Abstract
Oxygen supply is essential for the long-term viability and function of tissue engineered constructs in vitro and in vivo. The integration with the host blood supply as the primary source of oxygen to cells requires 4 to 5 weeks in vivo and involves neovascularization stages to support the delivery of oxygenated blood to cells. Consequently, three-dimensional (3D) encapsulated cells during this process are prone to oxygen deprivation, cellular dysfunction, damage, and hypoxia-induced necrosis. Here we demonstrate the use of calcium peroxide () and polycaprolactone (PCL), as part of an emerging paradigm of oxygen-generating scaffolds that substitute the host oxygen supply via hydrolytic degradation. The 35-day in vitro study showed predictable oxygen release kinetics that achieved 5% to 29% dissolved oxygen with increasing loading. As a biomaterial, the iterations of 0 mg, 40 mg, and 60 mg loaded scaffolds yielded modular mechanical behaviors, ranging from 5-20 kPa in compressive strength. The other controlled physiochemical features included swelling capacities of 22-33% and enzymatic degradation rates of 0.8% to 60% remaining mass. The 3D-encapsulation experiments of 3T3 fibroblasts, L6 rat myoblasts, and primary cardiac fibroblasts in these scaffolds showed enhanced cell survival, proliferation, and function under hypoxia. During continuous oxygen release, the scaffolds maintained a stable tissue culture system between pH 8 to 9. The broad basis of this work supports prospects in the expansion of robust and clinically translatable tissue constructs.
1. Introduction
Tissue engineering has remarkably advanced the existing biomedical strategies that are aimed to address the profound need of organ transplants and tissue substitutes. While progress has been achieved, the clinical demand for organ transplants and the lack of successful alternatives for patients persists in healthcare. As of September 2020, there are 109,000 patients that have been placed on the organ transplant waiting list in the United States. Yet, only 39,718 of these patients were removed from this waiting list, as reported by the Health Resources & Services Administration (OPTN/SRTR Annual Report)1. The evolution of biomaterials into various biomimetic and biocompatible structures has revealed diverse approaches that can support organ-scaled tissue constructs for these patients2-10. There has been consistent success in particular tissues, such as the trachea11. Among these tissues constructs, a variety of polycaprolactone (PCL)-based scaffolds have been employed to generate trachea replacements that are biocompatible and functional in the host system. The use of a biodegradable polymer, such as PCL, that is also intrinsically strong has made it possible to generate organ-sized tissue constructs11-15. This PCL-based synthetic trachea exhibited a high degree of epithelium restoration, but lacked cartilage formation in rat models in vivo11.
The translation of tissue engineered constructs from an in vitro to an in vivo system requires a multidimensional approach.
The success of in vivo tissue constructs relies on the proper integration with the host vasculature. The vascular integration of these constructs is responsible for delivering growth factors, cell signals, nutrients, and most critically, oxygen. The oxygen supply is essential for cell viability, proliferation, and function. The first 4 to 6 weeks post-implantation of a tissue construct involves tissue remodelling stages which are necessary for wound healing and neovascularization16. Regardless, the absence of vasculature during this time can halt cell growth and repair, as well as lead to further tissue damage by hypoxia-induced necrosis6, 17. Moreover, the cells within tissue constructs often become oxygen deficient the scaffold dimensions are greater than 100 μm to 200 μm due to diffusion limitations to oxygen transport18. Recent strategies to minimize these effects have involved treating cell samples and co-encapsulating cells in scaffolds with growth factors, such as vascular endothelial growth factor (VEGF), that promote vascularization19-22. The shortcoming of these methods is that they may lead to an undesired non-homogenous distribution of vascular networks in a three-dimensional (3D) biomaterial scaffolds5, 23-25. Other microfluidic approaches support neovascularization in vitro by generating vasculature-like structures within a tissue scaffold. These techniques while useful are also limited by level of resolution achieved and are time-consuming due to troubleshooting steps often required to optimize parameters that will result in a homogeneous channel formation throughout the 3D tissue construct18, 26-28. Using these methods in combination to work synergistically is possible; however, the lack of an immediate oxygen source until vascularization occurs will still risk cellular dysfunction or damage within the tissue construct.
Oxygen serves a critical factor in cell-cell communication, cell proliferation, and in cell differentiation as a signaling function6, 29-32. In clinical practice, hyperbaric oxygen therapy is example of an existing therapy which provides oxygen to a healing wound site via an external oxygen source33-35. The merits of hyperbaric oxygen therapy have been clinically validated as it preserves these key roles of oxygen to encourage wound healing33, 36-38. With the beneficial effects of high oxygen environments on tissue healing, the efforts to develop biomaterials that could generate, and release oxygen have garnered scientific impetus over the last decade5, 9, 39. Thus, oxygen-generating biomaterials have been developed to achieve homogenous results in organ-scale tissue constructs. Common materials that can generate oxygen include solid peroxides, liquid peroxides, and fluorinated compounds as oxygen carriers40-42. However, solid peroxides, such as calcium peroxide (), are preferred for their high yield of oxygen formation and low toxicity 6, 43, 44. Specifically, the hydrolysis of within hydrogel networks generates oxygen as the byproduct of the reaction as shown in the following two-step chemical reaction6, 45-47 :
Solid peroxides, however, pose a potential risk for uncontrollable burst of oxygen during hydrolysis, which can be damaging to surrounding tissues in vitro and in vivo17, 48, 49. A novel strategy to control the release of oxygen and offset this effect is to limit the rate of exposure of the solid peroxide to the water content in the cellular microenvironment by using a hydrophobic barrier around it17, 48, 50. The main advantage of this approach is the ability to provide adequate oxygen to the tissue culture system to support a 3D organ-sized construct during its integration in the host and alter it if necessary50.
Here we discuss the synthesis and characterization of novel oxygen-generating, microparticle-reinforced gelatin hydrogels. These microparticles included a hydrophobic component, polycaprolactone (PCL), to encapsulate as the source of oxygen generation. The cargo, , is known compound that can undergo hydrolysis which releases oxygen as a byproduct6. PCL compliments this component with its well-characterized biocompatibility, bioactivity, and hydrophobic properties. PCL based scaffolds have already successfully shown applications in tissue engineering scaffold design50-52. Therefore, to engineer our sustained oxygen generating scaffolds, PCL was chosen as the hydrophobic component along with . We extensively evaluated various versions of the oxygen-generating scaffolds. The different compositions of these scaffolds were assigned specific nomenclature for reference (Table 1). The effects of varying the oxygen content on the material properties, oxygen release kinetics, and cellular response were explored. The scaffolds were also assessed for their mechanical behavior, swelling, degradation, cytocompatibility, and metabolic activity. Finally, we evaluated the oxygen release kinetics and the biological performance in long-term cell cultures for up to 35 days in vitro by three-dimensional (3D) encapsulation different cell types. These cell types included primary rat cardiac fibroblasts, NIH/3T3 fibroblasts, and L6 rat myoblasts.
Table 1.
Nomenclature for oxygen generating scaffolds.
| Scaffold nomenclature |
mg of per mL of PCL |
mg of per mL of 5% w/v GelMA |
|---|---|---|
| 0CPO | 0 | 0 |
| 40CPO | 40 | 5.4 |
| 60CPO | 60 | 8.1 |
2. Materials & Methods
2.1. Materials
Polycaprolactone (PCL) was purchased from Scientific Polymer Products Inc., Mwt 55,000 GPC (Ontario, NY). PVA low molecular weight (98-99% hydrolyzed) was obtained from Alfa Aesar™ (Haverhill, MA). Calcium peroxide (), porcine skin gelatin, methacrylic anhydride, were all purchased from Sigma Aldrich (St. Louis, MO). Dulbecco’s phosphate buffered saline (DPBS), Dulbecco’s Modified Eagle’s Medium (DMEM- low glucose), fetal bovine serum (FBS), trypsin-ethylenediaminetetraacetic acid (EDTA) 0.25%, and penicillin/streptomycin (P/S) were all obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA). Alamar Blue Assay and Lactate Dehydrogenase (LDH) activity kit were bought from Invitrogen (Thermo Fisher Scientific, Pittsburgh, PA). 2-hydroxy-1- [4-(hydroxy ethoxy) phenyl]-2-methyl-1propanone (Irgacure 2959) was bought from BASF Corporation (Florham Park, NJ). Caspase glo 3/7 assay kit was procured from Promega (Madison, WI). All reagents were used as received without further purification. NeoFox Oxygen sensing probe was purchased from Ocean Optics Inc (Largo, FL). The hypoxia incubator chamber was purchased from StemCell Technologies (Vancouver, Canada).
2.2. Synthesis of the gelatin-based hydrogels
The oxygen-generating scaffolds included a gelatin-based hydrogel constituent in its material composition. This hydrogel composed of 5% (w/v) methacrylated gelatin (GelMA), and 0.5 % (w/v) Irgacure 2959 for photoinitiation. The hydrogel precursor solution was formulated by dissolving 10 g of porcine skin gelatin in 100 mL of DPBS at 50°C under magnetic stirring conditions. Following, a total of 8 mL methacrylic anhydride (MAA) was added dropwise and the solution continued to the stir for an additional 4 hours at 50°C and 200 rpm. This methacrylation reaction had been optimized for the methacrylation of the gelatin backbone in our previous report5. This reaction was quenched with 300 mL of DPBS. The methacrylated gelatin was dialyzed in nitrocellulose membranes and remained in distilled water for a week under magnetic stirring conditions (180 rpm) at 40°C. The dialyzed methacrylated gelatin was transferred into 50 mL conical tubes for temporary storage overnight −80°C. The solution was then lyophilized for one week to obtain the GelMA polymer in a foam form for use.
