ABSTRACT
Objectives
Cold plasma has shown efficacy in various dermatological applications by reduces inflammatory responses and modulating cytokine expression. Therefore, this study aimed to investigate the therapeutic effects of cold plasma on psoriasis.
Methods
In psoriasis HaCaT cells with cold plasma, we confirmed the expression of inflammatory cytokines involved in psoriasis formation and MAPK pathway, cell cycle, and apoptosis‐related factors. In psoriasis‐like BALB/c mice model, the effects of cold plasma treatment on skin were visually assessed. The expression of psoriasis‐related factors was confirmed through qPCR, Western blotting, and Immunohistochemistry.
Results
Cold plasma led to a reduction in inflammatory cytokines including IL‐17A, IL‐23A, IL‐24, IL‐1β, and TNF‐α in the psoriasis cell line. It also modulated factors involved in the MAPK pathway and the cell cycle. In the psoriasis‐like mice model, cold plasma resulted in improvements in skin thickness, erythema, scaling, and PASI. Additionally, decreases in inflammatory cytokines like INF‐γ, IL‐23, and S100a7 were observed, along with improvements in MAPK pathway activation, apoptosis, and other psoriasis‐related factors.
Conclusion
Through in vitro and in vivo studies, our research highlights the potential of cold plasma as a novel therapeutic approach for psoriasis. Furthermore, cold plasma could serve as an adjunctive treatment for skin immunological diseases.
Keywords: BALB/c, cold plasma, HaCaT cell, inflammation, psoriasis
1. Introduction
Recent advances in understanding physical plasma phenomena have emerged from the development of innovative plasma sources, alongside increased focus on medical applications of plasma. Active plasma components such as molecular, atomic ion, electron and photon, reactive species, ultraviolet, optical and infrared emission, activate complex biochemical procedures, and have control and catalytic functions [1]. Thermal and non‐thermal cold plasma, which has already been widely established in the medical field, are being used in various therapeutic applications.
Non‐thermal cold plasma is particularly noted for its lack of thermal damage to targeted tissues, as it induces cellular responses upon direct interaction with cells. Cold plasma has a potential possibility for treatment application applications in the dermatologic treatment fields. It has been shown to be effective not only in efficient disinfection and sterilization, but also in the treatment of various skin infections, wound healing, and tissue regeneration [2, 3]. In the field of dermatology, applications of plasma devices are expanding to various skin diseases including bedsores and atopic dermatitis [4, 5]. Despite these advances, treatment guidelines and optimized parameters to maximize the efficacy of plasma in dermatological applications remain undeveloped.
Psoriasis is a chronic inflammatory immune skin disease and characterized by erythema and thickening the skin due to excessive keratinization on the surface of the skin. The conventional treatment methods for psoriasis include topical agents, phototherapy, and systemic immunosuppressive agent. Recently, biologics treatment is effective for severe psoriasis. However, most psoriasis patients suffer from chronic progression, recurrence is frequent when treatment is stopped, and the disease course shows repeated deterioration and improvement courses [6]. In addition, most psoriasis patients need long‐term treatment, and they are usually treated by periodic, sequential, or simultaneous circulatory treatment methods to enhance effectiveness and reduce side effects of treatment. Therefore, a new treatment method capable of continuous treatment without irreversible side effects is needed [7].
In the pathophysiology of psoriasis, T cells are the main mediating factor of psoriasis. The activation of T cells increases excessively, secreting a large amount of immune‐mediated cytokines such as tumor necrosis factor‐alpha (TNF‐a), interleukin‐23 (IL‐23), and interleukin‐17 (IL‐17) and stimulates keratinocytes in the skin to induce hyperplasia of the epidermis [8, 9]. The translation of psoriasis‐related cytokines increases according to the increase of the rate of the STAT3 protein, and the increase of the keratinocyte proliferation cycle in psoriasis is caused by the increase of cyclin D1, CDK2, and CDK4, which are G1/S group regulatory proteins [10]. Phototherapy, a current treatment for psoriasis, effectively mitigates keratinocyte hyperplasia by inhibiting the G1 to S phase progression.
Previous studies have shown that cold plasma has the effect of inducing apoptosis and reducing IL‐12 [11, 12]. This has shown the potential as a new treatment for skin immune‐related diseases such as psoriasis by inhibiting hyperproliferation and controlling inflammation of keratinocytes. Therefore, in this experimental study, we aimed to examine the effect of cold plasma and confirm the treatment mechanism of cold plasma on the clinical severity, related cytokine profiles, and skin histology using animal models of psoriasis.
2. Materials and methods
2.1. Cold Plasma Device
Cold plasma device (RE:DERM PLASM, JNL, Korea) was used in this study. This device utilizes argon gas to generate low‐temperature plasma, emitting harmless ionized gas and a minimal amount of UV radiation. Therefore, the use of protective goggles and careful handling are required during operation (Figure 1A).
FIGURE 1.
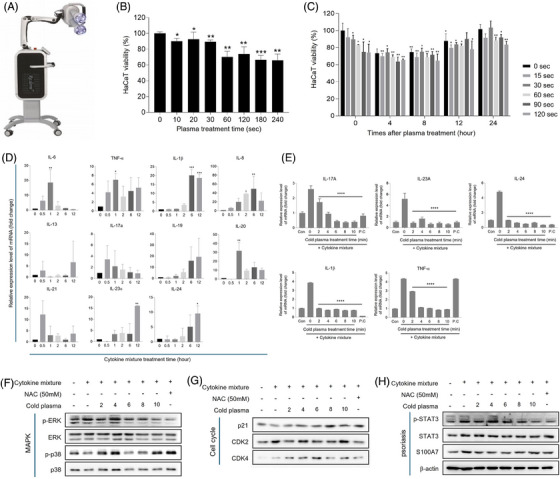
Effect of the cold plasma on psoriasis improvement in psoriasis cell lines from HaCaT cell. (A) Cold plasma device (RE:DERM PLASM, JNL, Korea). (B) HaCaT cell viability was measured after 0, 10, 20, 30, 60, 120, 180, and 240 s of cold plasma treatment and 24 h post‐treatment by using WST‐8 assay. (C) HaCaT cell viability was measured after 0, 10, 20, 30, 60, 120, 180, and 240 s of cold plasma treatment and 0, 4‐, 8‐, 12‐, and 24‐h post‐treatment by using WST‐8 assay. (D) The relative expression level of inflammatory cytokines related with psoriasis mRNA level after cytokine mixture treatment for 0, 0.5, 1, 2, 6, and 12 h. (E) The relative expression level of inflammatory cytokines related with psoriasis mRNA level after cytokine mixture treatment, cold plasma was applied for 0, 2, 4, 6, 8, and 10 min. (F–H) The expression level of MAPK pathway, cell cycle, psoriasis relative marker after cytokine mixture treatment for 0, 0.5, 1, 2, 6, and 12 h. The values represent the mean ± standard deviation. Significant values are indicated as *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001 versus Normal.
