux K, Wimbury D, Higgins P, Levine AP, Caplin B, Ferlin A, Yin P, Nelson CP, Stanescu H, et al. Galactosylation of IgA1 is associated with common variation in C1GALT1. J Am Soc Nephrol. 2017:28(7):2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken TA. Kinetic modeling confirms the biosynthesis of mucin core 1 (β-Gal(1-3) α-GalNAc-O-Ser/Thr) O-glycan structures are modulated by neighboring glycosylation effects. Biochemistry. 2004:43(14):4137–4142. [DOI] [PubMed] [Google Scholar]
- Gerken TA, Owens CL, Pasumarthy M. Determination of the site-specific O-glycosylation pattern of the porcine submaxillary mucin tandem repeat glycopeptide. Model proposed for the polypeptide:galnac transferase peptide binding site. J Biol Chem. 1997:272(15):9709–9719. [DOI] [PubMed] [Google Scholar]
- Gerken TA, Jamison O, Perrine CL, Collette JC, Moinova H, Ravi L, Markowitz SD, Shen W, Patel H, Tabak LA. Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J Biol Chem. 2011:286(16):14493–14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildersleeve JC, Wright WS. Diverse molecular recognition properties of blood group A binding monoclonal antibodies. Glycobiology. 2016:26(5):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MM, Suzuki H, Brooks MT, Tomana M, Moldoveanu Z, Mestecky J, Julian BA, Novak J, Herr AB. Recognition of galactose-deficient O-glycans in the hinge region of IgA1 by N-acetylgalactosamine-specific snail lectins: a comparative binding study. Biochemistry. 2010:49(27):5671–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich EC. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J Exp Med. 1994:180(6):2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. The ABO, Lewis and related blood group antigens; a review of structure and biosynthesis. FEMS Microbiol Immunol. 1989:1(6–7):321–330. [DOI] [PubMed] [Google Scholar]
- Green TJ, Lingo J, Knoppova B, Qiu S, Shang Q, Hall SD, Huang ZQ, Suzuki H, Novak J. Structure and function studies of IgG autoantibodies specific for aberrantly O-glycosylated IgA1 in IgA nephropathy. Glycobiology. 2023:33:1018. [Google Scholar]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011:208(1):67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji-Ghassemi O, Blackler RJ, Martin Young N, Evans SV. Antibody recognition of carbohydrate epitopes. Glycobiology. 2015:25(9):920–952. [DOI] [PubMed] [Google Scholar]
- Hall S, Gurganus G, Huang ZQ, Maillard N, Moldoveanu Z, Rizk DV, Julian BA, Renfrow MB, Novak J. Aberrantly O-glycosylated IgA1 in the pathogenic circulating immune complexes in patients with IgA nephropathy is associated with IgG and complement C3. Glycobiology. 2023:33:1014. [Google Scholar]
- Hamanova M, Chmelikova M, Nentwich I, Thon V, Lokaj J. Anti-Gal IgM, IgA and IgG natural antibodies in childhood. Immunol Lett. 2015:164(1):40–43. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Clausen H, Nielsen C, Teglbjaerg LS, Hansen LL, Nielsen CM, Dabelsteen E, Mathiesen L, Hakomori SI, Nielsen JO. Inhibition of human immunodeficiency virus (HIV) infection in vitro by anticarbohydrate monoclonal antibodies: peripheral glycosylation of HIV envelope glycoprotein gp120 may be a target for virus neutralization. J Virol. 1990:64(6):2833–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JE, Nielsen C, Arendrup M, Olofsson S, Mathiesen L, Nielsen JO, Clausen H. Broadly neutralizing antibodies targeted to mucin-type carbohydrate epitopes of human immunodeficiency virus. J Virol. 1991:65(12):6461–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JE, Jansson B, Gram GJ, Clausen H, Nielsen JO, Olofsson S. Sensitivity of HIV-1 to neutralization by antibodies against O-linked carbohydrate epitopes despite deletion of O-glycosylation signals in the V3 loop. Arch Virol. 1996:141(2):291–300. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Reily C, Novak J, Renfrow MB. Immunoglobulin A glycosylation and its role in disease. Exp Suppl. 2021:112:433–477. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Larson SB, Hasel KW, McPherson A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry. 1997:36(7):1581–1597. [DOI] [PubMed] [Google Scholar]