2.3. Synthesis of the oxygen-generating microparticles
The oxygen-generating scaffolds were reinforced with microparticles that contained the cargo for oxygen release. These microparticles were synthesized by encapsulating within a hydrophobic phase, PCL. In particular, the hydrophobic phase consisted of 13.5% (w/v) PCL in chloroform solution and was prepared under continuous stirring at room temperature until fully dissolved. Then, the was combined into the hydrophobic phase at varying concentrations of 0, 40, or 60 mg/mL. Based on the concentrations, the microparticle content was hypothesized to produce distinct oxygen-release profiles. In addition, the continued stirring of into PCL generated a complex for the microparticle fabrication. This study used an emulsification process which involved adding the PCL- solution into an aqueous phase. The aqueous phase composed of a low molecular weight polyvinyl alcohol (PVA) dissolved in deionized water at 80°C to form a 0.5% (w/v) solution. To this aqueous phase, the inner viscous phase, PCL-, was added dropwise from a micropipette under constant magnetic stirring (920 rpm). The microparticles at the bottom of the aqueous phase were transferred into 15 mL conical tubes. The samples were centrifuged at 800 rpm to separate the aqueous and hydrophobic phases by density. The supernatant containing the PVA solution was decanted and the particles were washed with chloroform to remove the residual PVA. The oxygen-generating microparticles were dried under vacuum for 4 hrs to evaporate the residual chloroform.
2.4. Fabrication of the oxygen-generating scaffolds
The previously synthesized microparticles were used to reinforce the GelMA hydrogels and fabricate the oxygen-generating scaffolds. Specifically, the microparticles were incorporated into the GelMA prepolymer solution at 0, 40, and 60 mg/mL concentrations. These precursor solutions were then pipetted into a 96-well plate for photocrosslinking. The scaffolds were photocrosslinked simultaneously at 700 mW/cm2 using an Omnicure S2000 (EXFO Photonic Solutions Inc., Ontario, Canada). For material characterization studies, macroscale hydrogels at 100 μL in volume were fabricated. The prepolymer solutions were photocrosslinked for 20-80 seconds at a power of 700 mW/cm2 for the pristine GelMA, 0CPO, 40CPO, and 60CPO gel conditions. All oxygen-generating scaffolds were submerged in DPBS for temporary storage.
2.5. Swelling and degradation analysis for the oxygen-generating scaffolds
The swelling and degradation behavior of the oxygen-generating scaffolds were evaluated using the macroscale scaffolds as previously mentioned. These scaffolds for the swelling analysis were placed in DPBS for 48 hours to achieve equilibrium swelling, with four replicates per composition. The scaffolds were then removed from the medium and the residual DPBS on the gel was absorbed using a Kimwipe. After, each of scaffold samples was weighed, transferred into 1.5 mL microcentrifuge tubes, and stored at −80°C for 24 hours. These samples were lyophilized to obtain the dry weight of the material. The swelling ratios of the gel samples were determined by dividing the wet weight after equilibrium swelling by their corresponding dry weights. The resulting ratios were reported as percentage values.
The degradation behavior of the oxygen-generating scaffolds was evaluated by enzymatic degradation. Specifically, collagenase type II was utilized as a physiologically relevant enzyme to facilitate degradation. The samples were prepared as described for the swelling experiments. These samples were transferred into pre-weighed 1.5 mL microcentrifuge tubes and stored at −80°C overnight. Following, the samples were lyophilized for 24 hours to dehydrate the material. The initial dry weight of a sample was determined by subtracting the weight of the empty microcentrifuge tubes from the total weight of the sample and its respective container. Subsequently, the samples were rehydrated by submerging the gels in 1 mL of DPBS for 24 hours. The DPBS was then removed and replaced with 1 mL of 2.5 U/mL of collagenase type II in DPBS. The samples in collagenase type II were placed in a 37°C incubator on a shaker at 70 rpm to initiate the degradation reaction. The degradation behavior was monitored by measuring the mass remaining at different time points (3, 6, 12, 18, 24, 36, and 48 hrs). At each time point, the samples were rinsed with DPBS to wash off the remaining enzymatic completely and prevent further degradation of the material. The samples were stored in a −80°C freezer temporarily overnight and lyophilized the following day to obtain the dry weight. The degradation experiments were performed in quadruplicates per scaffold composition. The degradation behavior was reported as the percent mass remaining after degradation. For each time point, this value was determined by dividing the dry weight after degradation by the initial weight of the hydrogel.
2.6. Mechanical testing for the oxygen-generating scaffolds
The mechanical performance of these oxygen-generating scaffolds was evaluated through their compressive moduli. The hydrogel scaffolds swelled in DPBS for 24 hours and were shaped using an 8-mm biopsy punch prior the dynamic mechanical analysis (DMA). Following, the samples were removed from the DPBS and excess moisture on the material was blotted using a Kimwipe. The parameters that were used in the DMA included, a preload force of 0.0010 N, isothermal temperature of 23 °C, soak time of 1 minute, force ramp rate of 0.1 N/min, and upper force limit of 2 N. The data yielded a stress-strain curve from which the slope of the linear region was used to determine the compressive modulus for each scaffold composition.
2.7. Morphology of oxygen-generating scaffolds
The microarchitecture of the synthesized oxygen-generating microparticles were assessed under a scanning electron microscope (JEOL 5200 SEM). Phase-contrast imaging by microscopy (Zeiss Axio Vert.A1, Berlin, Germany) was also utilized to further understand the morphology of the scaffolds and the average diameters of the microparticles. The scaffolds for SEM imaging were flash frozen in liquid nitrogen, lyophilized, and placed in argon atmosphere for gold coating. We used the NIH ImageJ software 1.52a to measure the percent porosity and pore size and distribution for all microparticle and scaffold compositions. The SEM images also revealed the morphological characteristics of the PCL- microparticles interfacing with the GelMA matrix in the scaffold.
2.8. Oxygen-release profiles of the scaffolds
The oxygen release profiles of the synthesized scaffolds were investigated in hypoxic cell culture conditions at 2% . These experiments used a NeoFox optical oxygen sensing probe to measure the percent (%) dissolved oxygen daily throughout a 35-day incubation period. The optical oxygen sensing probe was submerged in the media of the scaffolds with and without cells encapsulated in 3D. The amount of oxygen in the cell culture incubator was controlled using a hypoxia incubator chamber (StemCell Technologies). Therefore, the primary source of oxygen under hypoxic culture conditions was oxygen-generating microparticles. We also determined if the effect of catalase at 1 mg/mL concentration improved the oxygen release in our scaffolds. Catalase is of particular interest as it is a physiologically relevant enzyme that is involved in the decomposition of hydrogen peroxide to water and oxygen. Furthermore, catalase has been shown to improve the conversion efficiency to oxygen during the hydrolysis reaction53. Thus, this condition was necessary to investigates strategies that maximize oxygen release in our oxygen-generating scaffolds.
2.9. Modulation of the oxygen release kinetics
In the microparticles, the PCL content was modified to evaluate how the hydrophobic barrier alters the oxygen release kinetics of the scaffold. PCL concentrations analyzed ranged from 5% (w/v) PCL to 20% (w/v) PCL, with increasing increments of 1%. The oxygen-generating reagent content in the microparticles, , remained constant at 60 mg/mL in the PCL solution. The mixture stirred for 4 hours for a formation of the PCL- complex. These modified PCL- complexes were incorporated to generate other versions of the oxygen-generating microparticles. Then, the microparticles were fabricated using the emulsification process as previously described. In doing so, we hypothesized that these modified oxygen-releasing microparticles when incorporated into the scaffolds demonstrate modular and predictable oxygen-release kinetics in the in vitro 3D cell encapsulation studies. Similarly, the oxygen release was probed daily across a 35-day incubation period to determine the change in the cumulative oxygen release due to modifying the hydrophobic polymer in the microparticles.
2.10. Three-dimensional (3D) cell encapsulation in the oxygen-generating scaffolds
The cytocompatibility of the oxygen-generating scaffolds was extensively studied using three different cell types. Specifically, primary rat cardiac fibroblasts, NIH/3T3 fibroblasts, and L6 rat myoblasts were encapsulated in 3D within the scaffolds at passage 4. The cells were combined in the hydrogel precursor solutions at a density of 5x106 cells/mL. The hydrogel precursor solutions were prepared using 5% (w/v) GelMA in a photoinitiator solution (0.05% Irgacure 2959 in 1XDPBS) along with the microparticles for 0CPO, 40CPO, and 60CPO scaffolds. The cells were trypsinized, transferred into a conical tube, and centrifuged to form a cell pellet. The cell counts were obtained to determine the number of cells to resuspend in the prepolymer solutions at the desired cell density. The cell and microparticle laden prepolymer solution were pipetted at 40 μL at the bottoms of a 96-well plate before photocrosslinking. The 3D-encapsulated scaffolds were fabricated using a UV power of 700 mW/cm2 as in previous experiments (Fig. 1). These scaffolds were maintained in a hypoxia incubator chamber for 35 days. The different scaffold and cell types were analyzed for oxygen release behavior, cell proliferation, cytotoxicity, and apoptosis.
Fig 1.