2.2. Cell Viability
Human keratinocyte cells (HaCaT) were cultured at 37°C and 5% CO2 culture using Dulbecco's modified Eagle's medium containing 5% fetal bovine serum (FBS) (Invitrogen‐GIBCO‐BRL, Grand Island, NY DMEM) and 1% penicillin. After culturing 5 × 104 HaCaT cells in 12 well cell culture plate, in the CO2 incubator for 24 h, cell viability was measured. First, to determine the optimal investigation time for plasma, cold plasma was treated for 10, 20, 30, 60, 120, 180, and 240 s, and cell viability was quantified using a WST‐8 kit.
Second to evaluate the temporal effects on cell viability post‐treatment, cells were exposed to the same plasma treatment durations, and viability was assessed at 0, 4, 8, 12, and 24 h post‐treatment. For this, 10 µL of WST‐8 solution was added to each well, the plate was shielded from light and incubated at 37°C. The absorbance at 450 nm was measured using a microplate spectrophotometer (SpectraMax 340; Molecular Devices, Inc., CA, USA) 1 h later.
2.3. Cytokines Expression In Vitro
In order to establish cell lines with frequent biological characteristics similar to those of psoriasis, psoriasis cell lines were established using cytokines. TNF‐α, IL‐1α, IL‐6, IL‐17A, and IL‐22, which are known as cytokines that stimulate keratinocytes during the development of psoriasis, were mixed in 50 ng/mL and treated with normal HaCaT cells for 0, 0.5, 1, 2, 6, and 12 h. To confirm the establishment of psoriasis cell line, we investigated the expression level of inflammatory cytokines upregulated by psoriasis at the mRNA level. Furthermore, to check the potential improvement in psoriasis by cold plasma treatment, the cell was treated with a cytokine mixture and cold plasma was treated for 0, 2, 4, 6, 8, and 10 h. And then the expression level of inflammatory cytokines upregulated by psoriasis was examined and compared it with a positive control, N‐acetylcysteine, which is commonly used in psoriasis treatment.
2.4. Murine Model
BALB/c mice (6 weeks old, female) from Orient Bio Co. Ltd. (Sungnam, South Korea) were used in this study. By the day of the experiment, solid feed (no antibiotic added) and water were sufficiently supplied, adapted to the environment of 23°C ± 2°C temperature, 55% ± 10% humidity, and 12–12 h (light‐dark cycle) for 1 week, and used for the experiment. All animal experimental protocols were conducted in compliance with the National Institutes of Health's Principle of Laboratory Animal Care (NIH) and with the approval of the Institutional Animal Care and Use Committee of the Chung‐Ang University (IACUC 202401030015).
2.5. Study Design in Murine Model
As an animal model the experiment was conducted using the imiquimod‐induced psoriasis like inflammation model, which is generally used in psoriasis research. The dorsal fur of the mice was depilated after acclimation, psoriasis‐like inflammatory reactions was induced by applying 50 mg of Aldara cream (5% imiquimod) topically daily for a duration of 8 days. Cold plasma was irradiated daily for 2 days starting from the 7th day after the application of Aldara cream.
In this study, two murine model experiments were conducted. In first murine model experiment, the 18 female BALB/c mouse were obtained from Saeron Bio Inc. (Gyeonggi‐do, South Korea), and acclimated for 1 week before being used for the study. Mouse were divided into three groups: normal control (n = 6), psoriasis only (n = 6), psoriasis + plasma (n = 6). In this experiment, visual inspection was conducted to determine whether psoriasis had been improved. Additionally, measurements were taken for skin thickness, erythema, scale, and PASI (severity of psoriasis). In second murine model, the 24 female BALB/c mouse were obtained from Saeron Bio Inc. (Gyeonggi‐do, South Korea), and acclimated for 1 week before being used for the study. Mouse were divided into four groups: normal control (n = 6), psoriasis only (n = 6), psoriasis + plasma 10 min (plasma L, n = 6), and plasma 20 min (plasma H, n = 6).
2.6. qRT‐PCR
Total RNA was extracted using TRIzol (Invitrogen, CA, USA) to confirm that the expression of inflammatory cytokines was reduced by cold plasma treatment. The first strand cDNA synthesis from the entire RNA template was performed with PrimeScript RT master mix (Takara, Tokyo, Japan). The obtained cDNA was applied to RT‐PCR using qPCR 2X PreMIX SYBR (Enzynomics, Seoul, South Korea) and CFX‐96 thermocycler (Bio‐Rad, Hercules, CA, USA). The PCR conditions used to amplify all genes were 40 cycles at 95°C for 10 min, 95°C for 10 s, 60°C for 15 s, and 72°C for 20 s. The expression data was calculated as a cycle threshold (Ct) value using ΔCt, a quantification method. GAPDH was used in normalization. The primers used for qRT‐PCR are summarized in Table 1.
TABLE 1.