- Hassinen A, Pujol FM, Kokkonen N, Pieters C, Kihlstrom M, Korhonen K, Kellokumpu S. Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J Biol Chem. 2011:286(44):38329–38340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MC, Bursac Z, Julian BA, Villa Baca E, Featherston J, Woodford SY, Bailey L, Wyatt RJ. Life expectancy for patients from the southeastern United States with IgA nephropathy. Kidney Int Rep. 2018:3(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MC, Rizk DV, Kiryluk K, Nelson R, Zahr RS, Novak J, Wyatt RJ. IgA vasculitis with nephritis: update of pathogenesis with clinical implications. Pediatr Nephrol. 2022:37(4):719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, Shinzato T, Kobayashi Y, Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001:59(3):1077–1085. [DOI] [PubMed] [Google Scholar]
- Holodick NE, Rodriguez-Zhurbenko N, Hernandez AM. Defining natural antibodies. Front Immunol. 2017:8:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie A, Hiki Y, Odani H, Yasuda Y, Takahashi M, Kato M, Iwase H, Kobayashi Y, Nakashima I, Maeda K. IgA1 molecules produced by tonsillar lymphocytes are under-O-glycosylated in IgA nephropathy. Am J Kidney Dis. 2003:42(3):486–496. [DOI] [PubMed] [Google Scholar]
- Huang ZQ, Raska M, Stewart TJ, Reily C, King RG, Crossman DK, Crowley MR, Hargett A, Zhang Z, Suzuki H, et al. Somatic mutations modulate autoantibodies against galactose-deficient IgA1 in IgA nephropathy. J Am Soc Nephrol. 2016:27(11):3278–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Zhang Y, Tachibana K, Gotoh M, Kikuchi N, Kwon YD, Togayachi A, Kudo T, Kubota T, Narimatsu H. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J Biol Chem. 2003:278(8):5613–5621. [DOI] [PubMed] [Google Scholar]
- Jackson S. Immunoglobulin-antiimmunoglobulin interactions and immune complexes in IgA nephropathy. Am J Kidney Dis. 1988:12(5):425–429. [DOI] [PubMed] [Google Scholar]
- Jackson S, Montgomery RI, Julian BA, Galla JH, Czerkinsky C. Aberrant synthesis of antibodies directed at the Fab of IgA in patients with IgA nephropathies. Clin Immunol Immunopath. 1987a:45(2):208–213. [DOI] [PubMed] [Google Scholar]
- Jackson S, Montgomery RI, Mestecky J, Czerkinsky C. Normal human sera contain antibodies directed at Fab of IgA. J Immunol. 1987b:138(7):2244–2248. [PubMed] [Google Scholar]
- Janssen BJ, Christodoulidou A, McCarthy A, Lambris JD, Gros P. Structure of C3b reveals conformational changes that underlie complement activity. Nature. 2006:444(7116):213–216. [DOI] [PubMed] [Google Scholar]
- Jarrick S, Lundberg S, Welander A, Carrero JJ, Hoijer J, Bottai M, Ludvigsson JF. Mortality in IgA nephropathy: A nationwide population-based cohort study. J Am Soc Nephrol. 2019:30(5):866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash AA, Geetha Rani P, Banuprakash Reddy G, Banumathi S, Betzel C, Sekar K, Surolia A, Vijayan M. Crystal structure of the jacalin-T-antigen complex and a comparative study of lectin-T-antigen complexes. J Mol Biol. 2002:321(4):637–645. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Katiyar S, Swaminathan CP, Sekar K, Surolia A, Vijayan M. Structural basis of the carbohydrate specificities of jacalin: an X-ray and modeling study. J Mol Biol. 2003:332(1):217–228. [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc Natl Acad Sci USA. 2002:99(26):16613–16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005:437(7063):1252. [DOI] [PubMed] [Google Scholar]
- Ju T, Brewer K, D'Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 β1,3-galactosyltransferase. J Biol Chem. 2002:277(1):178–186. [DOI] [PubMed] [Google Scholar]
- Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004:13(2):171–179. [DOI] [PubMed] [Google Scholar]
- Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med. 1988:84(1):129–132. [DOI] [PubMed] [Google Scholar]
- Kabir S. Jacalin: a jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J Immunol Methods. 1998:212(2):193–211. [DOI] [PubMed] [Google Scholar]
- Kamei K, Ogura M, Sato M, Ito S, Ishikura K. Evolution of IgA nephropathy into anaphylactoid purpura in six cases—further evidence that IgA nephropathy and Henoch–Schönlein purpura nephritis share common pathogenesis. Pediatr Nephrol. 2016:31(5):779–785. [DOI] [PubMed] [Google Scholar]
- Kappler K, Hennet T. Emergence and significance of carbohydrate-specific antibodies. Genes Immun. 2020:21(4):224–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasbiullina NR, Shilova NV, Navakouski ME, Nokel AY, Knirel YA, Blixt O, Bovin NV. Repertoire of Abs primed by bacteria in gnotobiotic mice. Innate Immun. 2018:24(3):180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. 2014:124(6):2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int. 2011:80(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryluk K, Novak J, Gharavi AG. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med. 2013:64(1):339–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryluk K, Li Y, Moldoveanu Z, Suzuki H, Reily C, Hou P, Xie J, Mladkova N, Prakash S, Fischman C, et al. GWAS for serum galactose-deficient IgA1 implicates critical genes of the O-glycosylation pathway. PLoS Genet. 2017:13(2):e1006609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, Raska M, Renfrow MB, Julian BA, Novak J. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol. 2016:7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoppova B, Reily C, King RG, Julian BA, Novak J, Green TJ. Pathogenesis of IgA nephropathy: current understanding and implications for development of disease-specific treatment. J Clin Med. 2021:10(19):4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Okahashi N, Yamamoto T, Mizuno J, Inoue M, Hamada S. Purification and immunochemical characterization of Streptococcus sanguis ATCC 10557 serotype II carbohydrate antigen. Infect Immun. 1983:42(2):696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen S, Tolo H, Hirvonen M, Koistinen J. Long-term follow-up of anti-IgA antibodies in healthy IgA-deficient adults. J Clin Immunol. 1995:15(4):194–198. [DOI] [PubMed] [Google Scholar]
- Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, Glassock RJ. IgA nephropathy. Nat Rev Dis Primers. 2016:2(1):16001. [DOI] [PubMed] [Google Scholar]
- Landsteiner K. The specificity of serological reactions. New York, NY: Dover Publications, Inc.; 1945 [Google Scholar]
- de Las Rivas M, Lira-Navarrete E, Gerken TA, Hurtado-Guerrero R. Polypeptide GalNAc-Ts: from redundancy to specificity. Curr Opin Struct Biol. 2019:56:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KK, Suzuki H, Novak J, Wyatt RJ. Pathogenesis of Henoch-Schönlein purpura nephritis. Ped Nephrol. 2010:25(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mauff F, Razvi E, Reichhardt C, Sivarajah P, Parsek MR, Howell PL, Sheppard DC. The Pel polysaccharide is predominantly composed of a dimeric repeat of α-1,4 linked galactosamine and N-acetylgalactosamine. Commun Biol. 2022:5(1):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei MG, Jorgenson MA, Robbs EJ, Black IM, Archer-Hartmann S, Shalygin S, Azadi P, Lee CY. Characterization of Ssc, an N-acetylgalactosamine-containing Staphylococcus aureus surface polysaccharide. J Bacteriol. 2024:206(5):e0004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescar J, Sanchez JF, Audfray A, Coll JL, Breton C, Mitchell EP, Imberty A. Structural basis for recognition of breast and colon cancer epitopes Tn antigen and Forssman disaccharide by Helix pomatia lectin. Glycobiology. 2007:17(10):1077–1083. [DOI] [PubMed] [Google Scholar]
- Lim RS, Yeo SC, Barratt J, Rizk DV. An update on current therapeutic options in IgA nephropathy. J Clin Med. 2024:13(4):947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, Wang L. Structural diversity in salmonella O antigens and its genetic basis. FEMS Microbiol Rev. 2014:38(1):56–89. [DOI] [PubMed] [Google Scholar]
- Liu P, Lassen E, Nair V, Berthier CC, Suguro M, Sihlbom C, Kretzler M, Betsholtz C, Haraldsson B, Ju W, et al. Transcriptomic and proteomic profiling provides insight into mesangial cell function in IgA nephropathy. J Am Soc Nephrol. 2017:28(10):2961–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz V, Ditamo Y, Cejas RB, Carrizo ME, Bennett EP, Clausen H, Nores GA, Irazoqui FJ. Extrinsic functions of lectin domains in O-N-acetylgalactosamine glycan biosynthesis. J Biol Chem. 2016:291(49):25339–25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetscher RND, McKitrick TR, Gao C, Mehta AY, McQuillan AM, Kardish R, Boligan KF, Song X, Lu L, Heimburg-Molinaro J, et al. Unique repertoire of anti-carbohydrate antibodies in individual human serum. Sci Rep. 2020:10(1):15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015:26(7):1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixnerová D, Ling C, Hall S, Reily C, Brown R, Neprasova M, Suchanek M, Honsova E, Zima T, Novak J, et al. Galactose-deficient IgA1 and the corresponding IgG autoantibodies predict IgA nephropathy progression. PLoS One. 2019:14(2):e0212254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marglous S, Brown CE, Padler-Karavani V, Cummings RD, Gildersleeve JC. Serum antibody screening using glycan arrays. Chem Soc Rev. 2024:53(5):2603–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Saez N, Castro-Lopez J, Valero-Gonzalez J, Madariaga D, Companon I, Somovilla VJ, Salvado M, Asensio JL, Jimenez-Barbero J, Avenoza A, et al. Deciphering the non-equivalence of serine and threonine O-glycosylation points: implications for molecular recognition of the Tn antigen by an anti-MUC1 antibody. Angew Chem Int Ed Engl. 2015:54(34):9830–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Aryal RP, Heimburg-Molinaro J, Park SS, Wever WJ, Lehoux S, Stavenhagen K, van Wijk JAE, Van Die I, Chapman AB, et al. Identification and characterization of circulating immune complexes in IgA nephropathy. Sci Adv. 2022:8(43):eabm8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998:273(4):2260–2272. [DOI] [PubMed] [Google Scholar]
- Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Menard S, Candalh C, Ben-Khalifa K, Dugave C, Tamouza H, van Niel G, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008:205(1):143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr J, Lau K, Lai JCC, Gagarinov IA, Shi Y, McAtamney S, Chan RWY, Nicholls J, von Itzstein M, Haselhorst T. Unravelling the role of O-glycans in influenza A virus infection. Sci Rep. 2018:8(1):16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng EC, Goddard TD, Pettersen EF, Couch GS, Pearson ZJ, Morris JH, Ferrin TE. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 2023:32(11):e4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J, Russell MW. IgA subclasses. Monogr Allergy. 1986:19:277–301. [PubMed] [Google Scholar]
- Mestecky J, Schrohenloher RE, Kulhavy R, Wright GP, Tomana M. Site of J chain attachment to human polymeric IgA. Proc Natl Acad Sci USA. 1974:71(2):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J, Russell MW, Jackson S, Brown TA. The human IgA system: A reassessment. Clin Immunol Immunopathol. 1986:40(1):105–114. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Tomana M, Crowley-Nowick PA, Moldoveanu Z, Julian BA, Jackson S. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib Nephrol. 1993:104:172–182. [DOI] [PubMed] [Google Scholar]
- Mnich ME, van Dalen R, Gerlach D, Hendriks A, Xia G, Peschel A, van Strijp JAG, van Sorge NM. The C-type lectin receptor MGL senses N-acetylgalactosamine on the unique Staphylococcus aureus ST395 wall teichoic acid. Cell Microbiol. 2019:21(10):e13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu Z, Wyatt RJ, Lee J, Tomana M, Julian BA, Mestecky J, Huang W-Q, Anreddy S, Hall S, Hastings MC, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007:71(11):1148–1154. [DOI] [PubMed] [Google Scholar]
- Moldoveanu Z, Suzuki H, Reily C, Satake K, Novak L, Xu N, Huang ZQ, Knoppova B, Khan A, Hall S, et al. Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J Autoimmun. 2021:118:102593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro RC, Halbwachs-Mecarelli L, Roque-Barreira MC, Noel LH, Berger J, Lesavre P. Charge and size of mesangial IgA in IgA nephropathy. Kidney Int. 1985:28(4):666–671. [DOI] [PubMed] [Google Scholar]
- Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J, et al. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007:44(10):2598–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol. 2004:15(3):622–634. [DOI] [PubMed] [Google Scholar]
- Moura IC, Arcos-Fajardo M, Gdoura A, Leroy V, Sadaka C, Mahlaoui N, Lepelletier Y, Vrtovsnik F, Haddad E, Benhamou M, et al. Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. J Am Soc Nephrol. 2005:16(9):2667–2676. [DOI] [PubMed] [Google Scholar]
- Muthana SM, Gildersleeve JC. Factors affecting anti-glycan IgG and IgM repertoires in human serum. Sci Rep. 2016:6(1):19509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthana SM, Xia L, Campbell CT, Zhang Y, Gildersleeve JC. Competition between serum IgG, IgM, and IgA anti-glycan antibodies. PLoS One. 2015:10(3):e0119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa S, Imamura R, Kawamura M, Kato T, Abe T, Iwatani H, Yamanaka K, Uemura M, Kishikawa H, Nishimura K, et al. Evaluation of IgA1 O-glycosylation in Henoch-Schönlein purpura nephritis using mass spectrometry. Transplant Proc. 2019a:51(5):1481–1487. [DOI] [PubMed] [Google Scholar]
- Nakazawa S, Imamura R, Kawamura M, Kato T, Abe T, Namba T, Iwatani H, Yamanaka K, Uemura M, Kishikawa H, et al. Difference in IgA1 O-glycosylation between IgA deposition donors and IgA nephropathy recipients. Biochem Biophys Res Commun. 2019b:508(4):1106–1112. [DOI] [PubMed] [Google Scholar]
- New JS, Dizon BLP, Fucile CF, Rosenberg AF, Kearney JF, King RG. Neonatal exposure to commensal-bacteria-derived antigens directs polysaccharide-specific B-1 B cell repertoire development. Immunity. 2020:53:172–186.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Julian BA, Tomana M, Mestecky J. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001:21(5):310–327. [DOI] [PubMed] [Google Scholar]