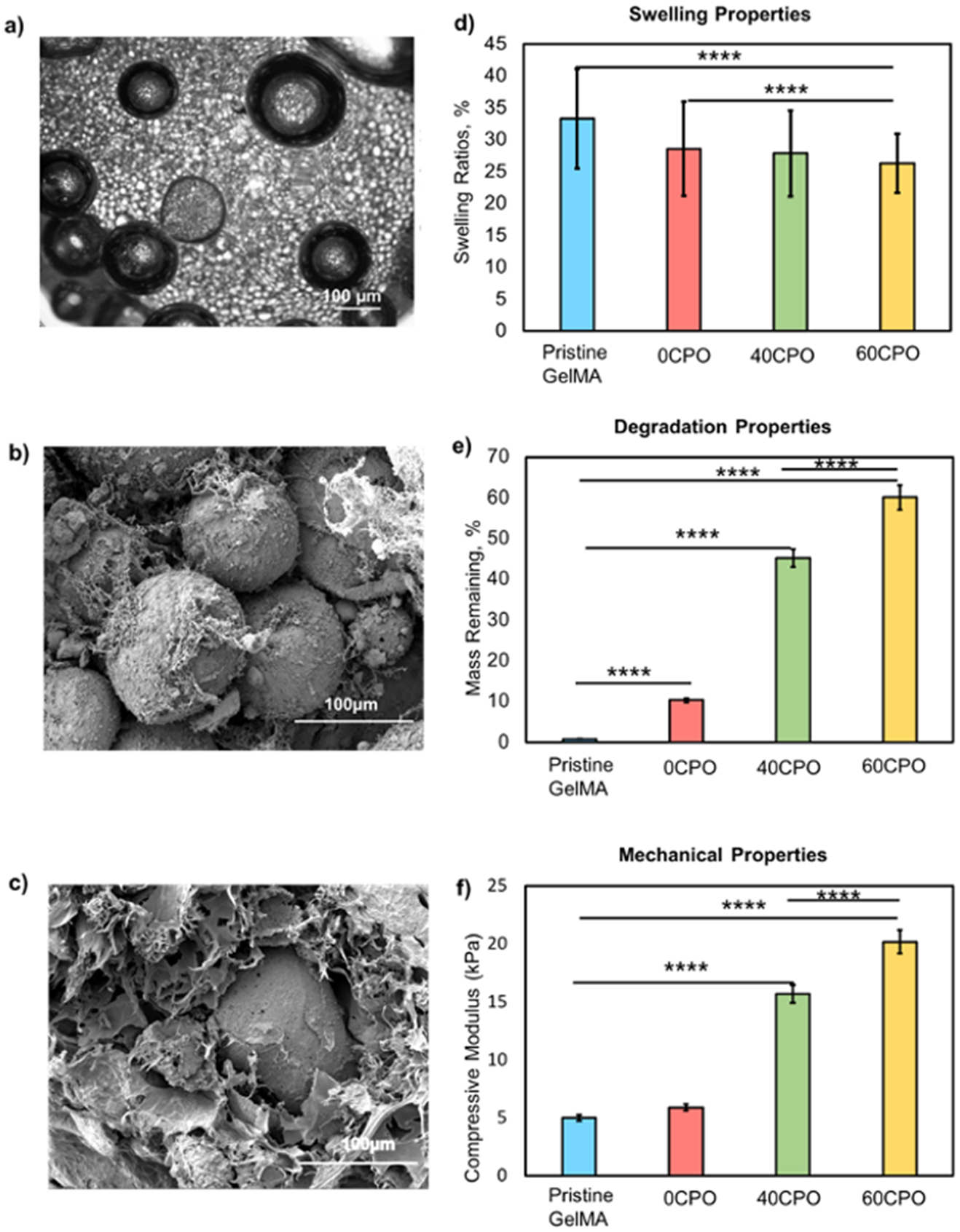
Characterization of the oxygen-generating scaffolds a) Phase contrast imaging of oxygen generating microparticles in GelMA hydrogel, b) SEM image of oxygen generating microparticles distributed within the GelMA network, and c) SEM images of oxygen-generating microparticle embedded within GelMA hydrogel, d) Swelling ratios of the oxygen-generating scaffolds, e) Degradation properties of the oxygen-generating scaffolds, and f) Mechanical properties by DMA compression test for the pristine GelMA, 0CPO, 40CPO, and 60CPO scaffolds which contained 0 mg, 0 mg, 5.4 mg, and 8.1 mg net in PCL per mL of the GelMA matrix, respectively.
2.11. Cell behavior and cytotoxicity in the oxygen-generating scaffolds
The tissue scaffolds were maintained using a cell culture media of DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) and 5% (v/v) penicillin/streptomycin. The cell cultures remained in an incubator at 37°C supplemented with 5% carbon dioxide (). The cell culture media was changed every 2-3 days. The oxygen-generating scaffolds were studied for viability and metabolic activity using the Alamar blue assay (Invitrogen, Pittsburgh, PA). Briefly, the Alamar blue reagent was incubated with the cells for 4 hours according to the manufacturer’s protocol. The colorimetric results were measured using a microplate reader. The fluorescence values were read using 560nm/590nm (Ex/Em).
The potential cytotoxicity of the composite scaffolds was examined using the lactate dehydrogenase (LDH) cytotoxicity assay (Invitrogen, Pittsburgh, PA). In brief, 25 mL of supernatant from the cell culture was transferred into a 96-well plate, and each sample reacted with the reaction analyte in a 1:1 ratio as per the manufacturer’s protocols. After 30 minutes, the stop solution was added, and the absorbance was read using a spectrophotometer at 560 nm wavelength. This experiment was performed in quadruplicates. In addition, we analyzed caspase 3 and 7 activities using the Caspase Glo 3/7 assay (Promega, Madison, WI). Similarly, the reaction substrate was added to the supernatant from the cell cultures in a 1:1 ratio and reacted for 45 minutes under light-protected conditions per the manufacturer’s instructions. The stop solution was added after 40 minutes to quench the reaction for luminescence readings. The luminescence was recorded for the reaction supernatant using a SpectraMaxiD3 microplate reader (San Jose, CA).
2.12. Statistical analysis
GraphPad Prism 6.0 (La Jolla, CA, USA) was used for the statistical analyses. The results were analyzed by performing a one-way ANOVA. Bonferroni post hoc tests were completed to analyze the statistically significant differences. A p-value < 0.05 was considered as a statistically significant difference in all shown analyses. All values are represented as averages ± standard deviation (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
3. Results
The oxygen release kinetics, cellular responses, and biocompatibility of the oxygen-generating scaffolds were comprehensively evaluated. This section describes the results of the mechanical characterization, swelling and degradation analysis, and oxygen-release kinetics of the oxygen-generating scaffolds. In addition, the biological performance of these scaffolds using three different cell types was studied by proliferation and cytotoxicity assays.
3.1. Synthesis and characterization of oxygen-generating scaffolds
The synthesized oxygen-generating scaffolds in this study included a gelatin-based hydrogel, reinforced with -loaded microparticles. Using an emulsification technique, microparticles containing varied amounts of in PCL were fabricated. These microparticles were then incorporated into GelMA hydrogels for iterations oxygen-generating scaffolds. Through phase contrast imaging by microscopy, it was determined that microparticle fabrication process yielded particles with an average diameter of 100 μm.The SEM images revealed the topography and microparticle integration within the GelMA matrix (Fig 1a-c). Other physical characterization techniques demonstrated that the swelling, degradation, and mechanical behavior of the composite constructs were based on scaffold composition. In particular, the swelling ratios of the 3D scaffolds decreased as the content increased in the oxygen-generating microparticles. This experiment determined swelling ratios of 33.34% ± 7.8, 28.58 % ± 7.39, 27.87% ± 6.7, and 26.31% ± 4.6 for the pristine GelMA, 0CPO, 40CPO, and 60CPO scaffolds respectively (Fig 1d). Similarly, the degradation behavior was also dependent on the concentration of the in the microparticles. After 12 hours, the percent mass remaining increased in response to the increased content in the microparticle with 0.76%, 10.32 %, 45.2%, and 60.1% for the pristine GelMA, 0CPO, 40CPO, and 60CPO scaffolds, respectively (Fig 1e). In the mechanical analysis, increasing the oxygen-releasing reagent content in the microparticles improved the compressive modulus of the composite scaffolds. Specifically, the compressive moduli were 5.01, 5.9, 15.7, and 20.2 kPa for the pristine GelMA, 0CPO, 40CPO and 60CPO scaffolds, respectively (Fig 1f). Therefore, these physical properties were tunable based on the microparticle composition which in turn also affected the biological response of the scaffolds.
3.2. Cellular response to oxygen generating scaffolds
The biological properties of the oxygen-generating scaffolds were evaluated with NIH/3T3 fibroblasts, L6 rat myoblasts, and primary cardiac fibroblasts 3D encapsulated. The in vitro cellular responses to the oxygen-generating scaffolds were assessed through the Alamar Blue, LDH, and Caspase Glo 3/7 activity assays. Figure 2 shows the results of these experiments for the pristine GelMA, 0CPO, 40CPO, and 60CPO scaffolds.
Fig 2.

Cellular response to the oxygen-generating scaffolds. Alamar Blue assay for evaluation of the metabolic activity of a) NIH/3T3 Fibroblasts b) L6 rat myoblasts and c) Cardiac fibroblasts.