Primer sequence used for qPCR analysis.
| Gene ID | Primer | Sequence (5′−3′) |
|---|---|---|
| Human IL‐6 | Forward | AGACAGCCACTCACCTCTTCAG |
| Reverse | TTCTGCCAGTGCCTCTTTGCTG | |
| Human TNF‐α | Forward | ACACCTTCAGCTGCCCAGACTG |
| Reverse | TCCGTGTTTGCTCTCCAGAGCA | |
| Human IL‐1β | Forward | ATGATGGCTTATTACAGTGGCAA |
| Reverse | GTCGGAGATTCGTAGCTGGA | |
| Human IL‐13 | Forward | ACGGTCATTGCTCTCACTTGCC |
| Reverse | CTGTCAGGTTGATGCTCCATACC | |
| Human IL‐17a | Forward | CGGACTGTGATGGTCAACCTGA |
| Reverse | GCACTTTGCCTCCCAGATCACA | |
| Human IL‐19 | Forward | CGTTCTACGTGGACAGGGTGTT |
| Reverse | CAGTGACACTGCCTCTGTTCCT | |
| Human IL‐20 | Forward | AGATCAGCAGCCTCGCCAATTC |
| Reverse | CAAAGTGACTCAGAATCTGGCTG | |
| Human IL‐21 | Forward | CCAAGGTCAAGATCGCCACATG |
| Reverse | TGGAGCTGGCAGAAATTCAGGG | |
| Human IL‐23α | Forward | GAGCCTTCTCTGCTCCCTGATA |
| Reverse | GACTGAGGCTTGGAATCTGCTG | |
| Human IL‐24 | Forward | CTTCTCTGGAGCCAGGTATCAG |
| Reverse | GGCACTCGTGATGTTATCCTGAG | |
| Human GAPDH | Forward | TGGAAATCCCATCACCATCTTC |
| Reverse | CGCCCCACTTGATTTTGG | |
| Mouse IFN‐γ | Forward | CACACTGCATCTTGGCTTTG |
| Reverse | TCCACATCTATGCCACTTGAG | |
| Mouse IL‐23 | Forward | CATGCTAGCCTGGAACGCACAT |
| Reverse | ACTGGCTGTTGTCCTTGAGTCC | |
| Mouse TNF‐ α | Forward | TCAAACCCTGGTATGAGCCC |
| Reverse | ACCCATTCCCTTCACAGAGC | |
| Mouse S100a7 | Forward | GATAGTGTGCCTCGCTTCATGG |
| Reverse | CTGGAGATGGTAGTCCTTCACC | |
| Mouse IL‐6 | Forward | TACCACTTCACAAGTCGGAGGC |
| Reverse | CTGCAAGTGCATCATCGTTGTTC | |
| Mouse GAPDH | Forward | AGGTCGGTGTGAACGGATTTG |
| Reverse | TGTAGACCATGTAGTTGAGGTCA |
2.7. Western Blot Analysis
To confirm the effect on cold plasma, the expression of MAPK, cell cycle‐related protein, and psoriasis marker protein was confirmed. HaCaT cells were dissolved as RIPA buffers containing 50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP‐40, 0.1% SDS, 0.5% deoxycholic acid, and 1 mM PMSF. HaCaT cells were centrifuged at 13 000 rpm for 10 min at 4°C by mixing protease inhibitors (Roche Applied Science, Indianapolis, IN, USA) in ice for 15 min. The supernatant was re‐centrifuged for 10 min, and BCA protein assay kit (Bio‐Rad, Hercules, CA, USA) was used to measure protein concentration. Protein (20 µg) was separated into 10% SDS‐PAGE (polyacrylamide gel electrophoresis) and adsorbed with PVDF (polyvinylidene fluoride) membrane (Millipore Corp, Bedford, MA, USA). The membrane was reacted in PBS containing 0.1% Tween‐20 and 5% skim milk (non‐fat milk) for 2 h at room temperature. And then, the membrane was diluted with 0.2% BSA (overnight at 4°C) using primary antibody MAPK‐related protein factors (p‐ERK, ERK, p‐p38, p38) and cell cycle‐related proteins (p21, CDK2, CDK4), psoriasis markers (p‐STAT3, STAT3, S100A7), and β‐actin (sc‑47778, Santa Cruz Biotechnology, Inc.). After washing three times, the membrane was reacted with an anti‐rabbit (PI‐1000) with HRP or a secondary antibody (Vector Labs Inc., Burlingame, CA, USA) with anti‐mouse (PI‐2000), and the band was visualized as an ECL Advance kit (ATTO, Tokyo, Japan). Immunodetection was performed using an Amersham ECL kit (GE Healthcare, IL, USA) according to the manufacturer's instructions. Since then, each gene expression has been standardized using the loading control group (β‐actin) expression. The antibodies used for western blot analysis are summarized in Table 2.
TABLE 2.
Antibodies used for western blot analysis.
| Antibodies | Product code | Company |
|---|---|---|
| Anti‐p‐ERK | #9101 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐ERK | #9102 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐p‐p38 | #4511 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐p‐38 | #9212 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐p‐STAT3 | #9131 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐STAT3 | #9139 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐S100A7 | #90393 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐β‐actin | #3700 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐JNK | #9252 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐p‐JNK | #4668 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐Caspase9 | #9504 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐Ki‐67 | #9449 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐E‐cadherin | #14472 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐CD34 | #37259 | Cell Signaling Technology Inc. (Beverly, MA) |
| Anti‐p21 | ab109199 | Abcam (Cambridge, UK) |
| Anti‐CDK2 | ab32147 | Abcam (Cambridge, UK) |
| Anti‐CDK4 | ab183685 | Abcam (Cambridge, UK) |
| Anti‐p53 | ab131442 | Abcam (Cambridge, UK) |
| Anti‐CyclinD1 | ab134175 | Abcam (Cambridge, UK) |
| Anti‐CDK6 | ab124821 | Abcam (Cambridge, UK) |
| Anti‐CD95 | ab82419 | Abcam (Cambridge, UK) |
| Anti‐Fas | ab271016 | Abcam (Cambridge, UK) |
| Anti‐Loricrin | ab183646 | Abcam (Cambridge, UK) |
| Anti‐Bcl2 | Ab203516 | Abcam (Cambridge, UK) |
2.8. Histopathologic Evaluation
To observe the improvement of psoriasis‐like inflammation in skin tissue by the cold plasma, skin tissue sample was fixed to 10% Neutral Buffered Formalin (NBF), and after a stepwise dehydration process with a low concentration to a high concentration ethanol solution, paraffin blocks were produced. Each tissue block was cut to a thickness of 5 µm, a tissue section was attached to the slide, and each tissue slide was deparaffinized with xylene and then hydrolyzed with alcohol. The thickness of the epidermis was observed using H&E stain. To confirm collagen and elastic fibers, the tissue was stained with Masson trichrome and Victoria blue. The stained tissue was observed using an optical microscope (DM750, Leica, Wetzlar, Germany).