- Novak J, Vu HL, Novak L, Julian BA, Mestecky J, Tomana M. Interactions of human mesangial cells with IgA and IgA-containing circulating immune complexes. Kidney Int. 2002:62(2):465–475. [DOI] [PubMed] [Google Scholar]
- Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005:67(2):504–513. [DOI] [PubMed] [Google Scholar]
- Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008:28(1):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Moldoveanu Z, Julian BA, Raska M, Wyatt RJ, Suzuki Y, Tomino Y, Gharavi AG, Mestecky J, Suzuki H. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Adv Otorhinolaryngol. 2011a:72:60–63. [DOI] [PubMed] [Google Scholar]
- Novak J, Raskova Kafkova L, Suzuki H, Tomana M, Matousovic K, Brown R, Hall S, Sanders JT, Eison TM, Moldoveanu Z, et al. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured mesangial cells. Nephrol Dial Transplant. 2011b:26(11):3451–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Julian BA, Mestecky J, Renfrow MB. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol. 2012:34(3):365–382. [DOI] [PubMed] [Google Scholar]
- Novak J, Barratt J, Julian BA, Renfrow MB. Aberrant glycosylation of the IgA1 molecule in IgA nephropathy. Semin Nephrol. 2018:38(5):461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odani H, Hiki Y, Takahashi M, Nishimoto A, Yasuda Y, Iwase H, Shinzato T, Maeda K. Direct evidence for decreased sialylation and galactosylation of human serum IgA1 Fc O-glycosylated hinge peptides in IgA nephropathy by mass spectrometry. Biochem Biophys Res Commun. 2000:271(1):268–274. [DOI] [PubMed] [Google Scholar]
- Odani H, Yamamoto K, Iwayama S, Iwase H, Takasaki A, Takahashi K, Fujita Y, Sugiyama S, Hiki Y. Evaluation of the specific structures of IgA1 hinge glycopeptide in 30 IgA nephropathy patients by mass spectrometry. J Nephrol. 2010:23(1):70–76. [PubMed] [Google Scholar]
- Odum J, Peh CA, Clarkson AR, Bannister KM, Seymour AE, Gillis D, Thomas AC, Mathew TH, Woodroffe AJ. Recurrent mesangial IgA nephritis following renal transplantation. Nephrol Dial Transplant. 1994:9:309–312. [PubMed] [Google Scholar]
- Ohyama Y, Nakajima K, Renfrow MB, Novak J, Takahashi K. Mass spectrometry for the identification and analysis of highly complex glycosylation of therapeutic or pathogenic proteins. Expert Rev Proteomics. 2020a:17(4):275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Yamaguchi H, Nakajima K, Mizuno T, Fukamachi Y, Yokoi Y, Tsuboi N, Inaguma D, Hasegawa M, Renfrow MB, et al. Analysis of O-glycoforms of the IgA1 hinge region by sequential deglycosylation. Sci Rep. 2020b:10(1):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Yamaguchi H, Ogata S, Chiurlia S, Cox SN, Kouri NM, Stangou MJ, Nakajima K, Hayashi H, Inaguma D, et al. Racial heterogeneity of IgA1 hinge-region O-glycoforms in patients with IgA nephropathy. iScience. 2022:25(11):105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S, Blixt O, Bergstrom T, Frank M, Wandall HH. Viral O-GalNAc peptide epitopes: a novel potential target in viral envelope glycoproteins. Rev Med Virol. 2016:26(1):34–48. [DOI] [PubMed] [Google Scholar]
- Papista C, Lechner S, Ben Mkaddem S, LeStang MB, Abbad L, Bex-Coudrat J, Pillebout E, Chemouny JM, Jablonski M, Flamant M, et al. Gluten exacerbates IgA nephropathy in humanized mice through gliadin-CD89 interaction. Kidney Int. 2015:88(2):276–285. [DOI] [PubMed] [Google Scholar]
- Person T, King RG, Rizk DV, Novak J, Green TJ, Reily C. Cytokines and production of aberrantly O-glycosylated IgA1, the main autoantigen in IgA nephropathy. J Interf Cytokine Res. 2022:42(7):301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzyk AJ, Bujacz A, Mak P, Potempa B, Niedziela T. Structural studies of Helix aspersa agglutinin complexed with GalNAc: A lectin that serves as a diagnostic tool. Int J Biol Macromol. 2015:81:1059–1068. [DOI] [PubMed] [Google Scholar]
- Pineau N, Aucouturier P, Preud'homme JL, Hagiwara K, Kobayashi K. Structural and functional variability of jacalin. Mol Immunol. 1991:28(1–2):185–187. [DOI] [PubMed] [Google Scholar]