3.2.1. Metabolic activity of various cell types
The metabolic activity of the different cell types in the oxygen- generating scaffolds demonstrated unique profiles over 35 days in long-term tissue cultures (Fig. 2a-c). The effects of the scaffold compositions on the metabolic activity were assessed on days 0, 1, 4, 7, 14, 21, 28, and 35 using the Alamar blue assay. Increasing trends in the metabolic activity were observed under hypoxic conditions (1% ) with 1 mg/mL catalase in the cell culture medium. This result is consistent with its physiological role in improving the conversion efficiency of intermediate hydrogen peroxide into water and oxygen. The 60CPO group exhibited the highest increase in metabolic activity for the NIH/3T3 fibroblasts on day 14 compared to the other scaffold compositions (Fig. 2a). In comparison, the NIH/3T3 fibroblasts in the 40CPO scaffolds demonstrated an increasing metabolic activity up to day 14, and a decline in metabolic activity past this time point.
Similarly, the L6 rat myoblasts in the scaffolds that contained less than 60 mg/mL per mL of PCL exhibited increasing metabolic activity only up to day 7 in vitro (Fig. 2b). There was success in the 60CPO group especially, which exhibited increasing metabolic activity in L6 rat myoblasts over time up to day 35. Overall, the metabolic activity of the L6 rat myoblasts in the 60CPO scaffolds remained the highest than that of the other scaffold compositions.
Similarly, the primary cardiac fibroblasts showed an increasing metabolic activity up to day 14 in the pristine GelMA, 0CPO, and 40CPO scaffolds whereas the 60CPO condition demonstrated increasing activity up to day 35 (Fig. 2c). According to these results, the 60CPO scaffold condition is most suitable for these cell types and has the capacity to maintain high metabolic activity in long-term tissue culture.
3.3.2. Lactate dehydrogenase (LDH) activity for measuring cytotoxicity
The cytotoxicity in this study was assessed through the LDH activity of the 3D encapsulated cells in the oxygen-generating scaffolds. The results demonstrate that the pristine GelMA, 0CPO, and 40CPO scaffolds elicited a significant increasing trend of LDH activity in the NIH/3T3 fibroblasts during the 35-day in vitro study (Fig. 3d-f).
Fig 3.
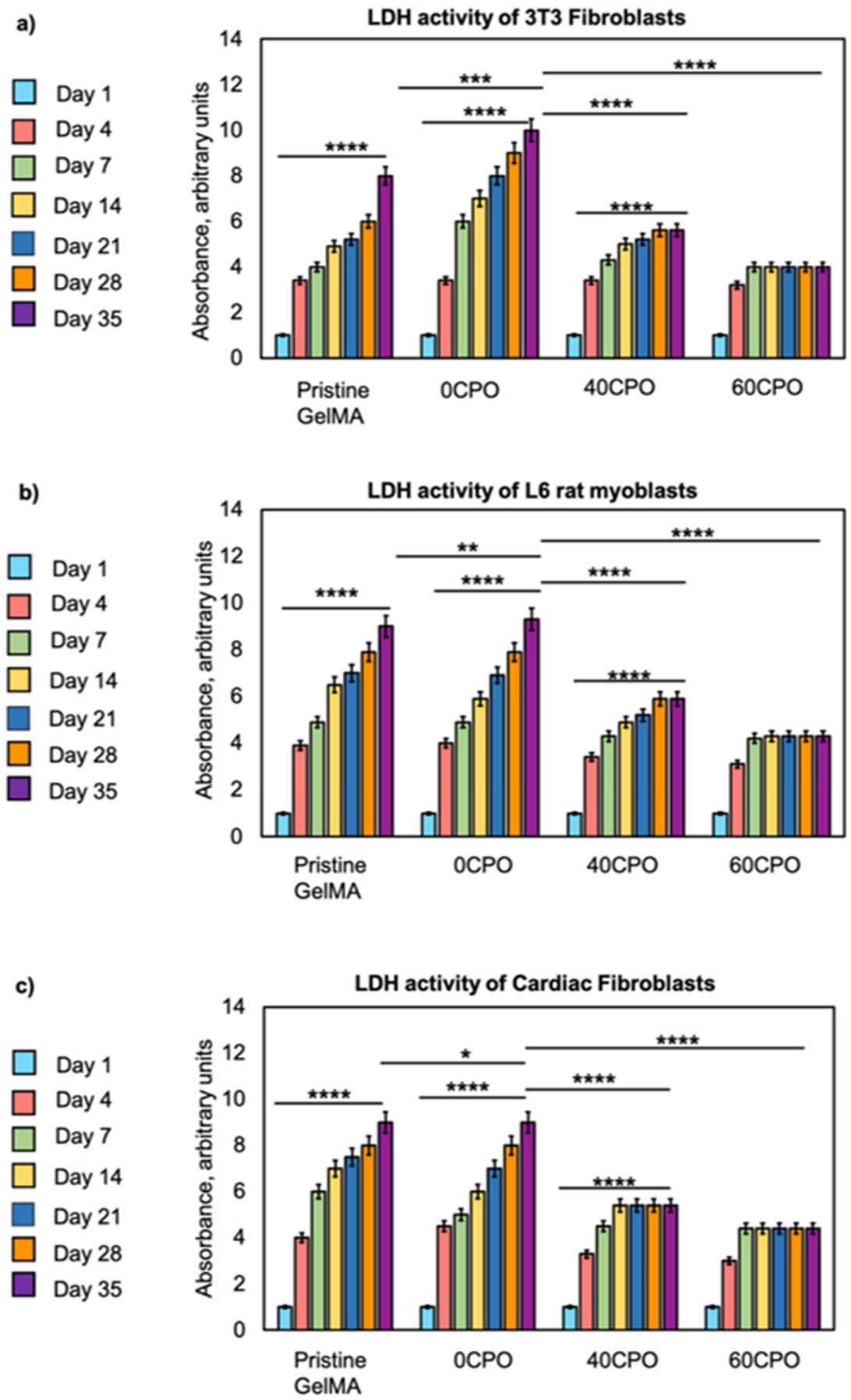
Lactate Dehydrogenase (LDH) assay for evaluation of cytotoxicity of d) NIH/3T3 Fibroblasts e) L6 rat myoblasts and f) Cardiac fibroblasts.
In contrast, there were modest effects with only steady increase in the LDH levels in NIH/3T3 fibroblasts in the 60CPO composition. This result is also comparable to trends in the LDH activities of the L6 rat myoblasts and the primary cardiac fibroblasts encapsulated in pristine GelMA and 0CPO. Interestingly, in the cardiac fibroblasts, there was an observed steady increase in LDH activity in response to the 40CPO scaffolds and no significant increase scaffold in the 60CPO scaffold. Therefore, a desirable lower LDH activity and cytotoxic effects are especially found in scaffolds with higher oxygen-generating content for primary cardiac fibroblasts.
3.3.3. Caspase Glo 3/7 assay for measuring apoptosis
In the 35-day in vitro study, both caspase 3 and 7 activity demonstrated an increasing trend in response to oxygen-generating scaffolds (Fig. 4g-f). Specifically, there was a moderate caspase 3 and 7 activity found in NIH/3T3 fibroblasts 3D-encapsulated in the 40CPO scaffold composition. Contrarily, the pristine GelMA and 0CPO scaffolds demonstrated lower caspase activity in comparison to 40CPO and 60CPO scaffolds. There were no significant changes in the caspase activity found in this study for the 60CPO composition. These cellular behaviors were also similar to scaffolds containing the L6 rat myoblasts and primary cardiac fibroblasts, suggesting that caspase activity may be irrespective to the cell types assessed in this study.
Fig 4.

Caspase Glow 3/7 assay for evaluation of cellular apoptosis of a) 3T3 Fibroblasts, b) L6 rat myoblasts, and c) Cardiac fibroblasts 3D encapsulated pristine GelMA, 0CPO, 40CPO, and 60CPO scaffolds which contained 0 mg, 5.4 mg, and 8.1 mg net in PCL per mL of the GelMA matrix, respectively.
3.4. Oxygen release kinetics of oxygen-generating scaffolds
The results oxygen-release kinetics were extrapolated from measurements of oxygen release in the tissue culture medium. These scaffolds were cultured in vitro for 35 days under hypoxia with the addition of catalase at 1 mg/mL. Furthermore, the tissue culture conditions also varied by cell type encapsulated within scaffold, which included NIH/3T3 fibroblasts, L6 rat myoblasts, primary cardiac fibroblasts, or no cells. The peak oxygen release differed between oxygen-generating scaffolds with and without cells, and between different cell types. The scaffolds in culture that were devoid of cells demonstrated higher average peak oxygen releases than scaffolds containing cells (Fig. 5). Without the presence of cells, the peak oxygen release was shown at later time points in the extended in vitro culture period (Fig. 5a). Specifically, both the pristine GelMA and 0CPO scaffolds show approximately the same peak oxygen release at day 1 (~5.02%). In contrast, the 40CPO and 60CPO scaffolds containing cells reached more than a 4-fold increase in peak oxygen release which occurred at later times points. Specifically, these measurements were 22% (day 22) and 29% (day 22) in the 40CPO and 60CPO scaffolds, respectively. According to the results, there was concentration dependency between the peak oxygen release and the concentration of in the scaffolds.
Fig 5.