2.9. Immunohistochemical Staining
Immunohistochemical staining was performed using UltraCision LP Large Volume Detection System HRP polymer kit (Thermo Scientific, Fremont, CA, USA). The paraffin fragment was removed using xylene, treated with ethanol, and hydrated. In order to suppress the activity of the peroxidase, it was reacted for 10 min using 3% hydrogen peroxide and washed four times using 1X TBS‐T. In order to suppress the non‐specific reaction, the reaction was performed at room temperature for 1 h with Ultra V Block. Each of the primary antibodies (S100A7, CD3, CD4) was uniformly added to the tissue and then reacted at 4°C for 24 h in a moist chamber. The slide was washed three times with TBS‐T, and then the primary antibody enhancer was reacted for 10 min and the HPR polymer was reacted for 15 min at room temperature. The slide was washed with TBS‐T, colored with DAB substrate for 30 s, compared with Mayer's hematoxylin, and then dehydrated and sealed to observe.
2.10. Assessment of Severity
We assessed the severity of the psoriasis using the Psoriasis Area and Severity Index (PASI). The severity of the lesion is divided into erythema, scale, and thickness, and is divided into five stages, from 0 to 4, respectively. The body surface area covered by psoriasis is divided into six stages: 0 = none, 1 = 1%–9%, 2 = 10%–29%, 3 = 30%–49%, 4 = 50%–69%, 5 = 70%–89%, and 6 = 90%–100%. It is calculated the weight assigned to each of the allocated weights.
2.11. Statistics
The experimental results are indicated by mean ± standard deviation (SD), and the significance test is performed by one‐way analysis of variation (ANOVA). The post‐hoc comparison between groups is performed by Turkey's HDS. A p‐value of 0.05 or less is considered statistically significant and indicated on the graphs with the following symbols: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
3. Results
3.1. In Vitro
3.1.1. Cell Viability
In the cell viability experiment according to the plasma treatment time, the cell viability decreased depending on the treatment time. It decreased significantly from 30 s, and the cell survival rate decreased to the maximum value by about 70% until the processing time was within 120 s. After that, it was confirmed that the decreasing trend slowed significantly in the time zone (Figure 1B).
In addition, when cell viability was confirmed immediately after plasma was treated by time, cell viability decreased depending on plasma treatment time. However, when the cell viability was confirmed after 4 h of plasma treatment, the cell viability was measured to be the lowest, and the level was maintained until 8 h passed. After 12 h, the trend showed a tendency to recover due to time. After 24 h, it was confirmed that the cell survival in a normal state. Therefore, cell viability decreased depending on plasma treatment time immediately after plasma treatment, but comparisons between groups measured after 4, 8, 12, and 24 h showed similar apoptosis and recovery, and there was no significant difference according to treatment time (Figure 1C).
Based on the results, there is minimal cell toxicity caused by plasma, but it can recover to normal cell viability. This can be inferred that plasma can be used to treat psoriasis through a mechanism that induces abnormal keratinocytes apoptosis and recovers to normal cells over time.
3.2. Cytokines Expression
When TNF‐α, IL‐1α, IL‐6, IL‐17A, and IL‐22 were administered to normal HaCaT cells at a concentration of 50 ng/mL to induce inflammation, we observed a rapid increase in the expression of inflammatory cytokines at specific times following cytokine exposure. IL‐17A and IL‐21 showed a rapid increase within 30 s; TNF‐α and IL‐6 within 1 h; IL‐1β and IL‐8 within 6 h; and IL‐13, IL‐19, IL‐23α, and IL‐24 within 12 h (Figure 1D).
When comparing the relative expression of inflammatory cytokines mRNA through qRT‐PCR, it was confirmed that the increased expression of inflammatory cytokines such as IL‐17A, IL‐23A, IL‐24, IL‐1β, and TNF‐α by cytokine mixture decreased in tendency with the treatment of cold plasma (Figure 1E).
3.3. Proteins Expression
Cell cycle regulatory proteins and psoriasis marker proteins were assessed to confirm how cold plasma is effective in regulating psoriasis. As a result, it was confirmed that the activation of p‐ERK that stimulates cell proliferation among MAPK proteins decreased as cold plasma was treated, and on the contrary, the activation of p‐p38 related to inflammatory reaction and apoptosis increased (Figure 1F).
The expression of p21 proteins, which is associated with linking DNA damage to cell cycle arrest, increased. The expression of CDK2 and CDK4 proteins, which promote GI/S transition as key players in cell cycle progression, CDK2 is activated while the activity of CDK4 is inhibited in psoriatic epidermis. So, in this experiment, CDK2 was decreased and CDK4 was increased by cold plasma treatment. Based on these results, the effect of cold plasma on inhibiting the cell proliferation cycle was confirmed (Figure 1G).
The activation of p‐STAT3 proteins involved in inflammatory reactions related to psoriasis was reduced when cold plasma was treated. Additionally, it was confirmed that the expression of S100A7 protein (known as psoriasin), a biomarker of psoriasis, decreased (Figure 1H).