- Pinheiro SP, Hankinson SE, Tworoger SS, Rosner BA, McKolanis JR, Finn OJ, Cramer DW. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses' health studies. Cancer Epidemiol Biomarkers Prev. 2010:19(6):1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Braddon F, Hendry B, Mercer A, Osmaston K, Saleem MA, Steenkamp R, Wong K, Turner AN, Wang K, et al. Long-term outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2023:18(6):727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek WJ, Yanagawa H, Makita Y, Renfrow MB, Julian BA, Rizk DV, Suzuki Y, Novak J, Suzuki H. Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS One. 2018:13(1):e0190967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard DG, Coligan JE, Speed SE, Gray BM. Carbohydrate fingerprints of streptococcal cells. J Clin Microbiol. 1981:13(1):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit S, Li T, Guan W, Song X, Song J, Tian Y, Li L, Sharma A, Dun B, Mysona D, et al. Multiplex glycan bead array for high throughput and high content analyses of glycan binding proteins. Nat Commun. 2018:9(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam FW. Structure of the human IgA subslasses and allotypes. Protides Biol Fluids. 1989:36:27–37. [Google Scholar]
- Putnam FW, Liu YS, Low TL. Primary structure of a human IgA1 immunoglobulin. IV. Streptococcal IgA1 protease, digestion, Fab and Fc fragments, and the complete amino acid sequence of the α1 heavy chain. J Biol Chem. 1979:254(8):2865–2874. [PubMed] [Google Scholar]
- Rajasekaran A, Green TJ, Renfrow MB, Julian BA, Novak J, Rizk DV. Current understanding of complement proteins as therapeutic targets for the treatment of immunoglobulin A nephropathy. Drugs. 2023:83(16):1475–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GP, Abeygunawardana C, Bush CA, Cisar JO. The cell wall polysaccharide of Streptococcus gordonii 38: structure and immunochemical comparison with the receptor polysaccharides of Streptococcus oralis 34 and Streptococcus mitis J22. Glycobiology. 1994:4(2):183–192. [DOI] [PubMed] [Google Scholar]
- Reily C, Rizk DV, Julian BA, Novak J. Assay for galactose-deficient IgA1 enables mechanistic studies with primary cells from IgA nephropathy patients. BioTechniques. 2018:65(2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reily C, Stewart T, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019:15(6):346–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reily C, Moldoveanu Z, Pramparo T, Hall S, Huang ZQ, Rice T, Novak L, Komers R, Jenkinson CP, Novak J. Sparsentan ameliorates glomerular hypercellularity and inflammatory-gene networks induced by IgA1-IgG immune complexes in a mouse model of IgA nephropathy. Am J Physiol Renal Physiol. 2024:326(5):F862–F875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem. 2005:280(19):19136–19145. [DOI] [PubMed] [Google Scholar]
- Renfrow MB, MacKay CL, Chalmers MJ, Julian BA, Mestecky J, Kilian M, Poulsen K, Emmett MR, A.G. M, Novak J. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Anal Bioanal Chem. 2007:389(5):1397–1407. [DOI] [PubMed] [Google Scholar]
- Revoredo L, Wang S, Bennett EP, Clausen H, Moremen KW, Jarvis DL, Ten Hagen KG, Tabak LA, Gerken TA. Mucin-type O-glycosylation is controlled by short- and long-range glycopeptide substrate recognition that varies among members of the polypeptide GalNAc transferase family. Glycobiology. 2016:26(4):360–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk DV, Saha MK, Hall S, Novak L, Brown R, Huang ZQ, Julian BA, Novak J. Glomerular immunodeposits of patients with IgA nephropathy are enriched for IgG autoantibodies specific for galactose-deficient IgA1. J Am Soc Nephrol. 2019:30(10):2017–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk DV, Novak L, Hall SD, Moldoveanu Z, Julian BA, Novak J, Haas M. Colocalization of IgG and IgA heavy chains with kappa and lambda light chains in glomerular deposits of IgA nephropathy patients using high-resolution confocal microscopy and correlation with Oxford MEST-C scores. J Clin Med. 2023:12(23):7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, Wilson IA, Daha MR, Dwek RA, Rudd PM. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003:278(22):20140–20153. [DOI] [PubMed] [Google Scholar]
- Ruhaak LR, Koeleman CA, Uh HW, Stam JC, van Heemst D, Maier AB, Houwing-Duistermaat JJ, Hensbergen PJ, Slagboom PE, Deelder AM, et al. Targeted biomarker discovery by high throughput glycosylation profiling of human plasma alpha1-antitrypsin and immunoglobulin A. PLoS One. 2013:8(9):e73082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, Lescar J, Chazalet V, Audfray A, Gagnon J, Alvarez R, Breton C, Imberty A, Mitchell EP. Biochemical and structural analysis of Helix pomatia agglutinin. A hexameric lectin with a novel fold. J Biol Chem. 2006:281(29):20171–20180. [DOI] [PubMed] [Google Scholar]