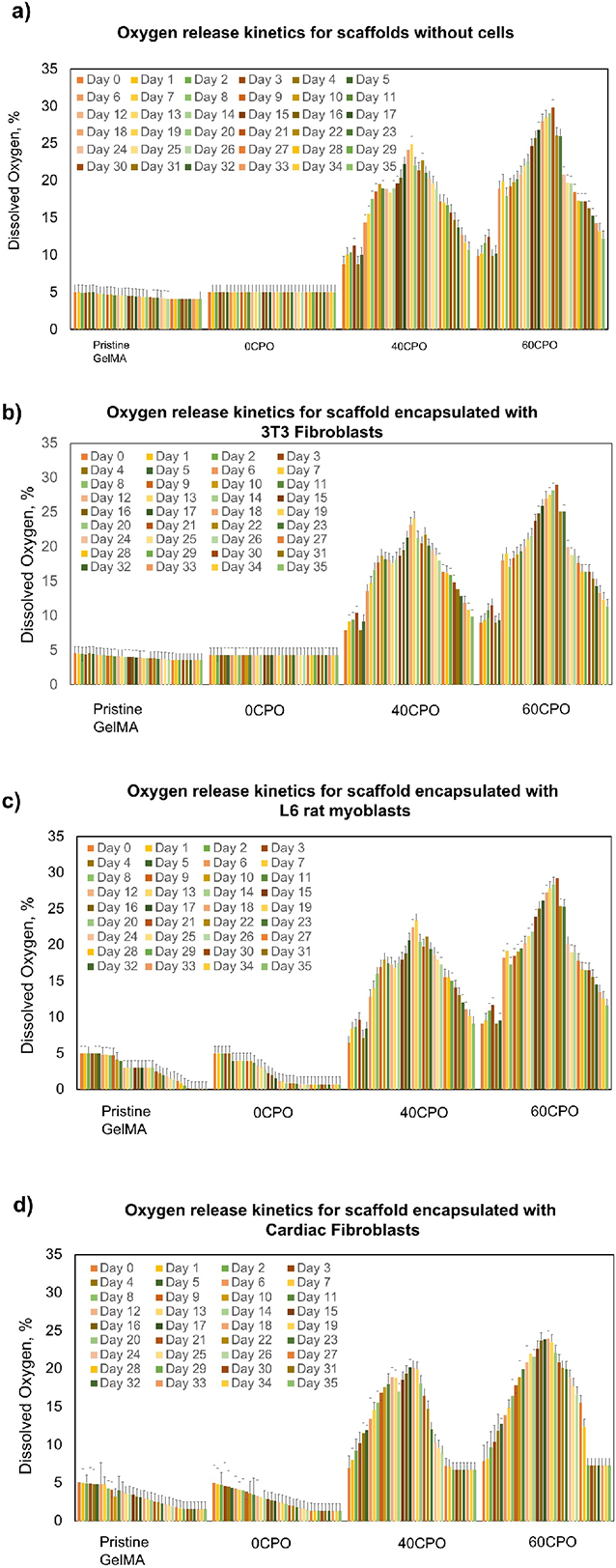
Release kinetics of oxygen-generating scaffolds cultured under hypoxia with catalase supplemented media a) without cells microencapsulated, b) with NIH/3T3 fibroblasts microencapsulated, c) with L6 rat myoblasts microencapsulated and d) with primary cardiac fibroblasts microencapsulated in 3D within pristine GelMA, 0CPO, 40CPO, and 60CPO.
In particular, the scaffolds containing microparticles with lower amounts reagent showed a lower oxygen release over time. The day 1 measurements across the various NIH/3T3 fibroblasts tissue constructs and cell culture conditions remained within the range of 4.27%-4.52%. In L6 rat myoblasts and primary cardiac fibroblasts, the initial measurements on day 1 were ~5.02% oxygen release. The day 1 oxygen release measurements of the pristine GelMA and 0CPO scaffolds also represented the peak oxygen release which declined over time. With NIH/3T3 fibroblasts, however, the decline in oxygen release in pristine GelMA and 0CPO is minimal as it continuously decreased to a range of 3.4%-3.59% by day 35 (Fig. 5b). The oxygen release trends between 3T3 fibroblasts in pristine GelMA and 0CPO were comparable to trends found in these scaffolds without cells. The data also supports that cell type affected the kinetics which was shown in the overall trend in all scaffold compositions with L6 rat myoblasts and primary cardiac fibroblasts (Fig. 5c-d). There was a significant decline from ~5.02% to ~0.1-1.5% within these tissue culture systems, suggesting the oxygen consumption rates were higher in the L6 rat myoblasts and primary cardiac fibroblasts than in the NIH/3T3 fibroblasts. In 40CPO and 60CPO scaffolds, we anticipate that our findings support that these scaffolds are compatible with other cell types which possess similar oxygen consumption rates.
As expected, the 40CPO and 60CPO which contained higher amounts of , improved the oxygen kinetics with respect to the control conditions. These findings mirrored the kinetics found in these scaffold compositions that were devoid of cells in Fig. 5a. In Fig. 5b, the peak oxygen in release of NIH/3T3 fibroblasts in 40CPO and 60CPO groups were 24% by day 19 and 29% by day 21, respectively. Our findings support that oxygen release kinetics were largely dictated by cell type and scaffold composition. In particular, increasing loading yielded scaffolds that demonstrated higher peak oxygen releases that occur past 14 days in vitro. Furthermore, a distinguishing feature of 60CPO scaffolds included a maximum oxygen release that occurs later than in the 40CPO scaffolds. Potentially, it is possible that these 60CPO scaffolds are sustainable solutions for oxygen delivery for longer than 35 days. Comparably, there was over a 4-fold improvement in the peak oxygen release in primary cardiac fibroblasts with a peak oxygen release of 20% (day 17) and 23% (day 18), respectively (Fig. 5d). Despite cell oxygen consumption, the profiles of 40CPO and 60CPO scaffolds still maintain predictable oxygen release kinetics with increasing amounts of . This behavior was also similar with respect to the L6 rat myoblasts 3D-encapsulated in these scaffold conditions. The peak oxygen release was presented at 23% at day 19 and 29% at day 21, in the 40CPO and 60CPO scaffolds (Fig. 5c). It is therefore evident that the observed oxygen release in the 40CPO and the 60CPO differed in terms of two following aspects: the magnitude of the % peak oxygen release and the day when the peak release was attained. We suspect that additionally the different oxygen consumption rates of the various cell types reduced the effects of the concentration on the occurrence and magnitude of the peak oxygen release in each of the scaffolds. However, the findings demonstrate that these oxygen-generating scaffolds deliver oxygen continuously under hypoxic conditions. Importantly, the oxygen release kinetics were controllable based on scaffold composition. The oxygen release kinetics is therefore adjustable to particular cell types. These features of our tissue construct recapitulate desirable properties in tunable tissue culture systems for extended periods.
3.4.1. Effect of varying PCL concentration on the cumulative oxygen release
The PCL component of the oxygen-generating microparticles served as the hydrophobic barrier to control the hydrolysis reaction that occurs between and the water content in the microenvironment. We varied PCL concentration in the microparticles to evaluate its effect on the overall oxygen release potential and kinetics in the scaffolds (Fig. 6). Specifically, this study used a PCL concentration range of 5% to 20% (w/v) in chloroform, with gradually increasing increments of 1% (w/v). In addition, the previous baseline of 13.5% (w/v) PCL in chloroform concentration was also included in this study. To these solutions, the was emulsified at a 60 mg/mL concentration as previously described. These modified microspheres reinforced the synthesized GelMA precursor solution to fabricate oxygen-generating scaffolds. The results were generated from scaffolds cultured without cells under induced hypoxia and 1 mg/mL catalase supplemented media. The lower PCL concentrations of yielded greater oxygen-release at more rapid rates than at higher PCL concentrations, as expected. However, in application, we also attribute the distinct oxygen-release profiles to myriad of factors, including the cell type, its oxygen consumption rate, the cell density and the concentration and crosslinking density of the hydrogel polymer. Due to mass transport constraints, we expect that the oxygen release kinetics have an additional dependency on the scaffold’s dimension, microparticle and cell distribution, and cell culture media.
Fig 6.

Cumulative oxygen release measured due to the change in the PCL concentration.
3.5. Effect of oxygen-generating scaffolds on pH of cell culture media
The change of pH was monitored in in vitro cell culture in this study as it is critical factor in cellular response and function (Fig. 7). In hydrolysis, one of the byproducts is which further degrades to form oxygen and water. However, it is also important to consider and characterize the other byproduct, calcium hydroxide () which can alter the pH of microenvironment. The pHs of the supernatants in the cell culture media were measured on days 1, 4, 7, 14, 21 and 35 with scaffolds containing or devoid of primary cardiac fibroblasts to study this effect. In the cell culture conditions, there were no significant changes in pH in the presence of the oxygen-generating scaffolds with or without cells 3D encapsulated. In pristine GelMA and 0CPO scaffolds devoid of cells, the pH remained in the range of pH 8-9 through the 35-day in vitro study. Furthermore, both the 40CPO and 60CPO scaffolds were also similar in that the pH and remain between pH 8-8.5 during the experiment. With cells encapsulated, the pH ranges mainly within pH 8 and 8.5 in pristine GelMA. However, on day 7, there was a slight increase to pH 9 before returning to the pH 8-8.5 range. In the primary cardiac fibroblasts, the 0CPO scaffolds remained in the range pH 8-8.5. Similarly, the 40CPO and 60CPO scaffolds remained primarily in the pH 8.5 range with increase to pH 9 between days 4 and 7. Although the changes in pH were measurable, the overall pH remained stable across all scaffold compositions and showed no significant increase over the 35-day culture period overall.
Fig 7.