3.4. In Vivo
3.4.1. Severity in Skin Lesions
The dorsal fur of the mice was depilated after stabilization, and psoriasis‐like inflammatory reactions were induced by applying 50 mg of Aldara cream (5% imiquimod) topically daily for a duration of 8 days. Cold plasma was irradiated daily for 2 days starting from the 7th day after the application of Aldara cream (Figure 2A).
FIGURE 2.
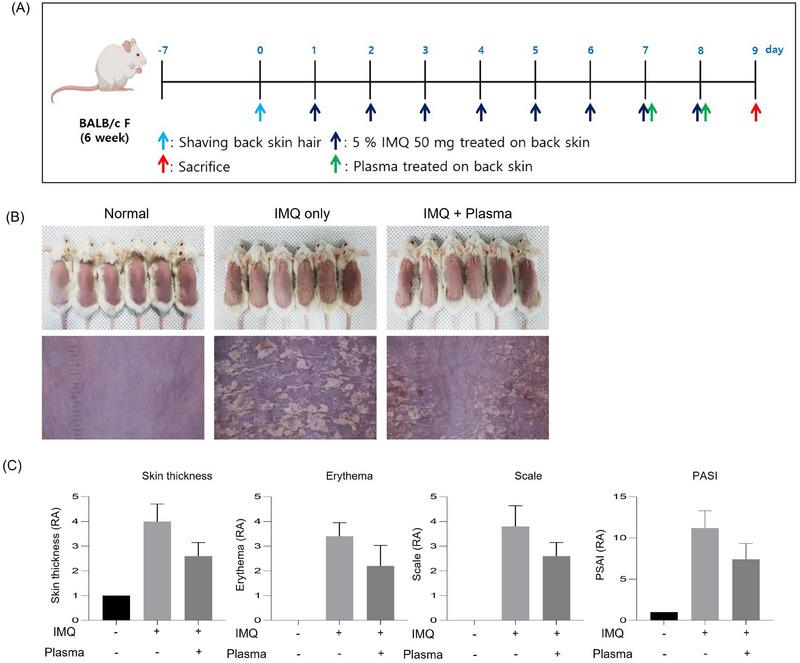
Cold plasma improves the symptoms of psoriasis in a psoriasis mimic BALB/c mice model. (A) A timetable of experimental treatments and sample collection. (B) Mouse photography by DSLR and Folliscope on Day 9. (C) The values of skin thickness, erythema, scale, and PASI (severity of psoriasis). Represent the mean ± standard deviation. Significant values are indicated as *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001 versus Normal.
In the first murine model experiment, the 18 female BALB/c mice were used and divided into three groups: normal control (n = 6), IMQ only (n = 6), and IMQ + plasma (n = 6). After treatment with plasma for 2 days, on the 9th day, psoriasis recovery was visually confirmed in the IMQ + plasma group compared to the IMQ‐only group by using DSLR and Folliscope (Figure 2B). Also, skin features were evaluated using the PASI score, a commonly used evaluation method in the clinical evaluation of psoriasis. In this model, the thickness of the skin, erythema, and the degree of scales were visually evaluated on a scale of 4 points each, and a total of 12 points were expressed. Compared to the IMQ‐only group, the PASI score was low in the test group treated with cold plasma (Figure 2C).
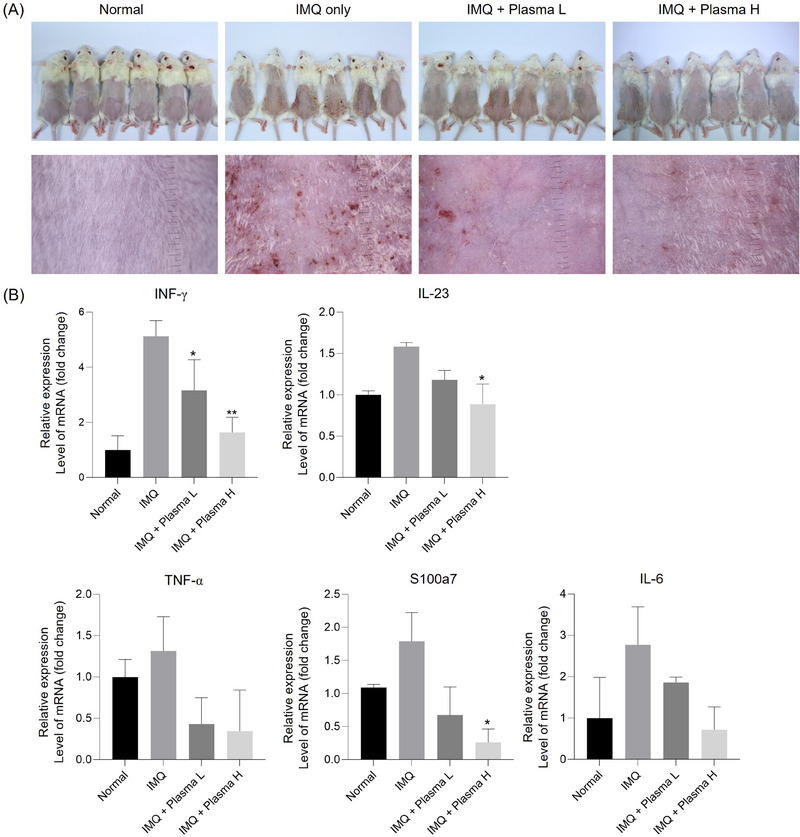
In the second murine model experiment, the 24 female BALB/c mice were used and divided into four groups: normal control (n = 6), IMQ only (n = 6), IMQ + plasma L (n = 6), IMQ + plasma H. After treatment with plasma for 2 days, on the 9th day, psoriasis recovery was visually confirmed in the IMQ + plasma group time‐dependently compared to the IMQ‐only group by using DSLR and Folliscope (Figure 3A).