- Sande C, Whitfield C. Capsules and extracellular polysaccharides in Escherichia coli and Salmonella. EcoSal Plus. 2021:9(2):eESP00332020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R, Sekar K, Banerjee R, Sharma V, Surolia A, Vijayan M. A novel mode of carbohydrate recognition in jacalin, a Moraceae plant lectin with a β-prism fold. Nat Struct Biol. 1996:3(7):596–603. [DOI] [PubMed] [Google Scholar]
- Sanz-Martinez I, Pereira S, Merino P, Corzana F, Hurtado-Guerrero R. Molecular recognition of GalNAc in mucin-type O-glycosylation. Acc Chem Res. 2023:56(5):548–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry MVK, Banarjee P, Patanjali SR, Swamy MJ, Swarnalatha GV, Surolia A. Analysis of saccharide binding to Artocarpus integrifolia lectin reveals specific recognition of T antigen (β-D-Gal(1–>3)D-GalNAc). J Biol Chem. 1986:261(25):11726–11733. [PubMed] [Google Scholar]
- Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, Miescher S, Simon HU, Pashov A, Vassilev T, et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med. 2015:7(269):269ra261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennhauser FH, Hosking CS, Jones CL, MacDonald RA, Mermelstein N, Roberton DM. Anti-IgA antibodies in IgA-deficient children. J Clin Immunol. 1988:8(5):356–361. [DOI] [PubMed] [Google Scholar]
- Seo HS, Cartee RT, Pritchard DG, Nahm MH. A new model of pneumococcal lipoteichoic acid structure resolves biochemical, biosynthetic, and serologic inconsistencies of the current model. J Bacteriol. 2008:190(7):2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Zhang J, Kowal P, Lu Y, Wang PG. Overexpression and biochemical characterization of β-1,3-N-acetylgalactosaminyltransferase LgtD from Haemophilus influenzae strain Rd. Biochem Biophys Res Commun. 2002:295(1):1–8. [DOI] [PubMed] [Google Scholar]
- Shimozato S, Hiki Y, Odani H, Takahashi K, Yamamoto K, Sugiyama S. Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol Dial Transplant. 2008:23(6):1931–1939. [DOI] [PubMed] [Google Scholar]
- Steffen U, Koeleman CA, Sokolova MV, Bang H, Kleyer A, Rech J, Unterweger H, Schicht M, Garreis F, Hahn J, et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. 2020:11(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenutz R, Weintraub A, Widmalm G. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol Rev. 2006:30(3):382–403. [DOI] [PubMed] [Google Scholar]
- Stephenson AE, Wu H, Novak J, Tomana M, Mintz K, Fives-Taylor P. The Fap1 fimbrial adhesin is a glycoprotein: antibodies specific for the glycan moiety block the adhesion of Streptococcus parasanguis in an in vitro tooth model. Mol Microbiol. 2002:43(1):147–157. [DOI] [PubMed] [Google Scholar]
- Sterner E, Flanagan N, Gildersleeve JC. Perspectives on anti-glycan antibodies gleaned from development of a community resource database. ACS Chem Biol. 2016:11(7):1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner E, Peach ML, Nicklaus MC, Gildersleeve JC. Therapeutic antibodies to ganglioside GD2 evolved from highly selective germline antibodies. Cell Rep. 2017:20(7):1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TJ, Takahashi K, Whitaker RH, Raska M, Placzek WJ, Novak J, Renfrow MB. IgA1 hinge-region clustered glycan fidelity is established early during semi-ordered glycosylation by GalNAc-T2. Glycobiology. 2019:29(7):543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TJ, Takahashi K, Xu N, Prakash A, Brown R, Raska M, Renfrow MB, Novak J. Quantitative assessment of successive carbohydrate additions to the clustered O-glycosylation sites of IgA1 by glycosyltransferases. Glycobiology. 2021:31(5):540–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr SJ, Royle L, Chapman CJ, Hamid UM, Robertson JF, Murray A, Dwek RA, Rudd PM. The O-linked glycosylation of secretory/shed MUC1 from an advanced breast cancer patient's serum. Glycobiology. 2008:18(6):456–462. [DOI] [PubMed] [Google Scholar]
- Stuchlova Horynova M, Raska M, Clausen H, Novak J. Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell Mol Life Sci. 2013:70(5):829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchlova Horynova M, Vrablikova A, Stewart TJ, Takahashi K, Czernekova L, Yamada K, Suzuki H, Julian BA, Renfrow MB, Novak J, et al. N-acetylgalactosaminide α2,6-sialyltransferase II is a candidate enzyme for sialylation of galactose-deficient IgA1, the key autoantigen in IgA nephropathy. Nephrol Dial Transplant. 2015:30(2):234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009:119(6):1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011:22(10):1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, Tomino Y, Gharavi AG, Novak J. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem. 2014:289(8):5330–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wall SB, Suzuki H, Smith AD, Hall S, Poulsen K, Kilian M, Mobley JA, Julian BA, Mestecky J, et al. Clustered O-glycans of IgA1: defining macro- and micro-heterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010:9(11):2545–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Smith AD, Poulsen K, Kilian M, Julian BA, Mestecky J, Novak J, Renfrow MB. Naturally occurring structural isomers in serum IgA1 O-glycosylation. J Proteome Res. 2012:11(2):692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarp MA, Sorensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, Clausen H. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 2007:17(2):197–209. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Burchell JM, Graham R, Beatson R. Latest developments in MUC1 immunotherapy. Biochem Soc Trans. 2018:46(3):659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Carroll K, Inker LA, Floege J, Perkovic V, Boyer-Suavet S, Major RW, Schimpf JI, Barratt J, Cattran DC, et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019:14(3):469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomana M, Niedermeier W, Mestecky J, Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry. 1976:13(4):325–328. [DOI] [PubMed] [Google Scholar]
- Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997:52(2):509–516. [DOI] [PubMed] [Google Scholar]
- Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999:104(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Dell A, Haslam SM, Tissot B, Canis K, Azadi P, Backstrom M, Costello CE, Hansson GC, Hiki Y, et al. Comparison of methods for profiling O-glycosylation: human proteome organisation human disease Glycomics/proteome initiative multi-institutional study of IgA1. Mol Cell Proteomics. 2010:9(4):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo FB. Is Henoch-Schönlein purpura the systemic form of IgA nephropathy? Am J Kidney Dis. 1988:12(5):373–377. [DOI] [PubMed] [Google Scholar]
- Wandall HH, Irazoqui F, Tarp MA, Bennett EP, Mandel U, Takeuchi H, Kato K, Irimura T, Suryanarayanan G, Hollingsworth MA, et al. The lectin domains of polypeptide GalNAc-transferases exhibit carbohydrate-binding specificity for GalNAc: lectin binding to GalNAc-glycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology. 2007:17(4):374–387. [DOI] [PubMed] [Google Scholar]
- Wang L, Radic MZ, Galili U. Human anti-Gal heavy chain genes. Preferential use of VH3 and the presence of somatic mutations. J Immunol. 1995:155(3):1276–1285. [PubMed] [Google Scholar]
- Weisel NM, Weisel FJ, Farber DL, Borghesi LA, Shen Y, Ma W, Luning Prak ET, Shlomchik MJ. Comprehensive analyses of B-cell compartments across the human body reveal novel subsets and a gut-resident memory phenotype. Blood. 2020:136(24):2774–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006:208(2):270–282. [DOI] [PubMed] [Google Scholar]
- Wu AM, Wu JH, Lin LH, Lin SH, Liu JH. Binding profile of Artocarpus integrifolia agglutinin (Jacalin). Life Sci. 2003:72(20):2285–2302. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013:368(25):2402–2414. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Novak J, Gaber LW, Lau KK. IgA nephropathy and Henoch-Schönlein purpura nephritis. In: Kher KK, Schnapper HW, Makker SP, editors. Clinical Pediatric nephrology. 2nd ed. London, UK: Informa UK Ltd; 2006. pp. 213–222 [Google Scholar]
- Yanagawa H, Suzuki H, Suzuki Y, Kiryluk K, Gharavi AG, Matsuoka K, Makita Y, Julian BA, Novak J, Tomino Y. A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One. 2014:9(5):e98081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo EM, Morrison SL. IgA: an immune glycoprotein. Clin Immunol. 2005:116(1):3–10. [DOI] [PubMed] [Google Scholar]
- Yoo EM, Coloma MJ, Trinh KR, Nguyen TQ, Vuong LU, Morrison SL, Chintalacharuvu KR. Structural requirements for polymeric immunoglobulin assembly and association with J chain. J Biol Chem. 1999:274(47):33771–33777. [DOI] [PubMed] [Google Scholar]
- Yu G, Zhang Y, Meng B, Xie X, Wang Z, Ying W, Lv J, Zhang H. O-glycoforms of polymeric immunoglobulin A1 in the plasma of patients with IgA nephropathy are associated with pathological phenotypes. Nephrol Dial Transplant. 2021:37(1):33–41. [DOI] [PubMed] [Google Scholar]
- Zhang S, Sun H, Zhang Z, Li M, Guo Z, Ye W, Cai G, Sun W, Li M. Diagnostic potential of plasma IgA1 O-glycans in discriminating IgA nephropathy from other glomerular diseases and healthy participants. Front Mol Biosci. 2022:9:871615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012:82(7):790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]