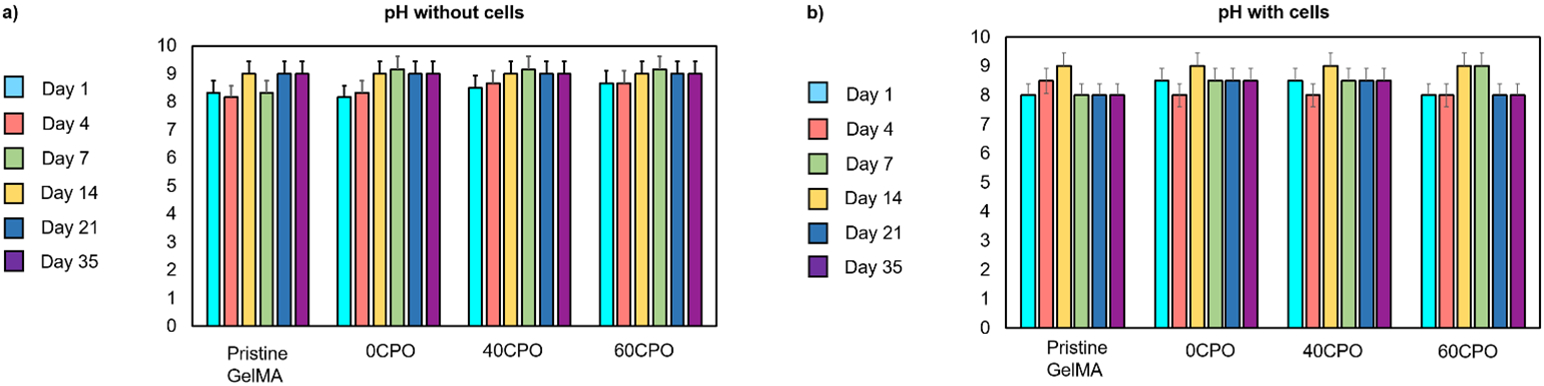
Effect of oxygen generating scaffolds on pH of the culture media as observed on days 1, 4, 7, 14, 21, and 35 when cultured a) without cells under hypoxia and b) with primary cardiac fibroblasts microencapsulated. The data is represented for the pristine GelMA, 0CPO, 40CPO, and 60CPO scaffolds which contained 0 mg, 5.4 mg, and 8.1 mg net in the GelMA matrix, respectively.
4. Discussion
4.1. Synthesis, characterization, and mechanical testing of oxygen-generating scaffolds
The synthesized oxygen-generating microparticles served as the vehicle to deliver oxygen in the microenvironment within tissue scaffolds. The microparticle sizes included a diameter distribution range of 50-250 μm. We attribute the range of the diameters of these oxygen-generating microspheres to the (w/v) ratio of in PCL within the scaffolding biomaterial matrix. Using these parameters, a mean microparticle size of 100 μm was chosen for microencapsulation at a 13.5% (w/v) in the GelMA prepolymer solution. Specifically, this average size and the (w/v) ratio were optimized to maintain adequate oxygen availability to cells seeded at a 5x106 cells/mL density. Furthermore, this microsphere composition directly affected the biomaterial properties of the oxygen-generating scaffold. Therefore, the other physical properties such as swelling, degradation, and compressive strength are also in turn affected by material composition (Fig. 1d-f). The swelling analysis demonstrated that the behavior decreased with increasing concentrations of in PCL, and therefore, were controlled by modifying this component (Fig 1d). The oxygen-generating scaffolds included hydrogel polymer networks that are hydrophilic which can absorb and retain water. In Fig. 1e, the swelling behavior can be explained by the amount of volume occupied in the hydrogel matrix that is hydrophilic.
For instance, in scaffolds reinforced with microparticles with higher concentrations of in PCL, there was less hydrophilic content than in scaffolds than in scaffolds that were reinforced with microparticles with lower concentrations of in PCL. The oxygen- generating scaffolds contained primarily two components: a hydrophilic GelMA hydrogel matrix and the PCL- composite microparticles. The hydrophilicity and porosity of the material supported the absorption and retention of aqueous media5, 54. The structure of the matrix allowed the aqueous solution to remain in the polymer network and to cause the hydrogels to swell.
This swelling capacity was considerably reduced with the addition of oxygen-generating microparticles. Specifically, both the hydrophilic surface and volume within the polymeric matrix were affected. Therefore, these microparticle-reinforced hydrogels exhibited lower swelling ratios. As the concentration increases, the scaffolds become densely filled with non-hydrophilic content which decreased the net hydrophilic surface area within the hydrogel matrix. Consequently, these composition modifications resulted in significantly swelling ratios than pristine GelMA in the results.
Moreover, the increasing amount of the constituent in this study increased the solid, water-soluble content in the scaffold. The control of swelling is desirable, especially in tissue constructs that must remain implanted in physiological environments long term. In mineralized tissues, such as bone, the interconnected pores of hydrogels may collapse in response to over swelling55. However, these oxygen-generating scaffolds allow for the manipulation and restriction of this property by modifying the amount of . Similarly, the degradation analysis revealed the relationship between percent mass remaining and the scaffold composition (Fig 1e).
Additionally, the techniques involved in the scaffold synthesis yielded unique crosslinking densities, stiffnesses, and morphologies. As in other tissue construct types, these material properties largely impact cell viability, proliferation, and cell spreading within the 3D scaffold matrix. At a cellular level, these material characteristics offer biological, chemical, and mechanical cues that will dictate mechano-transduction, guided growth, and differentiation56, 57. This study robustly covered the physical, chemical, and biological analyses to understand the biomaterial behavior. The mechanical behaviors are relevant biomaterial properties that facilitate proper tissue construct viability and cellular functions. The swelling analysis demonstrated that the behavior decreased with increasing concentrations of in PCL, and therefore, were controlled by modifying this component (Fig 1d). The oxygen-generating scaffolds included hydrogel polymer networks that are hydrophilic which can absorb and retain water. In Fig. 1e, the swelling behavior can be explained by the amount of volume occupied in the hydrogel matrix that is hydrophilic. For instance, in scaffolds reinforced with microparticles with higher concentrations of in PCL, there was less hydrophilic content than in scaffolds than in scaffolds that were reinforced with microparticles with lower concentrations of in PCL.
The percent mass remaining increased with increased (w/v) ratios of in PCL. In comparison, the oxygen-generating scaffolds reinforced with microspheres containing more content yielded faster degradation rates. It is important to consider that this analysis utilized collagenase type II to facilitate enzymatic degradation of the gelatin component of the scaffold. Therefore, scaffolds with higher concentrations of in PCL possessed a lower amount of degradable gelatin content. Conversely, the scaffolds reinforced with microparticles with lower content included higher amounts of degradable gelatin content. Other findings shown in mechanical testing experiments demonstrated another correlation between the material composition and compressive modulus of each scaffold composition (Fig 1f). Our findings support that mechanical strength is another physical characteristic that is modifiable during the microparticle synthesis. Further investigation using SEM imaging and phase-contrast imaging revealed the morphology of oxygen-generating scaffolds (Fig. 1a-c). Specifically, the hydrogel matrix appeared as a lace-like surface that covered the microparticles throughout the scaffold. This result shows that the microparticles are fully encapsulated and integrated in the gelatin-based scaffold. The hydrogel covering over the microspheres show that the oxygen-generating particles fully integrate within the hydrogel matrix6. Overall, these findings demonstrated how the synthesized oxygen-generating microspheres interface with the hydrogel matrix and affect the material properties.
4.2. Oxygen release kinetics
The oxygen-generating scaffold compositions provided unique oxygen-release profiles. The kinetics found in this study revealed that the peak release is greater and occurs at later time points in vitro with increased concentration of in the PCL (Fig. 5). This behavior was expected based on the dynamics involved in the breakdown of via hydrolysis. With increased content, there was more reactant available to undergo hydrolysis, which would, therefore, lead to more oxygen release over time. Understanding the rate of delivery of this nutrient supply is important in volumetric bone defects that require rapid healing of a bone graft without causing necrosis at the site of injury. In this study, the peak oxygen release was also determined in hypoxic cell culture conditions with the addition of catalase. Based on the results (Fig. 5), the catalase supported and improved the hydrolysis of . As shown, the peak oxygen releases were found higher and at later time points in the 40CP0 and 60CPO constructs than in the pristine GelMA and 0CPO constructs. In this experiment, was a rate-limiting step in the breakdown of in the microspheres rather than catalase which was held at a 1 mg/mL concentration across all conditions. Therefore, once the was depleted, a decline was expected to occur following the peak release because of the lack of reactant during hydrolysis. The oxygen-generating scaffolds with and without microencapsulated cells presented this oxygen release behavior. However, the average amount oxygen release at peak measurements was found higher in scaffolds without microencapsulated cells. This finding was expected as there were no cells to consume the dissolved oxygen in the cell culture media during the extended in vitro study. Therefore, the presence of 3D-encapsulated cells was another factor that influenced the lower rates of oxygen release.
The oxygen release kinetics was also controlled by the hydrophobic barrier, PCL, in the microparticles. To understand this aspect, the concentration of PCL in the microspheres was varied while holding in the particles at a constant concentration (i.e., 60 mg/mL). Using increasing range of 5-20%, a gradual and predictable cumulative oxygen release over time is shown in Fig. 6. There was a positive correlation between the PCL concentration and the oxygen release over time. Consequently, it is feasible to both adjust the amount of oxygen-releasing content, , as well the hydrophobic barrier, PCL, in the microparticles for fine-tuned scaffold properties. We would also consequently expect that changing the intricate balance between the , PCL or the oxygen generating microparticle concentrations within the hydrogel matrix, or changing the gel concentration or properties, could all lead to varied release kinetics which allow for immense tunability over the oxygen release potential and kinetics of these scaffolds.