FIGURE 3.
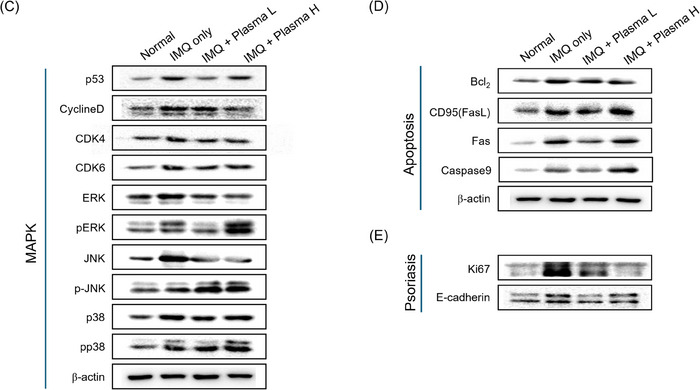
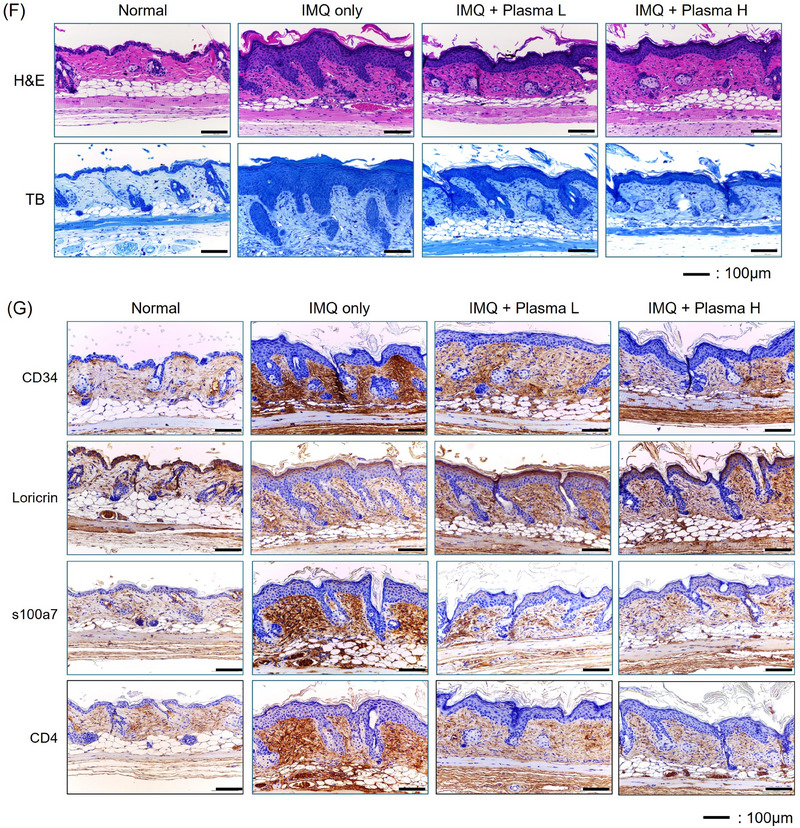
Cold plasma decreased the cytokines and factors related with psoriasis in a psoriasis mimic BALB/c mice model. (A) Mouse photography by DSLR and Folliscope on Day 9. (B) The relative expression level of inflammatory cytokines related with psoriasis mRNA level on Day 9 mouse back skin. (C–E) The expression level of MAPK pathway, cell cycle, psoriasis relative markers on Day 9 mouse back skin. (F) Representative images of H&E and MT‐stained of each treated mouse's skin on day 9. (G) IHC staining of CD34, Loricrin, s100a7, and CD4. Represent the mean ± standard deviation. Scale bar = 100 µm. Significant values are indicated as *p < 0.05 versus IMQ only.
3.5. Cytokines Expression
RNA was extracted from skin tissue and cytokines known to increase in psoriasis skin tissue, such as S100a7, INF‐ɤ, TNF‐α, IL‐6, and IL‐23, were analyzed through qPCR. Compared to the normal group, all cytokines expression increased in the psoriasis group and decreased in plasma treatment groups. The decrease in cytokines expression was greater in the plasma H group than in the plasma L group (Figure 3B).
3.6. Proteins Expression
The apoptosis‐related pathway was identified to check the cell cycle arrest caused by cold plasma treatment and to assess the improvement of hyperkeratosis in psoriasis‐like inflammation lesions. Cell cycle suppressor proteins increased in the group that induced only psoriasis but decreased in the group that treated cold plasma. Through this, it was confirmed that treating the murine model with plasma‐induced cell cycle arrest (Figure 3C). When plasma is treated, apoptosis is induced through various steps. When FAS or FASL is expressed inside the nucleus, apoptosis is induced by BCL‐2 is blocked, BAX of mitochondria is activated, and apoptosome is activated, and then caspase9 increases. When the protein expression of the inducing factors was confirmed, apoptosis‐related protein expressions increased, leading to the apoptosis of keratinocytes (Figure 3D). In addition, protein expression of Ki‐67 and E‐cadherin, indicators of psoriasis, was confirmed. The protein expression of Ki‐67 and E‐cadherin increased in the group that induced only psoriasis but decreased when cold plasma was treated. It was confirmed that cold plasma treatment relieved psoriasis in the imiquimod‐induced psoriasis‐like inflammation murine model (Figure 3E).
3.7. Histopathologic and Immunohistochemical Evaluations
Histologically, H&E and Toluidine blue stains were performed to confirm the expression of psoriasis and improvement by cold plasma treatment. In the psoriasis group, there were hyperkeratosis, parakeratosis, and pustulosis in H&E stains. In the plasma treatment groups, the thickened epidermal layer was reduced (Figure 3F).