4.3. Cellular response in vitro to the oxygen-generating scaffolds
4.3.1. Metabolic activity of microencapsulated cells
The biological performance was improved in scaffolds reinforced with microspheres composed of higher content. The results of the Alamar blue assay support that the 60CPO composition provided the most auspicious microenvironment for the studied cell types (Fig. 2). In contrast, the cell microencapsulated pristine GelMA and 0CPO compositions exhibited similar metabolic activities. As these scaffolds did not contain , the cell culture systems were deficient of a source of oxygen under induced hypoxia. However, there were observable differences between the metabolic activities of the NIH/3T3 fibroblasts, L6 rat myoblasts, and primary cardiac fibroblasts. Generally, these cell types were expected to demonstrate unique metabolic activities as they are derived from different cell lineages. In NIH/3T3 fibroblasts, the pristine GelMA and 0CPO scaffolds gradually increased up to day 14. The L6 rat myoblasts and primary cardiac fibroblasts demonstrated similar results in the pristine GelMA and 0CPO scaffolds. In these scaffolds, the metabolic activities of these cells remained the same, irrespective to cell type. Based on these observations, these scaffolds may be solely support cell viability but lack the required extracellular oxygen and other biological and physical cues required that support energy-intensive cellular activities and aerobic metabolism. In contrast, the NIH/3T3 fibroblasts in 40CPO and 60CPO scaffolds both demonstrated increase in metabolic activity past 14 days. However, both the myoblasts and cardiac fibroblasts microencapsulated in 40CPO showed an increase in metabolic activity up to day 7 before declining and plateauing for the remainder of the culture period. These results suggest that the oxygen-generating scaffolds provided the oxygen as essential nutrient to support metabolism over extended days in in vitro cell culture. However, cells such as myoblasts and cardiac fibroblast required more of this nutrient supply to sustain metabolic activities past 7 days. The 60CPO showed superior metabolic activity across different cell types continuously up to day 35. Based on the cell lineages, these oxygen-generating scaffolds may more suitable for supporting the metabolic activities of embryonic cells such as NIH/3T3 fibroblasts for longer cell culture periods rather than other undifferentiated cells derived from skeletal or connective tissue such as, L6 rat myoblasts and primary cardiac fibroblasts.
4.3.2. LDH – Cell Cytotoxicity
The presence of LDH was utilized as a biomarker to determine plasma damage and cytotoxicity. Specifically, the LDH levels were indicative of membrane damage which can determine the cytotoxicity of the biomaterial. In this study, the NIH/3T3 fibroblasts, L6 rat myoblasts, primary cardiac fibroblasts exhibited higher LDH levels in the pristine GelMA and 0CPO groups, with rapid increases in levels over time (Fig. 3). These results suggest that these scaffold compositions were most cytotoxic among the scaffold compositions tested. While gelatin-based hydrogels are biocompatible, it is possible the cells were not well-suited in these microenvironments in long-term cell culture. Contrarily, the addition of oxygen-generating microsphere has a positive effect on improving the cytotoxicity of the tissue scaffold for long-term cell culture purposes. The fibroblasts and myoblasts yielded significantly lower LDH levels in the 40CPO and 60CPO scaffolds. The LDH levels in the 40CPO composition, however, also showed a gradual increasing trend in LDH up to 28 days. In comparison, these lower LDH levels were maintained up to 35 days when 3D-encapsulated in 60CPO scaffolds. The oxygen source provided in the scaffolds supported cell viability and metabolic activity which in turn helped overcome potential cytotoxic effects of the hypoxic cell culture environment in extended in vitro cell culture. Evidently, these results demonstrated significant improvement in scaffolds in the 40CPO and 60CPO scaffolds. For instance, the LDH levels of the primary cardiac fibroblasts plateaued in the 40CPO and 60CPO groups at days 14 and day 7, respectively, rather than continuing to increase. Overall, these biological responses suggest that including an oxygen-generating component in these scaffolds reduced the cytotoxic effects of cellular microenvironment. The results also support that 60CPO scaffold composition yielded the most desirable biological performance in vitro in this study. Based on the results, we anticipate that different oxygen consumption profiles would be generated based on the cell type if the scaffold composition and cell seeding density were held constant.
4.3.3. Caspase assay for presence of apoptosis
The caspase 3/7 activity assay revealed the potential for apoptosis in using these oxygen-generating tissue scaffolds (Fig. 4). The luminescence in this assay correlated to the amount of apoptosis in a particular cell culture. In the reaction, the substrate binds to DEVD, a tetrapeptide sequence which produces a quantifiable luminescence. In pristine GelMA and 0CPO, the NIH/3T3 fibroblast, L6 rat myoblasts, and primary cardiac fibroblasts demonstrated increasing apoptotic activity over time. This finding was expected as these tissue scaffolds were entirely oxygen-deprived under induced hypoxia. Although short-term cell viability was maintained, later time points, especially beyond 7 days, in the cell culture conditions were not permissible for extended periods. Moreover, this analysis coincided with lower metabolic activities and higher LDH activities also found in these compositions (Fig. 2-3). Without oxygen, necessary cellular functions were not supported which most likely led to induction of apoptotic cell death pathways. At later time points in in vitro cell culture, such as at day 21, caspase activity dramatically increased. Among the cell types investigated, the L6 rat myoblasts demonstrated to be the most susceptible to apoptosis when microencapsulated in pristine GelMA and 0CPO under induced hypoxia. Overall, this apoptotic behavior was significantly mitigated in 40CPO and 60CPO scaffolds. Across the cell types, caspase activity decreased over time but eventually increased at day 28. As expected, the 60CPO scaffold supported all cell types in long-term cell culture with predominantly low caspase activity over time. After day 21, the caspase activity plateaued in the 60CPO groups, and stopped increasing in apoptotic activity. Again, this behavior corresponded with previous experiment as LDH levels remained constant after day 14 for this cell culture condition (Fig. 3). Therefore, the oxygen source of the scaffolds without the supplement of additional biochemical factors may be detrimental to long-term cell culture system and avoid cellular dysfunction and cell death. Interestingly, a distinguishable characteristic was shown in the primary cardiac fibroblasts, which maintained constant caspase activity after day 7 in both the 40CPO and 60CPO groups. These findings support that the oxygen-generating scaffolds support cell viability across various cell types under hypoxic conditions.
4.4. Effect of pH
The oxygen-generating scaffolds supported a stable cell culture system with no significant fluctuations in the in vitro cell culture environment. Importantly, the pH of the supernatant of the cell culture remained stable throughout the 35-day tissue culture period. Therefore, the byproducts of hydrolytic degradation, and , did not modify the pH of the cell culture system detrimentally. These byproducts are essentially temporary reaction intermediates, which may result in a more basic pH in the supernatant. However, as expected, the in vitro cell culture with the pH of pristine GelMA scaffold which lacked any had no change in pH (Fig. 7). Generally, DMEM in the cell culture often contains supplements of with sodium bicarbonate regardless, which results in more basic cellular environments. Furthermore, some changes in the pH of the cell culture media may be due cell metabolism as cells grow and proliferate. We have demonstrated the capitalizing of the hydrolytic degradation of does not compromise a stable cellular microenvironment in vitro.
5. Conclusions
In this study, we developed and robustly characterized novel oxygen-generating scaffolds. Our tissue scaffolds composed of a gelatin-based hydrogel matrix reinforced with oxygen-generating microparticles. We have developed a modular microparticle synthesis process using an emulsification technique that produced a portfolio of oxygen-generating scaffolds with distinct oxygen-release kinetics. These scaffolds released oxygen consistently over 4 weeks with long-lasting dissolved oxygen levels for up to 35 days in culture under hypoxia. Other material properties such as compressive moduli, swelling, and degradation were controlled by altering the in the oxygen-generating microspheres. These scaffolds were also tailorable to diverse cell types, including NIH/3T3 fibroblasts, L6 rat myoblasts, and primary cardiac fibroblasts. The in vitro results support that the oxygen- generating component provides functional benefits for long-term cell culture. The metabolic activity and apoptotic activity were controlled by the oxygen-generating scaffolds, with consistent success in the 60CPO scaffolds. Overall, stable cellular microenvironments were achieved, without causing major fluctuations in pH. These oxygen-generating scaffolds offer a promising novel biomaterial for sustained delivery of oxygen in 3D tissue constructs. We anticipate that these scaffolds will be easily modified and customized to obtain a wide array of oxygen release kinetics and biological properties for different tissue-engineered applications. This technology, along with efforts to improve vascularization strategies, has the capacity to tremendously advance the in vivo clinical performance of tissue constructs and is broadly
Acknowledgements
This research was funded by the Transformational Project Award from the American Heart Association (AHA) (19TPA34910111) and the University of Massachusetts Lowell start-up funds. The authors thank Mr. Alex Viglione for technical help. The patent application for this work has been filed but not yet published.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.H. R. a. S. Administration, 2020.