IHC staining was performed on CD34 and S100A7, which are known to increase during psoriasis induction, and loricrin, which is known to decrease. CD34 and S100A7 were strongly induced in the psoriasis group but decreased in the plasma treatment group. The decrease was greater in the plasma treatment group for 20 min than in the plasma treatment group for 10 min. On the other hand, loricrin decreased in the psoriasis group and increased in the plasma treatment group. It was confirmed that it was time‐dependent as it was seen that the increase was greater in the plasma treatment group for 20 min than in the plasma treatment group for 10 min (Figure 3G).
4. Discussion
Plasma has various composite of active components including molecular, charged particles, electrons and photons, ultraviolet radiation, and reactive species. Plasma has been proven to have various effects through these various components. In recent studies, the effectiveness of plasma was demonstrated in antimicrobial, anti‐inflammatory, tissue‐stimulating, blood flow‐enhancing, and proapoptotic effects. Plasma devices may be divided into high‐temperature plasma and cold plasma according to the method of being emitted plasma. In general, high‐temperature plasma devices have been used for sterilization of medical devices and implants. Cold plasma devices could be used for the treatment of viable tissues and be applied to a wide range of medical research fields. Cold plasma devices can be subdivided according to indirect or direct discharge [13, 14].
The cold atmospheric plasma device, which type was used in this experimental study. The cold plasma devices using direct discharge have dielectric barrier discharge, therefore the isolated high‐voltage electrode is contacted directly to the surface to be treated biological sample or living tissue [15]. Otherwise, the indirect discharge type of cold atmospheric plasma devices produces remotely and delivers indirect plasma components to the targeted treatment tissues via a carrier gas. Partially ionized gas at atmospheric pressure and room temperature in cold atmospheric plasma devices generates plasma components such as reactive oxygen species, reactive nitrogen species, electronics, ions, UV, and visible rays [16]. This cold plasma could be applied to wide medical fields including oncology, otolaryngology, gastroenterology, and odontology.
In dermatologic fields, there has been research for atopic eczema, itch and pain relief, disinfection (bacteria/fungi/viruses), treatment of epidermal barrier defects such as ichthyosis, wound healing, and treatment of scar or skin tumor [17]. The application of cold plasma is able to be easily accessible to skin disease because of the combination of the different active agents and their broad range of positive effects. In this experimental study, the effect of cold atmospheric plasma treatment on HaCaT cells and murine models of psoriasis‐like inflammation was investigated.
In vitro, after treating cytokine mixtures on HaCaT cells, cold plasma treatment was applied to identify the expression of inflammatory cytokines, cell cycle proteins, and psoriasis markers. As a result, it was confirmed that cold plasma treatment on cytokine mixture pretreated HaCaT induced the decrease of expression of inflammatory cytokines and both cell cycle proteins and psoriasis markers. The effect of cold plasma on inhibiting the cell proliferation cycle was confirmed. Hence, the possibility of controlling inflammatory response and overexpressed cell cycle in the cold plasma treatment for psoriasis could be confirmed.
We also evaluated the effect of cold plasma in imiquimod‐induced psoriasis‐like skin inflammation in mice. In clinical features, the thickness of skin, erythema, and scale were improved by cold plasma treatment. This improvement in clinical features was consistent with histologically improved findings. Additionally, CD34 and S100A7 expression were decreased by cold plasma treatment. And the effects of plasma treatment for psoriasis‐like inflammation were more pronounced during the longer duration of plasma treatment. To suggest the mechanism by which cold plasma has a therapeutic effect on psoriasis, the expression of cell cycle and apoptosis‐related protein was evaluated. As a result, treatment with cold plasma induced cell cycle arrest and apoptosis in the hyperkeratotic skin lesions. Additionally, the expression of the Ki‐67 and E‐cadherin in psoriasis‐like inflammatory skin lesions and the expression of cytokine inflammation were reduced by cold plasma treatment.
Therefore, we could suggest that cold plasma treatment ameliorates imiquimod‐induced psoriasis‐like skin inflammation in murine models by regulating inflammatory cytokines and cell proliferation cycles. The active components of plasma could induce cell death via apoptotic pathways and suppress keratinocyte hyperproliferation and T‐cell activation through the reduction of inflammatory cytokines.
Recently, there have been a few reported papers suggesting the cold plasma effect in psoriasis. Kim et al. reported the portable cold atmospheric plasma patch alleviated the psoriatic symptoms by inducing the opening of calcium channels in keratinocytes, thereby restoring abnormal keratinocyte differentiation [18]. In other experimental studies, surface air plasma showed the effects of induction cell death and cytokine release of human keratinocytes [19]. And there was a case report that the treatment of cold atmospheric plasma led to the disappearance of psoriasis skin lesions [20]. Additionally, recent papers have reported potential applications of cold plasma in dermatology, ranging from onychomycosis to melanoma [21, 22, 23]
5. Conclusion
Our study has seen the possibility of cold plasma as a new treatment method for psoriasis through in vivo and in vitro studies. Furthermore, it is believed that cold plasma can be used as an adjuvant treatment modality for skin immune diseases. In order to confirm the effects and safety and identify the mechanisms of treatment effects, it is thought that a large population clinical study of psoriasis patients will be helpful.
Ethics Statement
This study's protocol was approved by the Institutional Animal Care and Use Committee of the Chung‐Ang University (IACUC. 202401030015).
Conflicts of Interest
The authors have no conflict of interest to declare.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B5075412).
Yu‐Jin Kim and Beom Joon Kim contributed equally to this work.
Funding: This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B5075412).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, CSY, upon reasonable request.
References
- 1. Korachi M., Ozen F., Aslan N., et al., “Biochemical Changes to Milk Following Treatment by a Novel, Cold Atmospheric Plasma System,” International Dairy Journal 42 (2015): 64–69. [Google Scholar]
- 2. Haertel B., Hähnel M., Blackert S., Wende K., von Woedtke T., and Lindequist U., “Surface Molecules on HaCaT Keratinocytes After Interaction With Non‐Thermal Atmospheric Pressure Plasma,” Cell Biology International 36, no. 12 (2012): 1217–1222. [DOI] [PubMed] [Google Scholar]
- 3. Suschek C. V. and Opländer C., “The Application of Cold Atmospheric Plasma in Medicine: The Potential Role of Nitric Oxide in Plasma‐Induced Effects,” Clinical Plasma Medicine 4, no. 1 (2016): 1–8. [Google Scholar]
- 4. Zhang J. P., Guo L., Chen Q. L., et al., “Effects and Mechanisms of Cold Atmospheric Plasma on Skin Wound Healing of Rats,” Contributions to Plasma Physics 59, no. 1 (2019): 92–101. [Google Scholar]
- 5. Heinlin J., Isbary G., Stolz W., et al., “Plasma Applications in Medicine With a Special Focus on Dermatology,” Journal of the European Academy of Dermatology and Venereology 25, no. 1 (2011): 1–11. [DOI] [PubMed] [Google Scholar]
- 6. Hasse S., Duong Tran T., Hahn O., et al., “Induction of Proliferation of Basal Epidermal Keratinocytes by Cold Atmospheric‐Pressure Plasma,” Clinical and Experimental Dermatology 41, no. 2 (2016): 202–209. [DOI] [PubMed] [Google Scholar]
- 7. Burden A., Boon M. H., Leman J., Wilson H., Richmond R., and Ormerod A., “Diagnosis and Management of Psoriasis and Psoriatic Arthritis in Adults: Summary of SIGN Guidance,” British Medical Journal 341 (2010): c5623. [DOI] [PubMed] [Google Scholar]
- 8. Arndt S., Landthaler M., Zimmermann J. L., et al., “Effects of Cold Atmospheric Plasma (CAP) on ß‐Defensins, Inflammatory Cytokines, and Apoptosis‐Related Molecules in Keratinocytes In Vitro and In Vivo,” Public Library of Science ONE 10, no. 3 (2015): e0120041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Alcantara C. C., Reiche E. M. V., and Simão A. N. C., “Cytokines in Psoriasis,” Advances in Clinical Chemistry 100 (2021): 171–204. [DOI] [PubMed] [Google Scholar]
- 10. Zhou Z.‐S., Li M., Gao F., et al., “Arecoline Suppresses HaCaT Cell Proliferation Through Cell Cycle Regulatory Molecules,” Oncology Reports 29, no. 6 (2013): 2438–2444. [DOI] [PubMed] [Google Scholar]
- 11. Turrini E., Laurita R., Stancampiano A., et al., “Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T‐Lymphoblastoid Leukemia Cells,” Oxidative Medicine and Cellular Longevity 2017 (2017): 4271065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee C.‐H., Wu S.‐B., Hong C.‐H., Yu H.‐S., and Wei Y.‐H., “Molecular Mechanisms of UV‐Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV‐Based Phototherapy,” International Journal of Molecular Sciences 14, no. 3 (2013): 6414–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gan L., Jiang J., Duan J. W., et al., “Cold Atmospheric Plasma Applications in Dermatology: A Systematic Review,” Journal of Biophotonics 14, no. 3 (2021): e202000415. [DOI] [PubMed] [Google Scholar]
- 14. Bernhardt T., Semmler M. L., Schäfer M., Bekeschus S., Emmert S., and Boeckmann L., “Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology,” Oxidative Medicine and Cellular Longevity 2019 (2019): 3873928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Šimončicová J., Kryštofová S., Medvecká V., Ďurišová K., and Kaliňáková B., “Technical Applications of Plasma Treatments: Current state and Perspectives,” Applied Microbiology and Biotechnology 103 (2019): 5117–5129. [DOI] [PubMed] [Google Scholar]
- 16. Rezaeinezhad A., Eslami P., Mirmiranpour H., and Ghomi H., “The Effect of Cold Atmospheric Plasma on Diabetes‐Induced Enzyme Glycation, Oxidative Stress, and Inflammation; In Vitro and In Vivo,” Scientific Reports 9, no. 1 (2019): 19958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamsteeg M., Jansen P., Van Vlijmen‐Willems I., et al., “Molecular Diagnostics of Psoriasis, Atopic Dermatitis, Allergic Contact Dermatitis and Irritant Contact Dermatitis,” British Journal of Dermatology 162, no. 3 (2010): 568–578. [DOI] [PubMed] [Google Scholar]
- 18. Kim N., Lee S., Lee S., et al., “Portable Cold Atmospheric Plasma Patch‐Mediated Skin Anti‐Inflammatory Therapy,” Advanced Science 9, no. 34 (2022): 2202800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhong S., Dong Y., Liu D., et al., “Surface Air Plasma‐induced Cell Death and Cytokine Release of human Keratinocytes in the Context of Psoriasis,” British Journal of Dermatology 174, no. 3 (2016): 542–552. [DOI] [PubMed] [Google Scholar]
- 20. Gareri C., Bennardo L., and De Masi G., “Use of a New Cold Plasma Tool for Psoriasis Treatment: A Case Report,” SAGE Open Medical Case Reports 8 (2020): 2050313X20922709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahn H. J., Kim T. E., Lee Y. J., Jeong S. J., Shin M. K., and Jeong K. H., “A Pilot Study of Semiquantitative Treatment Evaluation Following Nonthermal Atmospheric‐Pressure Plasma Administration for Onychomycosis,” Skin Research and Technology 29, no. 1 (2023): e13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yazdani Z., Pasandi M. S., Golpour M., Eslami M., and Rafiei A., “Effect of Cold Atmospheric Plasma on Changing of Biomolecular Structures Involved in Apoptosis Pathways of Melanoma Cancer,” Skin Research and Technology 30, no. 1 (2024): e13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang N., Yan T., Mei X., Liu J., Lei Y., and Yang C., “Cold Atmospheric Plasma Therapy for Malassezia Folliculitis: Laboratory Investigations and a Randomized Clinical Trial,” Skin Research and Technology 30, no. 7 (2024): e13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, CSY, upon reasonable request.