- 2.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ and Atala A, Nature Biotechnology, 2016, 34, 312–319. [DOI] [PubMed] [Google Scholar]
- 3.Jung JW, Lee J-S and Cho D-W, Scientific Reports, 2016, 6, 21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Suvarnapathaki S, Walsh K and Camci-Unal G, MRS Communications, 2018, 8, 1–14. [Google Scholar]
- 5.Suvarnapathaki S, Nguyen MA, Wu X, Nukavarapu SP and Camci-Unal G, RSC Advances, 2019, 9, 13016–13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suvarnapathaki S, Wu X, Lantigua D, Nguyen MA and Camci-Unal G, NPG Asia Materials, 2019, 11, 65. [Google Scholar]
- 7.Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S and Lewis JA, Science Advances, 2019, 5, eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camci-Unal G, Newsome D, Eustace BK and Whitesides GM, Adv Healthc Mater, 2016, 5, 641–647, 626. [DOI] [PubMed] [Google Scholar]
- 9.Lantigua D, Nguyen M, Wu X, Suvarnapathaki S, Kwon S, Camci-Unal G and Gavin W, Soft Matter, 2020. [DOI] [PubMed] [Google Scholar]
- 10.Suvarnapathaki S, Wu X, Lantigua D, Nguyen MA and Camci-Unal G, Macromolecular Bioscience, 2020, 2000176. [DOI] [PubMed] [Google Scholar]
- 11.Park J-H, Yoon J-K, Lee JB, Shin YM, Lee K-W, Bae S-W, Lee J, Yu J, Jung C-R, Youn Y-N, Kim H-Y and Kim D-H, Scientific Reports, 2019, 9, 2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwivedi R, Kumar S, Pandey R, Mahajan A, Nandana D, Katti DS and Mehrotra D, J Oral Biol Craniofac Res, 2020, 10, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung JW, Lee J-S and Cho D-W, Scientific reports, 2016, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H-HG, Rumma RT, Ozaki CK, Edelman ER and Chen CS, Cell stem cell, 2018, 22, 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BS, Jang J, Chae S, Gao G, Kong J-S, Ahn M and Cho D-W, Biofabrication, 2016, 8, 035013. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Jia S, Chen W, Liu X and Zhang S, Materials Science and Engineering: C, 2019, 101, 1–14. [DOI] [PubMed] [Google Scholar]
- 17.Gholipourmalekabadi M, Zhao S, Harrison BS, Mozafari M and Seifalian AM, Trends Biotechnol, 2016, 34, 1010–1021. [DOI] [PubMed] [Google Scholar]
- 18.Suvarnapathaki S, Ramos R, Sawyer SW, McLoughlin S, Ramos A, Venn S and Soman P, Journal of Materials Research, 2018, 33, 2012–2018. [Google Scholar]
- 19.Pashneh-Tala S, MacNeil S and Claeyssens F, Tissue Eng Part B Rev, 2016, 22, 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinlan E, López-Noriega A, Thompson EM, Hibbitts A, Cryan SA and O'Brien FJ, Journal of tissue engineering and regenerative medicine, 2017, 11, 1097–1109. [DOI] [PubMed] [Google Scholar]
- 21.Fu J and Wang D-A, Trends in biotechnology, 2018, 36, 834–849. [DOI] [PubMed] [Google Scholar]
- 22.Yan Y, Chen H, Zhang H, Guo C, Yang K, Chen K, Cheng R, Qian N, Sandler N and Zhang YS, Biomaterials, 2019, 190, 97–110. [DOI] [PubMed] [Google Scholar]
- 23.Dew L, MacNeil S and Chong CK, Regen Med, 2015, 10, 211–224. [DOI] [PubMed] [Google Scholar]
- 24.Goranov V, Shelyakova T, De Santis R, Haranava Y, Makhaniok A, Gloria A, Tampieri A, Russo A, Kon E and Marcacci M, Scientific reports, 2020, 10, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rana D, Zreiqat H, Benkirane-Jessel N, Ramakrishna S and Ramalingam M, Journal of tissue engineering and regenerative medicine, 2017, 11, 942–965. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Lai BFL, Xie R, Huyer LD, Montgomery M and Radisic M, Nature protocols, 2018, 13, 1793–1813. [DOI] [PubMed] [Google Scholar]
- 27.Fenech M, Girod V, Claveria V, Meance S, Abkarian M and Charlot B, Lab on a Chip, 2019, 19, 2096–2106. [DOI] [PubMed] [Google Scholar]
- 28.Hancock MJ, Piraino F, Camci-Unal G, Rasponi M and Khademhosseini A, Biomaterials, 2011, 32, 6493–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao B, Li Z, Peng R and Ding J, Biomaterials, 2015, 64, 21–32. [DOI] [PubMed] [Google Scholar]
- 30.Goossens GH and Blaak EE, Frontiers in endocrinology, 2015, 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpe CMO, Villar-Delfino PH, dos Anjos PMF and Nogueira-Machado JA, Cell death & disease, 2018, 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belikov AV, Schraven B and Simeoni L, Journal of biomedical science, 2015, 22, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sunkari VG, Lind F, Botusan IR, Kashif A, Liu ZJ, Yla-Herttuala S, Brismar K, Velazquez O and Catrina SB, Wound Repair Regen, 2015, 23, 98–103. [DOI] [PubMed] [Google Scholar]
- 34.Huang C-C, Chia W-T, Chung M-F, Lin K-J, Hsiao C-W, Jin C, Lim W-H, Chen C-C and Sung H-W, Journal of the American Chemical Society, 2016, 138, 5222–5225. [DOI] [PubMed] [Google Scholar]
- 35.Montazeri L, Hojjati-Emami S, Bonakdar S, Tahamtani Y, Hajizadeh-Saffar E, Noori-Keshtkar M, Najar-Asl M, Ashtiani MK and Baharvand H, Biomaterials, 2016, 89, 157–165. [DOI] [PubMed] [Google Scholar]
- 36.Grassmann J, Schneppendahl J, Hakimi A, Herten M, Betsch M, Lögters T, Thelen S, Sager M, Wild M, Windolf J, Jungbluth P and Hakimi M, 2015, 33, 513–520. [DOI] [PubMed] [Google Scholar]
- 37.Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE and Weibel S, Cochrane Database Syst Rev, 2015, DOI: 10.1002/14651858.CD004123.pub4, Cd004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thistlethwaite KR, Finlayson KJ, Cooper PD, Brown B, Bennett MH, Kay G, O'Reilly MT and Edwards HE, Wound Repair Regen, 2018, 26, 324–331. [DOI] [PubMed] [Google Scholar]
- 39.Alemdar N, Leijten J, Camci-Unal G, Hjortnaes J, Ribas J, Paul A, Mostafalu P, Gaharwar AK, Qiu Y and Sonkusale S, ACS biomaterials science & engineering, 2017, 3, 1964–1971. [DOI] [PubMed] [Google Scholar]
- 40.McQuilling JP, Sittadjody S, Pendergraft S, Farney AC and Opara EC, Biomater Sci, 2017, 5, 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.J. OL Jeung Soo Huh, 2015, 05. [Google Scholar]
- 42.Patil PS, Fountas-Davis N, Huang H, Michelle Evancho-Chapman M, Fulton JA, Shriver LP and Leipzig ND, Acta Biomater, 2016, 36, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Zhao Y, Li T, Chen Z, Wang Y and Qin C, Chemical Engineering Journal, 2016, 303, 450–457. [Google Scholar]
- 44.Khorshidi S, Karkhaneh A and Bonakdar S, Journal of Biomedical Materials Research Part A, 2020, 108, 136–147. [DOI] [PubMed] [Google Scholar]
- 45.Steg H, Buizer AT, Woudstra W, Veldhuizen AG, Bulstra SK, Grijpma DW and Kuijer R, J Mater Sci Mater Med, 2015, 26, 207. [DOI] [PubMed] [Google Scholar]
- 46.Chandra PK, Ross CL, Smith LC, Jeong SS, Kim J, Yoo JJ and Harrison BS, Wound Repair Regen, 2015, 23, 830–841. [DOI] [PubMed] [Google Scholar]
- 47.Rastinfard A, Nazarpak MH and Moztarzadeh F, RSC Advances, 2018, 8, 91–101. [Google Scholar]
- 48.Farris AL, Rindone AN and Grayson WL, Journal of Materials Chemistry B, 2016, 4, 3422–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim HY, Kim SY, Lee H-Y, Lee JH, Rho G-J, Lee H-J, Lee H-C, Byun J-H and Oh SH, Biomacromolecules, 2019, 20, 1087–1097. [DOI] [PubMed] [Google Scholar]
- 50.Kamaly N, Yameen B, Wu J and Farokhzad OC, Chemical Reviews, 2016, 116, 2602–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho WW, Kim BS, Ahn M, Ryu YH, Ha DH, Kong JS, Rhie JW and Cho DW, Advanced Healthcare Materials, 2020, 2001693. [DOI] [PubMed] [Google Scholar]
- 52.Ha D-H, Chae S, Lee JY, Kim JY, Yoon J, Sen T, Lee S-W, Kim HJ, Cho JH and Cho D-W, Biomaterials, 2020, 266, 120477. [DOI] [PubMed] [Google Scholar]
- 53.Trusek-Holownia A and Noworyta A, Biotechnology Reports, 2015, 6, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Stroll SI, Lantigua D, Suvarnapathaki S and Camci-Unal G, Biomaterials science, 2019, 7, 2675–2685. [DOI] [PubMed] [Google Scholar]
- 55.Portnov IV, Möller M, Richtering W and Potemkin II, Macromolecules, 2018, 51, 8147–8155. [Google Scholar]
- 56.Vining KH and Mooney DJ, Nature reviews Molecular cell biology, 2017, 18, 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai P, Hu B, Leow WR, Wang X, Loh XJ, Wu YL and Chen X, Advanced materials, 2018, 30, 1800572. [DOI] [PubMed] [Google Scholar]


